An antiplatelet drug clopidogrel is a prodrug, the actions of which may be related to an adenosine diphosphate (ADP) receptor on platelet cell membranes (8). The drug specifically and irreversibly inhibits the P2Y12 subtype of the ADP receptor, which is important to the aggregation of platelets and cross-linking by the protein fibrin (9). The blockade of this receptor inhibits platelet aggregation by blocking activation of the glycoprotein IIb/IIIa pathway. We have previously reported that clopidogrel aggravated the severity of NSAID-induced antral ulcers in refed rats, notably converting non-hemorrhagic into hemorrhagic lesions (10). More recently, we also found using a rat model that gastric lesions induced by acidified ASA (25 mM ASA in 50 mM HCl) were markedly aggravated by pretreatment of the animals with clopidogrel (11, 12). However, neither omeprazole nor cimetidine had any effect on these responses, because the gastric ulcerogenic and bleeding responses in this model were caused by ASA acidified with exogenous HCl. Thus, it is desirable to develop a new animal model that better reflects gastric bleeding in humans and can be used to screen for the drugs effective in preventing GI bleeding.
Here, we set up a new experimental model in rats for evaluating gastric bleeding and ulceration in the stomach in response to low-dose ASA plus clopidogrel under conditions where the animals are infused with histamine, the most potent and direct stimulant of gastric acid secretion (13). We also examined using this model the prophylactic effect of various antiulcer agents, both antisecretory and mucosal protective drugs, on these responses induced under such conditions.
Animals
Male Sprague-Dawley rats (220-260 g; Nippon Charles River, Shizuoka, Japan) were acclimatized to standard laboratory conditions (12:12-h lightdark cycle, temperature 22ħ1°C). Experiments were carried out using 4~6 rats per group under urethane anesthesia (1.5 g/kg, i.p.). All experimental procedures involving animals were approved by the Experimental Animal Research Committee of Kyoto Pharmaceutical University.
General Procedures
Under urethane anesthesia, two catheters were inserted into the stomach, one from an incision in the esophagus and another through the pylorus via an incision in the duodenum, and the stomach was then perfused with saline at a rate of 0.1 ml/min using an infusion pump under histamine-stimulated acid secretion, and the perfusate was collected every 15 min for determination of hemoglobin concentration as well as acid output (12) (Fig. 1). After an equilibration period with saline perfusion for 2 hours, the stomach was perfused with 25 mM ASA for another 90 min. Gastric bleeding was evaluated as the hemoglobin concentration in the perfusate. At the end of the experiment (90 min after the onset of ASA perfusion) the animals were killed for examination of the gastric mucosa. Clopidogrel (30 mg/kg) was given p.o. 24 h before the perfusion. The doses of ASA and clopidogrel were determined based on our previous studies (11, 12). Antisecretory drugs such as a H2RA, famotidine (1~10 mg/kg), and a PPI, omeprazole (30 mg/kg), and mucosal protective drugs such as rebamipide (30 mg/kg), irsogladine (10 mg/kg) and teprenone (300 mg/kg), were given i.d. 30 min before the ASA perfusion. In some cases, the gastric mucosa was examined with a light microscope following ASA perfusion, with or without clopidogrel pretreatment, under stimulation of acid secretion caused by histamine infusion. The tissue samples were then immersed in 10% neutralized formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E).
