
-ketoglutarate
(AKG), an intermediate of the Krebs cycle, is a common scavenger of amino groups.
Binding amino groups AKG transforms the ammonia to non toxic aminoacids like
glutamate or glutamine. AKG plays also important role in non-enzymatic oxidative
decarboxylation during hydrogen peroxide (H
2O
2)
decomposition. Combined, these effects lead to suppressed oxygen radical generation
and prevention of lipids peroxidative damage (1). It was also presented that
AKG inhibits oxidative stress induced by H
2O
2
in erythrocytes and cultured neurons (2, 3). Moreover AKG is also plays an important
role in the inhibition of mtDNA damage induced by hydrox radical (OH
·)
in mice brain (4). In addition to its protective activity on redox homeostasis,
AKG also possesses the prooxidative characteristics, forming the active complexes
with iron in brain homogenates (5). Nevertheless, the precise organism mechanisms
activated by AKG still remain poorly understood.
Deficiency of antioxidant molecules leads to the oxidative stress, which can be defined as a redox homeostasis aberration related to the oxidation process. The presence of antioxidant enzymes, like superoxide dismutase (SOD) or glutathione peroxidase (GPx) prevents the oxidative damage in cells. SOD catalyzes the conversion of superoxide to H
2O
2 and O
2, reducing its dangerous activity (6). The function of SOD as a cell molecule protector is well established. Only a few studies demonstrated SOD toxic characteristics (7). Glutathione peroxidase (GPx) reduces hydrogen peroxide and lipid peroxides to H
2O and lipid alcohols and oxidizes glutathione to glutathione disulfide (8, 9).
There are many conflicting reports on the age associated alterations in antioxidant enzyme activity. Inconsistent results may arise from the differences in strains, age of the investigated animals as well as the difference in animal maintenance conditions and experimental procedures. Previous studies showed that the activity of antioxidant enzymes (SOD, GPx) decline with age in liver, kidney and heart (10-12). However, other studies show that the activity of antioxidant enzymes either increases or remains unchanged with age (13-15).
Peroxidation of cell membrane lipids caused by reactive oxygen species (ROS) leads to malonyldialdehyde production (MDA). Lipid peroxidation products are the well-established lipid markers of oxidative stress and tissue damage (16). Estimation of MDA levels bases on its chemical reactivity with thiobarbituric acid (TBARS) (17). Number of studies present that lipid peroxidation increases with age in different tissues (12, 18). Total antioxidant status (TAS) is the commonly used marker of oxidative stress, presenting the antioxidant defense state. The redox state is not only determined by the activity or concentration of particular antioxidants but also by their synergistic effects (19).
The experimental evidences suggest that oral administration of AKG may reverse or prevent arterial stiffening in aged animals (20). Arterial hardness associated with cardiovascular (CV) risk factors, is considered to be the independent predictor of cardiovascular disease and mortality in ageing organisms (21). However it was already shown that ROS plays the pivotal role in the mechanism of vascular damage (22, 23). Indeed, an imbalance between free radicals and antioxidant levels contributes to an oxidative stress, which is believed to be an important mechanism in the development of atherosclerosis and ageing processes (24). Atherosclerotic lesions increase the production of ROS, they induce the lipids molecules oxidation, participate in inflammation and reduce the bioavailability of nitric oxide. Furthermore, other studies indicate that oxidized low-density lipoprotein (LDL) is associated with the coronary artery disease (25), while the oxidative modification of lipids and proteins and their precipitation to the arterial sub-endothelial space, leads to the reduction of arterial stiffening (26, 27).
Harisson
et al. (20) proposed different mechanisms of AKG related blood vessels flexibility. It is well known that the AKG induces proline synthesis. As blood vessels stiffening is associated with the abnormal accumulation of collage type I and III in their walls, proline plays the key role in the synthesis of collagen. Slow turnover of collagen reduces arterial elasticity. Therefore the compound that increases cellular metabolism and improves collagen synthesis, may be beneficial in maintaining the youthful characteristics of blood vessel in the older organisms.
Therefore, the objective of this study was to evaluate the effect of oral treatment with

-ketoglutarate on blood redox state in mice and to estimate the association between age-dependent oxidative stress and altered arterial elasticity.
MATERIALS AND METHODS
Animals
The experiments were conducted on mice derived from the Department of Animal
Breeding and Genetics, Warsaw University of Life Sciences and selected for body
weight. The mice featured the shortened lifespan. When compared with the ‘light’
mice from the same outbred stock, ‘heavy’ mice lived 100 days shorter on average.
The selection for body weight was not associated with the mice skin fat level
(28). Mice with the initial body weight of 36,0±3 g and final body weight of
38.4±4 g, were housed individually under standard conditions; 12/12 hour light-dark
cycle, temperature of 22±1°C and humidity of 60±1%. Mice in the control group
were fed
ad libidum with the standard diet (Labofeed H, Kcynia, Poland)
prepared according to Pastuszewska
et al. (29). Mice in the experimental
groups were fed with the standard diet supplemented with either Ca-AKG or Na-AKG
(2%), delivered by Feeds and Concentrates Production Plant in Kcynia (Poland).
Mice were provided with free access to drinking water. Mice were divided into
4 groups with 10 individuals in each group: control I (12 months old), control
II (2 months old), experimental group I fed with Ca-AKG (12 months old) and
experimental group II fed with Na-AKG (12 months old). The feed and water intake
was controlled on everyday bases. Body weight was controlled on weekly basis.
After 1 (control II) or 6 (other groups) months the animals were terminated
and tissues were collected for analysis. At the end of the experiment the number
of animals in each group was subsequently: Ca-AKG – 10 animals, Na-AKG – 8 animals
and control I – 8 animals. Each investigated animal died naturally (ageing),
what was confirmed by pathological examination. The experimental procedures
used in this study were approved by the local ethics committee.
Preparation of blood plasma, erythrocytes and liver
Venous blood was collected to heparinized tubes and centrifuged at 2000 g for 15 min at 4°C to facilitate blood plasma and erythrocyte separation. Erythrocytes were washed with 0.9% NaCl and immediately used for the measurement of activity of SOD and GPx. Plasma was aliquoted into Eppendorf tubes and liver tissue was frozen and stored at 80°C for the TAS and TBARS analysis.
Measurement of total antioxidant capacity
ABTS (2,2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) was incubated with
a peroxidase (metmyoglobin) and H
2O
2
for ABTS
·+ production. The color of the species
was detected at a wavelength of 600 nm. Antioxidants inhibit the color change
what is directly proportional to their concentration (30) (TAS NX 2332, Randox,
Crumlin, UK). A Tecan Infinite M200 analyzer was used for the measurements.
The procedure accuracy was controlled using the respective control specimens
of the same manufacturer (TAS NX 2331).
Measurement of antioxidant enzymes
SOD was detected by spectrophotometric method, using the Ransod
®
kits. This method employs xanthine and its oxidase (XOD) to generate superoxide
radicals. The radicals react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium
chloride (I.N.T.) and form a red formazan dye. SOD activity was measured
via
reaction inhibition and detected at wavelength of 505 nm (Ransod SD 125, Randox,
Crumlin, UK) (31).
GPx activity was measured by the modified Kraus and Gather method (32). In the
presence of glutathione reductase (GR) and NADPH, the oxidized glutathione (GSSG)
is converted to the reduced form with a concomitant oxidation of NADPH to NADP
+.
The absorbance was measured at wavelength 340 nm (Ransel RS 505, Randox, Crumlin,
UK).
Measurement of lipid peroxidation indices
Malondialdehyde, the most abundant product of all lipid peroxidation products, was measured using thiobarbituric acid (TBA) according to Uchiyama and Mihara technique (33). The absorbance at wavelength 535 nm was measured with a Tecan Infinite M200 analyzer. The results represent the concentration of thiobarbituric acid reactive substances (TBARS) in samples. The liver tissue was homogenized in 1% potassium chloride and centrifuged at 2000 g for 15 min at 4°C. The supernatant was used for the analysis and the tissues were placed in the reaction solution (1% phosphoric acid, 2% butylated hydroxytoluene, 1% potassium chloride and 0.4% TBA). The solution was stored at 95°C for 60 min prior to the analysis.