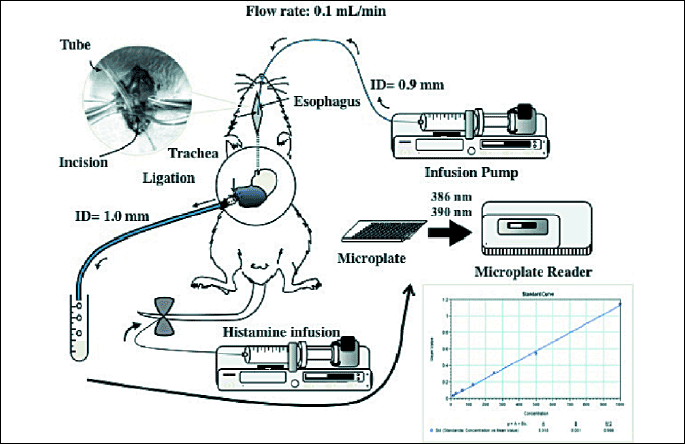 |
| Fig. 1. Schematic illustration showing the perfusion system for determining gastric bleeding in urethane-anesthetized rats. A soft catheter (ID 0.8 mm) was inserted into the stomach through an incision in the cervical esophagus and held in place by a ligature. This catheter was connected to an infusion pump, and saline or 25 mM ASA maintained at 37°C was perfused through the gastric lumen at a rate of 0.1 ml/min. Gastric outflow was collected through a cannula (ID 3.0 mm) that was inserted into the stomach via an incision in the duodenum and held in place by two ligatures around the duodenum near the pylorus. Gastric bleeding was measured as the hemoglobin concentration in the gastric perfusate. During the experimental period, acid secretion was stimulated by continuous i.v. infusion of histamine (4-16 mg/kg/h). |
Determination of histamine-stimulated acid secretion
Gastric acid secretion was stimulated by histamine in the stomach under perfusion during the entire experiment (Fig. 1). The stomach was perfused with saline at a flow rate of 0.1 ml/min for 210 min, and the perfusate was collected every 15 min for determination of acid output. In some experiments, the stomach was perfused with 25 mM ASA for the last 90 min in the animals pretreated with clopidogrel (30 mg/kg). Histamine (4-16 mg/kg/h) was continuously infused i.v. from the tail at a flow rate of 1 ml/h, and acid secretion was measured by titrating the gastric perfusate at pH 7.0 with 100 mM NaOH using a pH-stat method (Hiranuma Comtite-8, Tokyo, Japan). Acid output was calculated as the product of the acidity and volume of the gastric perfusate, and the results were presented as µEq/15 min or µEq/120 min. The following drugs, famotidine (1~10 mg/kg), omeprazole (30 mg/kg), rebamipide (30 mg/kg), irsogladine (10 mg/kg), and teprenone (300 mg/kg), were given i.d. 30 min before the start of histamine infusion or the onset of ASA perfusion.
Macroscopic evaluation of gastric lesions
Animals with various treatments were killed for examination of the gastric mucosa at the end of the experiments. The stomach was excised, treated with 2% formalin for fixation of the tissue walls, and then opened along the greater curvature, and the mucosa was examined for damage under a dissecting microscope (x10). The area (mm2) of macroscopically visible lesions was measured, summed for each tissue, and used as a lesion score. The person measuring the lesions did not know the treatments given to the animals.
Determination of hemoglobin concentrations
Gastric bleeding was determined by an increase in the hemoglobin concentration of the gastric perfusate (11, 12). To this end the gastric perfusate was collected every 15 min during the experiment where the stomach was perfused with saline or 25 mM ASA under histamine (8 mg/kg/h)-stimulated acid secretion, in the absence or presence of clopidogrel pretreatment (30 mg/kg). The concentration of hemoglobin was assessed on a Hitachi spectrophotometer (U-2000, Ibaraki, Japan), according to standard curves made by adding various amounts of rat hemoglobin (sigma Chemicals, St. Louis, MO) to saline or 25 mM ASA solutions. The absorption maximum of hemoglobin differed in the different solutions. Since in the present study the pH of the luminal perfusate, even when the stomach was perfused with saline, was around 3.5 under histamine-stimulated acid secretion. When rat hemoglobin was dissolved in saline of pH 3.5, maximal absorption occurred at 386 nm, whereas in the ASA solution of pH 3.5, maximal absorption was at 390 nm (14). These two wavelengths were used for the estimation of hemoglobin in the respective gastric perfusates. The appropriate perfusion solution adjusted to pH 3.5 was used as a blank. Data were analyzed using the Short Softmax Program and the results were presented as microgram per milliliter or microgram per 90 min.
Measurement of myeloperoxidase activity
Myeloperoxidase (MPO) activity in the antral mucosa was measured as described by Krawisz et al. (15) with some modifications. The animals were killed 90 min after the onset of 25 mM ASA perfusion under histamine (8 mg/kg/h) -stimulated acid secretion, with clopidogrel pretreatment (30 mg/kg, p.o.) given 24 h before the perfusion. In some cases, famotidine (10 mg/kg), omeprazole (30 mg/kg), rebamipide (30 mg/kg), irsogladine (10 mg/kg), or teprenone (300 mg/kg) was given i.d. 30 min before the ASA. All blood was withdrawn from the heart by perfusing with saline, and the stomach was excised and opened along the greater curvature. After the tissue was rinsed with cold saline, the antral mucosa was scraped with glass slides, weighed, and homogenized in a 50 mmol phosphate buffer containing 0.5% hexadecyl-trimethyl-ammonium bromide (pH 6.0; Sigma Chemicals, St. Louis, MO) and centrifuged at 2,000 rpm for 10 min at 4°C. MPO activity in the supernatant was determined using o-dianisidine dihydrochloride (Sigma-Aldrich). The changes in absorbance at 450 nm were recorded on a microplate reader (VERSAmax; Molecular Device, Sunnyvale, CA). Sample protein content was estimated by spectro- photometric assay (Pierce Protein Assay Kit, IL). The MPO activity was obtained from the slope of the reaction curve, based on the following equation: specific activity (µmol H2O2/min/mg protein) = (OD/min)/OD/µmol H2O2×mg protein).