Aorta preparation
Mice were anaesthetized by exposure to 95% CO
2
and terminated by cervical dislocation. The dissected portion of the abdominal
aorta, prior to the right and left common iliac arteries, was carefully cleaned
to remove adhering tissues. The aorta was cut into 4 mm pieces and each piece
was attached at one end to a force transducer and metal pin on a mounting block
(34, 35). The average weight of each aorta piece was 2.75 mg. Aorta sections
were immersed into oxygenated and thermostatically controlled chambers (37°C),
having an internal depth of 5.5 cm and diameter of 3.2 cm. The sections were
stored in phosphate buffered saline (0.15 M PBS, pH 7.4) consisting of NaCl,
KCl, Na
2HPO
4
and NaH
2PO
4.
Force was measured using a FTO3 force displacement transducer (Grass Instrument,
West Warwick, RI) connected to a home-built bridge amplifier, which was interfaced
with 8S PowerLab A/D Converter (ADInstruments, Chalgrove, Oxfordshire, UK).
The transducer had a functional range from 0 to 50 g, with a reliable force
of 2 mg (equivalent to 0.004% of the functional range). The PowerLab 8S A/D
converter was connected to an iBook G4 running Chart v. 5.4 Software (AD Instruments,
Australia). The data recording was performed at a sampling speed of 40,000 data
samples per second (40 KHz) and the input impedance of the amplifier was 200
M differential (20).
Force measurements
Aorta sections were suspended vertically, in duplicates. The recorded signal was adjusted to zero for aorta sections with no tension with the aid of an offset dial mounted on the pre-amplifier unit. Each aorta section was exposed to a step-wise increase in tension (0.09 N or 10 g), measured using the FT03 Grass Force transducer. The aorta sections were then allowed to relax before being exposed to repeat step-wise increasing in tension. Aorta sections were subsequently removed and weighed. Immediately after a step-wise tension increase, the recording trace was observed to fall as the aorta tissue exerted the degree of elastic recoil. This fall in the recording trace was measured over the time using the Average Slope calculation available as part of Chart v. 5.4 Software (AD Instruments, Australia). Average Slope (g ms-1) is a time derivative of the data points in a trace selection and it was calculated from the least-square line of best fit (20).
Elastic recoil calculations
It was assumed that the tension of the aorta wall is the equivalent of the recorded
force transducer as the result of a manual stretch. The fall in the recording
trace that was observed immediately after a step-wise increase in tension was
then measured as a degree of elastic recoil in the aorta sections. The measurement
of average slope (g ms-1) obtained for each aorta sample was subsequently converted
into Newtons (N ms
-1) before being adjusted for
sample weight to give a final elastic recoil value of N ms
-1
mg
-1 wet wt (20).
Statistical analyses
Data are presented as the mean ±S.E.M. The results were analyzed by one-way analysis of variance using StatGraphics 4.1 Plus (StatPoint, Inc., USA) and were tested for Gaussian Normal Distribution. Data were found to be normally distributed with the equal variance. A difference of p<0.05 between means was considered to be significant.
RESULTS
Redox state analysis
Based on the conducted analysis we found that the old mice in control I had
the significantly lower antioxidant potential then the young animals of in control
II. Nevertheless the addition of Ca-AKG increased TAS level in the blood plasma
comparing to the control I. This effect was not observed after the addition
of Na-AKG (
Fig. 1). The old mice from control I showed the significantly
lower erythrocyte SOD activity comparing to the young animals from control II.
SOD activity in mice fed with AKG was significantly lower then in control I
group. With Na-AKG intake, SOD activity was reduced comparing to the Ca-AKG
group (
Fig. 2). GPx activity in young mice was the lowest comparing to
the other control groups. Furthermore, Na-AKG had the significant effect on
GPx activity, having the highest activity recorded among the investigated groups
(
Fig. 3). Mice fed with Ca-AKG presented lower GPx activity than the
other AKG treated groups. As it was expected young mice had the significantly
lower TBARS in blood serum comparing to the old mice. Similarly animals fed
with AKG also demonstrated lower TBARS concentration comparing to the control
I, however only the animals fed with Ca-AKG showed significant difference (
Fig.
4). Also in the liver of young animals TBARS concentration was statistically
lower then in the control I group. AKG significantly decreased lipid peroxydation
in liver comparing to the old mice received feed without the supplement. Ca-AKG
appeared to be the most efficient in lipid peroxidation inhibition, decreasing
TBARS concentration almost to the 50% of the value of control I (
Fig. 5).
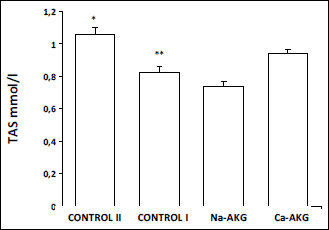 |
Fig. 1. The effect of Na-AKG
and Ca-AKG treatment on TAS level in mice blood plasma. *p<0.05 vs.
control I. **p<0,05 vs. Ca-AKG. |
 |
Fig. 2. The effect of Na-AKG
and Ca-AKG treatment on SOD activity in mice erythrocytes. *p<0.01
vs. control I. **p<0.05 vs. AKG treatment groups,***p<0.01
vs. Ca-AKG. |
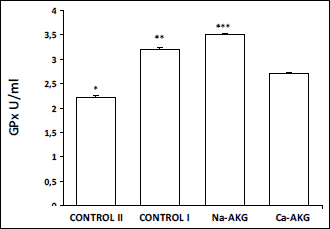 |
Fig. 3. The effect of Na-AKG
and Ca-AKG treatment on SOD activity in mice erythrocytes. *p<0.01
vs. control I. **p<0.05 vs. AKG treatment groups, ***p<0.01
vs. Ca-AKG. |
Tension measurements
An average manual step increases in tension generating 0.09 N or 10 g (4.95
x 10
-3 N mg´
1 wet
wt.). Aorta sections were found to recoil by 0.015 N or 1.5 g, which is the
value that represents approximately 15-16% of the manually applied tension.
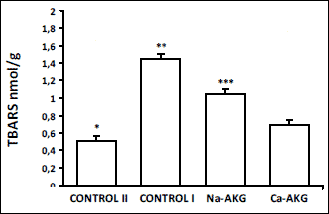 |
Fig. 4. The effect of Na-AKG
and Ca-AKG treatment on TBARS level in the mice blood plasma. *p<0.01
vs. control I. **p<0.05 vs. Ca-AKG. |
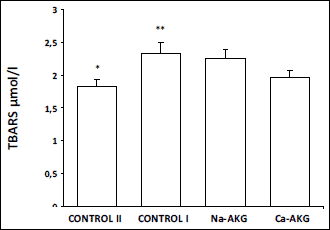 |
Fig. 5. The effect of Na-AKG
and Ca-AKG treatment on TBARS level in mice liver.
*p<0.01 vs. control I. **p<0,01 vs. AKG treatment
groups. ***P<0,01 vs. Ca-AKG. |
Old control mice
versus the animal treated with Na-AKG and Ca-AKG.
The aorta elasticity in the mice of the control group I was 2.91±0.32 x 10
-6
N ms
-1 mg
-1 wet
wt. After Na-AKG intake, the elasticity of aorta sections increased by almost
32% to 3.84±0.61 x 10
-6 N ms
-1
mg
-1 wet wt., although the value was not significantly
higher then the control. After Ca-AKG treatment, the elasticity of aorta sections
increased by 91% to 5.57±1.31 x 10
-6 N ms
-1
mg
-1 wet wt. Furthermore, Ca-AKG intake had a
significant effect on arterial elasticity compared to the control I mice (
Fig.
6).
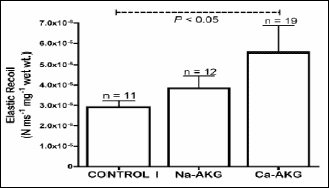 |
Fig. 6. Elastic recoil recordings
from aorta sections from Control I, Na-AKG and Ca-AKG treated old mice.
Recordings were made via force transducer attached to A/D converter at
the sampling rate of 40,000 samples/s. Each point represents the mean
±S.E.M. of 11, 12 and 19 aorta replicate samples, respectively. |
Stretch series and arterial robustness. In all studied arteries, the initial
stretch series (
e.g. application of tension followed by relaxation) lead
to the decrease in elasticity with subsequent applications of tension.