Preparation of drugs
The drugs used were urethane (Tokyo Kasei, Tokyo, Japan), aspirin (Sigma Chemicals, St. Louis, MO), clopidogrel (Sanofi-Aventis, Tokyo, Japan), histamine 2HCl, famotidine (Nacalai Tesqure, Kyoto, Japan), omeprazole (Astra Zeneca, Mondal, Sweden), rebamipide (Otsuka, Tokyo, Japan), teprenone (Eisai, Tokyo, Japan) and irsogladine (Nippon-Shinyaku, Kyoto, Japan). Omeprazole was suspended in a 0.5% carboxymethylcellulose solution. Other agents were dissolved in saline. All drugs were prepared immediately before use and administered p.o. (orally), i.p. (intraperitoneally), or i.d. (intraduodenally) in a volume of 0.5 ml/100 g body weight, or infused i.v. (intravenously) in a volume of 1 ml/h. Control animals received the vehicle alone.
Statistics
Data are presented as the meanħS.E. for 4 to 6 rats per group. Statistical analyses were performed using a two-tailed unpaired t-test and Dunnetts multiple comparison test, and values of P<0.05 were regarded as significant.
Gastric ulcerogenic and bleeding responses to aspirin with or without clopidogrel pretreatment under acid-stimulated conditions
Acid secretion was stimulated by histamine infused i.v. throughout the experiments. The secretion was increased by histamine (4-16 mg/kg/h) in a dose- dependent fashion, reaching maximal values of 19.8ħ4.2 µEq/15 min, 35.7ħ6.1 µEq/15 min and 45.3ħ8.1 µEq/15 min, respectively, at 4, 8 and 16 mg/kg/h (data not shown). To determine the most effective dose of histamine, we next examined the effect of various doses (4, 8 and 16 mg/kg/h) on the gastric bleeding and lesions caused by 25 mM ASA with or without clopidogrel pretreatment (30 mg/kg). Perfusion of the stomach with 25 mM ASA alone did not cause much bleeding even when acid secretion was stimulated with histamine at either dose (Figs. 2A and 2B). Likewise, the perfusion with ASA did not generate many hemorrhagic lesions in the stomach even with the acid secretion, although some damage was observed in both the corpus and antrum of the stomach when histamine was used at 16 mg/kg/h (Fig. 2C). However, when the animals were pretreated with clopidogrel (30 mg/kg, p.o.), the ASA perfusion caused gastric bleeding on the stimulation of acid secretion, the degree being dependent on the dose of histamine (Figs. 2A and 2B). In addition, the pretreatment with clopidogrel also significantly aggravated the severity of gastric lesions caused by ASA in the presence of acid secretion; the effect was significant when gastric lesions were induced by ASA perfusion concomitant with histamine infusion at 8 and 16 mg/kg/h (Fig. 2C). The ASA perfusion without stimulation of acid secretion did not cause gastric bleeding and injury even in the presence of clopidogrel (data not shown). As shown in Fig. 2D, the gastric lesions produced by 25 mM ASA when acid secretion was stimulated by histamine (8 mg/kg/h) were markedly aggravated by pretreatment with clopidogrel (30 mg/kg), converting the lesions from non-hemorrhagic to hemorrhagic.