DISCUSSION
The process of ageing is commonly known to be associated with high ROS level, the decreased antioxidative cell abilities and affected redox homeostasis (36, 37). The elevated amount of free radicals is considered to be the main cause of cellular metabolism impairment at the state when the antioxidant defense system is no longer capable to remove free radicals (38). The recent studies show that antioxidant enzymes and antioxidant molecules may decrease in the consequence of ageing (12, 39, 40). These observations lead to the hypothesis that the ageing process is associated with both the decrease of the antioxidant status and the age dependent increase in lipid peroxidation (the consequence of diminished antioxidant protection). Oxidative processes in ageing organism may be not only the reason of the disease but also the effect of alteration arising from the organism physiological disturbance. Reactive oxygen forms creation in older organism was shown to be the effect of high ammonia concentration. This may arise from insufficient hepatocytes activity, hyperphosphatemia after kidney dysfunction or improper phosphor balance (41). AKG as a strongly ammonia and phosphate binding factor may indirectly stabilize redox state in organism (1, 42). The results of the presented study show the beneficial effect of

-ketoglutarate treatment on the antioxidant potential in mice, what appear to be dependent on AKG chemical structure. Old mice treated with Ca-AKG showed the significantly higher TAS level than the control and Na-AKG treated animals. TAS determines the synergistic effect of all antioxidants. Such a deficiency in any of the antioxidants may result in the reduction of TAS. Thereby, it is not surprising that previous studies reported TAS as an important diagnostic indicator of redox state related diseases (43, 44).
We observed that TBARS concentration in young mice and those fed with Ca-AKG was significantly lower compared to the control and Na-AKG treated animals. The study also shows that the liver TBARS concentration was elevated in aged animals. This result stays in the agreement with the findings of Sverko
et al. who observed that liver TBARS concentration in 3 months old mice was 0.68 nmol/mg comparing to 1.21 nmol/mg in 12 months old animals (45). Velvizhi investigated the effect of AKG on TBARS level in liver in rats treated with alcohol or ammonia (1, 2). The beneficial influence of AKG was shown in both cases, what supports the result of the presented experiments conducted on ageing mice. Sokolowska
et al. (2) suggested that

-ketoglutarate inhibits oxidative stress when it is induced by hydrogen peroxide in human erythrocytes. In the present study, the analysis of redox state markers (TAS and TBARS) suggests that

-ketoglutarate improves serum redox homeostastis and most probably protects arteries from vascular damage caused by free radicals. Indeed, the decreased TBARS concentration in blood plasma and liver, confirms proper antioxidative state. The presented AKG activity, associated with the elimination of potential environmental oxidants, supports non-enzymatic mechanism of ageing.
A number of studies present the age related high oxidative damage accompanied
by decreased activity of antioxidant enzymes such as SOD or GPx (10-12). However,
different studies demonstrated that the activity of antioxidant enzymes increases
or stays on the same level independently on age (13-15). Thus the importance
of SOD in the organism response to free radicals remains controversial. The
high level of SOD or low level GPx activity in erythrocytes are considered to
be the valuable risk predictors in diseases like pneumonia (46). An increase
in SOD activity facilitates the conversion of the superoxide anion to hydrogen
peroxide. However, Loo
et al. presented that the inhibition of SOD does
not necessarily affect O
2-
generation (47). Despite of this, the decreased SOD activity may impact carcinogenesis
(45). Moreover, it was also shown that the activity of the antioxidant enzymes
Cu/Zn-superoxide dismutase (SOD) declines in aged animals (14). Other study
shows that level of superoxide dismutase activity may change dependently on
species. Expression of SOD is comparably low in rats (48). While the increase
in SOD activity is most probably the result of high ROS level (49), the present
study compares the decreased SOD in each group of aged mice with young mice.
The high SOD activity in young mice most probably elevates H
2O
2
production what stimulates cell proliferation (50). Moreover, while the animal
and human studies show the opposite changes in SOD activity values, we conclude
that the presented results should be evaluated also by additional redox markers
(51, 52). The antioxidant status in animals fed with AKG, decreased lipid peroxidation
and lower SOD activity are interpreted as a beneficial change in redox homeostasis
stabilization. Thus, the presented positive effect of AKG treatment on arterial
elasticity, along with the observed elevated SOD and GPx activity, remain in
agreement with the previous studies (53). GPx relies on reduced glutathione
(GSH) to convert hydrogen peroxide to water. In the present study we noticed
the increased GPx level in each of the old mice groups. Guo
et al. (14)
shows elevated activity of GPx in the aortas of old mice, fed
ad libitum.
The mechanism responsible for elevation of GPx level still needs to be investigated.
We assume that the high GPx activity in rats receiving Na-AKG was the result
of a high concentration of GSH (AKG is involved in GSH synthesis). Decreased
level of glutathione reduces activity of GPx and GR (54). Indeed, we expect
that dependently on type of AKG salt and its availability in the alimentary
tract, the level of intermediate available for glutathione aminoacid synthesis
may be affected.
Tension of the artery wall depends on the amount of fibers and muscle layer thickness. Traditional antihypertensive factors were reported to reduce arterial stiffness, mostly
via lowering blood pressure. The relative resistance of peripheral arteries to stiffening in ageing animals is considered to be the effect of decreased ratio of elastin to smooth muscle or collagen. It was also suggested that the different stiffening blood vessels characteristics reflects self-remodeling ability of arteries (55). AKG has recently been identified as the natural ligand for a G-protein-coupled-receptor (GPR99), expressed in a kidney, testis and smooth muscles (56). As the receptor ligand, AKG may form a link between TCA-cycle intermediates and it may influence both metabolic status and protein/collagen synthesis. This may be a reason of the beneficial effect of AKG on aorta wall elasticity, reported in the study. Discrepancies in redox homeostasis in ageing animals may be the critical mechanism responsible for processes of tissue degeneration. Reactive oxygen species can modify aminoacid chains, form protein aggregates, cleave peptide bonds and make proteins more susceptible for the proteolytic degradation (57). Thus, the increase in oxidative stress in arterial wall with increasing age, may lead to the elevated level of particular cytokines production. Such compounds are known to stimulate the activity of elasteases responsible for elastin production. In this way, changes in elastic fiber composition within the ageing arterial wall may be associated with high tendency to calcification (58). Thus, with increasing age, aortic wall may become stiffer and harder as a direct result of high oxidative stress what induces changes in blood vessel wall composition. In support of which, a recent study indicates that AKG may improve blood vessel elasticity through the alteration in its composition (20). Based on the presented results, we hypothesize that the increased arteries elasticity of elderly mice after AKG treatment is not only the consequence of redox state stabilization and but also the result of protection of connective tissue from oxidation processes.
The presented results of redox state analysis stay in consistence with the observations of blood vessel elasticity. In both cases the most beneficial results were observed after Ca-AKG treatment. We hypothesize that the inhibition of ROS level decreases elastin degradation and it may improve aminoacid absorption. In our studies AKG administration caused the enhancement of redox homeostasis in ageing animals.
As in the alimentary tract Ca-AKG dissolves slower then Na-AKG, consequently it remains available for the longer period of time. This improves the efficiency of absorption. Even though the presented hypothesis requires further investigations, the presented study supports the following conclusions; 1) AKG dependently of its salt may contribute to different pathways of the organism redox state stimulation 2) Ca-AKG reduces lipid peroxidation and it enhances organism potential 3) AKG (mainly as Na-AKG) modulates activity of antioxidant enzymes and stabilizes redox homestasis in older mice to the level observed in young animals, 4) AKG facilitates the arterial elasticity.
Acknowledgements:
We appreciate the dedication and skilled technical assistance of B. Holle in
measurements of arterial elasticity.
Conflict of interests: None declared.
REFERENCES
- Velvizhi S, Kadiyala B, Dakshayani KB. Effects of
 -ketoglutarate on antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Nutrition 2002; 18: 747-750.
-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Nutrition 2002; 18: 747-750.