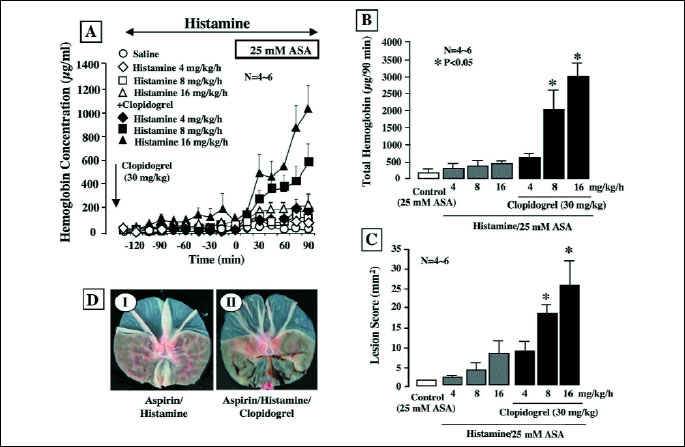 |
| Fig.
2. Effect of histamine on gastric bleeding (A&B) and ulcerogenic
(C&D) responses induced by luminal perfusion of ASA in the
absence or presence of clopidogrel in urethane-anesthetized rats. Histamine
(4-16 mg/kg/h) was given by continuous i.v. infusion from the tail during
the experimental period. The stomach was perfused with saline for 2 h
and then with 25 mM ASA for another 90 min. Clopidogrel (30 mg/kg) was
given p.o. 24 h before the onset of ASA perfusion. Fig. 2A shows
the time-course of change in the hemoglobin concentration in the luminal
perfusate, and the data are presented as the mean±S.E. of values
determined every 15 min from 4-6 rats. Fig. 2B shows total hemoglobin
output in the perfusate for the last 90 min, and the data are presented
as the mean±S.E. for 4-6 rats. Fig. 2C shows gastric lesions
generated by 25 mM ASA under various conditions, and the data are presented
as the mean±S.E. for 4-6 rats. Fig. 2D shows the macroscopic
appearance of hemorrhagic gastric lesions caused by 25 mM ASA in the absence
(I) or presence (II) of clopidogrel (30 mg/kg) pretreatment when acid
secretion was stimulated by histamine (8 mg/kg/h). * Significant difference from the corresponding groups without clopidogrel pretreatment. |
In the subsequent experiments, we therefore perfused the stomach with 25 mM ASA in the presence of clopidogrel (30 mg/kg) when acid secretion was stimulated by the i.v. infusion of histamine at 8 mg/kg/h.
Effect of various drugs on histamine-stimulated gastric acid secretion
Since the presence of luminal acidity is essential for ASA-induced gastric lesions (12, 16) and since the present model consists of luminal perfusion with ASA and stimulation acid secretion, in addition of clopidogrel pretreatment, we examined the effects of various drugs at the doses used in the present study on histamine- induced acid secretion.
Acid secretion was increased by the i.v. infusion of histamine at 8 mg/kg/h, reaching a plateau of about 40 µq/15 min within 40 min (Fig. 3A). The acid response to histamine was dose-dependently reduced by famotidine (110 mg/kg), an H2RA, given i.d. 30 min before the histamine infusion, the effect being significant even at 1 mg/kg. Total acid output for 2 h after the histamine infusion was also dose-dependently and significantly decreased by famotidine, the inhibition being 58.3%, 61.2%, and 79.2%, respectively, at 1, 3 and 10 mg/kg (Fig. 3B). Likewise, the acid secretory response to histamine (8 mg/kg/h) was almost completely inhibited by omeprazole (30 mg/kg, i.d.), a PPI, given 30 min before histamine (Fig. 4A), and the inhibition of total acid output was 92.3% (Fig. 4B). By contrast, the acid response to histamine was not significantly affected by either rebamipide (30 mg/kg), irsogladine (10 mg/kg) or teprenone (300 mg/kg) given i.d. 30 min before the histamine infusion.