- Sokolowska M, Oleszek A, Wlodek L. Protective effect of alpha-ketoacids on the oxidative hemolysis. Pol J Pharmacol 1999; 51: 429-434.
- Desagher S, Glowinski J, Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci 1997; 17: 9060-9067.
- Yamamoto HA, Mohanan PV. Effect of alph
 -ketoglutarate and oxaloacetate on brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Toxicol Lett 2003; 143: 115-122.
-ketoglutarate and oxaloacetate on brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Toxicol Lett 2003; 143: 115-122.
- Puntel RL, Roos DH, Grotto D, Garcia SC, Nogueira CW, Rocha JB. Antioxidant properties of Krebs cycle intermediates against malonate pro-oxidant activity in vitro: a comparative study using the colorimetric method and HPLC analysis to determine malondialdehyde in rat brain homogenates. Life Sci 2007; 81: 51-62.
- Valdivia A, Perez-Alvarez S, Aroca-Aguilar JD, Ikuta I, Jordan J. Superoxide dismutases: a physiopharmacological update. J Physiol Biochem 2009; 65: 195-208.
- Gardner R, Salvador A, Moradas-Ferreira P. Why does sod overexpression sometimes enhance, sometimes decrease, hydrogen peroxide production? A minimalist explanation. Free Rad Biol Med 2002; 32: 1351-1357.
- Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 2004; 44: 381-386T.
- Mehta JL, Li D. Epinephrine upregulates superoxide dismutase in human coronary artery endothelial cells. Free Radic Biol Med 2001; 30: 148-153.
- Pieri C, Falasca M, Marcheselli F, et al. Food restriction in female Wistar rats: V. Lipid peroxidation and antioxidant enzymes in liver. Arch Gerontol Geriatr 1992; 14: 93-99.
- Chen, LH, Snyder DL. Effects of age, dietary restriction and germ-free environment on glutathione-related enzymes in Loubund-Wistar rats. Arch Gerontol Geriatr 1992; 14: 17-26.
- Xia E, Rao G, Van Remmen H, Heydari AR. Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J Nutr 1995; 125: 195-201.
- Rao G, Xia E, Nadakavukaren MJ. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr 1990; 120: 602-609.
- Guo ZM, Yang H, Hamilton ML, Van Remmen H. Richardson A. Effects of age and food restriction on oxidative DNA damage and antioxidant enzyme activities in the mouse aorta. Mech Ageing Dev 2001; 122: 1771-1786.
- Leutner S, Eckert A, Muller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm 2001; 108: 955-967.
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement and significance. Am J Clin Nutr 1993; 57: 715-725.
- Slater TF, Cheeseman KH, Davies MJ, et al. Free radical mechanisms in relation to tissue injury. Proc Nutr Soc 1987; 46: 1-12.
- Davis, LJ, Tadolini B, Biagi PL, Walford R. Licastro L. Effect of age and extent of dietary restriction on hepatic microsomal lipid peroxidation potential in mice. Mech Ageing Dev 1993; 72: 155-163.
- Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol 1994; 234: 279-293.
- Harrison A.P, Bruggemann D, Bartels EM, Andrea K, Pierzynowski S, et al. Healthy ageing: the beneficial effect of dietary supplementation with alph
 -ketoglutarate on arterial elasticity in elderly mice. JPCCR 2009; 3: 24-30.
-ketoglutarate on arterial elasticity in elderly mice. JPCCR 2009; 3: 24-30.
- Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC Study. Hypertension 1999; 34: 201-206.
- Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004; 84: 1381-1478.
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 2007; 100: 460-473.
- McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in hypertension: the role of superoxide anion. Hypertension 1999; 34: 539-545.
- Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol 2003; 41: 360-370.
- Kals J, Kampus P, Kals M, et al. Impact of oxidative stress on arterial elasticity in patients with atherosclerosis. Am J Hypertension 2006; 19: 902-908.
- Kampus P, Kals J, Unt E, et al. Association between arterial elasticity, C-reactive protein and maximal oxygen consumption in well-trained cadets during three days extreme physical load: a pilot study. Physiol Meas 2008; 29: 429-437.
- Wirth-Dzieciolowska E, Czuminska K. Longevity and aging of mice from lines divergently selected for body weight for over 90 generations. Biogerontology 2000; 1: 169-176.
- Pastuszewska B, Ochtabinska A, Morawski A. A note on the nutritional adequacy of stock diets for laboratory rats and mice. J Anim Feed Sci 2000; 9: 533-542.
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status of premature neonates. Clin Sci 1993; 84: 407-412.
- Arthur JR, Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 1985; 36: 1569-1575.
- Kraus RJ, Gather HE. Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun 1980; 96: 1116-1122.
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissue by thiobarbituric acic test. Anal Biochem 1978; 86: 271-278.
- Harrison AP, Nielsen OB, Clausen T. Role of Na+-K+
pump and Na+ channel concentrations in the
contractility of rat soleus muscle. Am J Physiol 1997; 272: 1402-1408.
- Harrison AP, Flatman JA. Measurement of force and both surface and deep M wave properties in isolated rat soleus muscles. Am J Physiol 1999; 277: R1646-R1653.
- Floyd RA. Oxidative damage to behavior during aging. Science 1991; 254: 1597.
- Sohal RS, Orr WC. Relationship between antioxidants, prooxidants, and the aging process. Ann NY Acad Sci 1992; 663: 74-84.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997; 82: 291-295.
- Visioli F, Hagen TM. Nutritional strategies for healthy cardiovascular aging: focus on micronutrients. Pharmacol Res 2007; 55: 199-206.
- Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 2009; 11: 2685-2700.
- Kuro-o. M. A potential link between phosphate and aging - lessons from Klotho-deficient mice. Mech Ageing Dev 2010; 131: 270-275.
- Birck R, Zimmermann E, Wassmer S, Nowack R, van der Woude FJ. Calcium ketoglutarate versus calcium acetate for treatment of hyperphosphataemia in patients on maintenance haemodialysis: a cross-over study. Nephrol Dial Transplant 1999; 14: 1475-1479.
- Miller NJ, Rice-Evans C, Davies MJ. A new method for measuring antioxidant activity. Biochem Soc Trans 1993; 21: 95S.
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 1993; 84: 407-412.
- Sverko V, Balog T, Sobocanec S, Gavella M, Marotti T. Age-associated alteration of lipid peroxidation and superoxide dismutase activity in CBA and AKR mice. Exp Gerontol 2002; 37: 1031-1039.
- Park EM, Ramnath N, Yang GY, et al. High superoxide dismutase and low glutathione peroxidase activities in red blood cells predict susceptibility of lung cancer patients to radiation pneumonitis. Free Radic Biol Med 2007; 42: 280-287.
- Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 2006; 290: 2600-2605.
- Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 2004; 24: 1367-1373.
- Hansberg W, de Groot H, Sies H. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic Biol Med 1993; 14: 287-293.
- Galaris D, Mantzaris M, Amorgianiotis Ch. Oxidative stress and aging: the potential role of iron. Hormones 2008; 7: 114-122.
- Salvioli S, Olivieri F, Marchegiani F, et al. Genes, ageing and longevity in humans. Problems, advantages and perspectives Free Radic Res 2006; 40: 1303-1323.
- Voss P, Siems W. Clinical oxidation parameters of aging. Free Radic Res 2006; 40: 1339-1349.
- Jones DP, Eklow L, Thor H, Orrenius S. Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Arch Biochem Biophys 1981; 210: 505-516.
- Lee HC, Wei YH. Oxidative stress, mtochondrial DNA mutation, and apoptosis in aging. Exp Biol Med 2007; 232: 592-606.
- McEniery CM, Wilkinson IB, Avolio AP, et al. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol 2007; 34: 665-671.
- He WH, Miao FJP, Lin DCH, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004; 429: 188-193.
- Vucevic D, Radosavljevic T, Zunic S, et al. The role of oxidative stress in the pathogenesis of pulmonary emphysema. Med Pregl 2005; 58: 472-477.
- Atkinson J, Capdeville-Atkinson C, Chillon JM., Giummelly P, Lartaud-Idjouadiene I. Cardiovascular aging: physiology, pathology, pharmacology and therapeutics. IDrugs 1998; 1: 650-651.