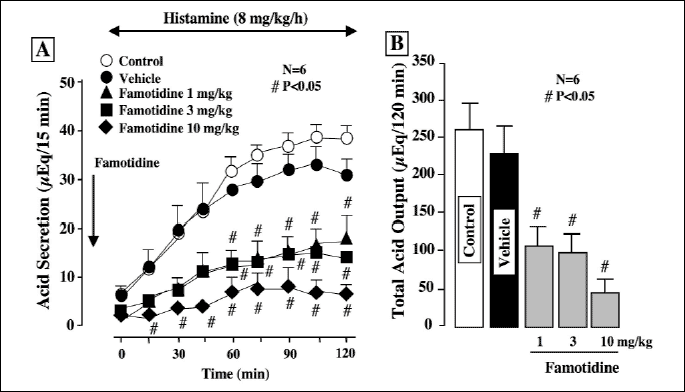 |
| Fig. 3. Effect of famotidine on histamine-stimulated acid secretion in urethane- anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h). Famotidine (1-10 mg/kg) was given i.d. 30 min before the histamine infusion. Fig. 3A shows the time-course of change in acid secretion, and the data are presented as the mean±S.E. of values determined every 15 min from 6 rats. Fig. 3B shows total acid output for 2 h, and the data are presented as the mean±S.E. for 6 rats. # Significant difference from vehicle, at P<0.05. |
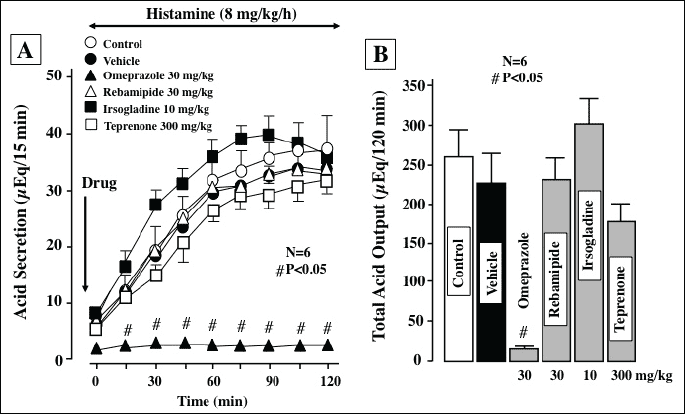 |
| Fig. 4. Effect of various antiulcer drugs on histamine-stimulated acid secretion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h). Omeprazole (30 mg/kg), rebamipide (30 mg/kg), irsogladine (10 mg/kg) or teprenone (300 mg/kg) was given i.d. 30 min before the histamine infusion. Fig. 4A shows the time-course of change in acid secretion, and the data are presented as the mean±S.E. of values determined every 15 min from 6 rats. Fig. 4B shows total acid output for 2 h, and the data are presented as the mean±S.E. for 6 rats. # Significant difference from vehicle, at P<0.05. |
Effect of famotidine on gastric bleeding and ulcerogenic responses to aspirin plus clopidogrel under stimulation of acid secretion
Histamine (8 mg/kg/h) was infused i.v. for 210 min in the animals pretreated with clopidogrel (30 mg/kg), and the stomach was perfused with saline for the first 120 min and with 25 mM ASA for the last 90 min. Following the histamine infusion, acid secretion was increased and reached about 40 µEq/15 min, while ASA perfusion significantly raised acid output because of the titratable acidity due to ASA, with values reaching ~55 µEq/15 min (Fig. 5). Famotidine (1-10 mg/kg, i.d.), given 30 min before ASA, dose-dependently and significantly decreased acid output, and the inhibitory effect was observed even after the stomach was perfused with ASA.
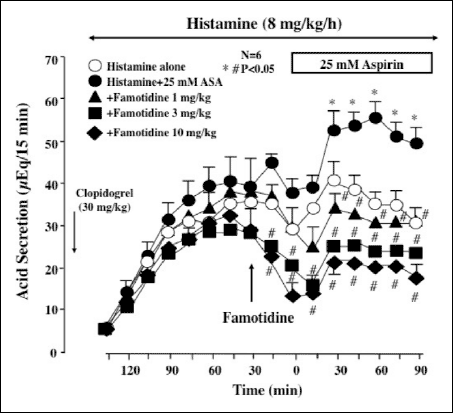 |
Fig. 5. Effect of famotidine on histamine-stimulated acid secretion in the absence and presence of luminal ASA perfusion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h). ASA (25 mM) was perfused in the stomach for the last 90 min of the experimental period. Clopidogrel (30 mg/kg) was given p.o. 24 h before the ASA perfusion. Famotidine (1-10 mg/kg) was given i.d. 30 min before ASA. Data are presented as the mean±S.E. of values determined every 15 min from 6 rats. Significant difference at P<0.05; * from histamine alone (without ASA perfusion); # from vehicle. |
The luminal perfusion of ASA in the presence of clopidogrel increased gastric bleeding when acid secretion was stimulated by histamine, the response being especially pronounced from 30 min after the onset of ASA perfusion (Fig. 6A), and the total amount of hemoglobin was almost 10 times greater than that in control animals without clopidogrel pretreatment (Fig. 6B). The bleeding in response to ASA was dose-dependently prevented by famotidine (1-10 mg/kg, i.d.), given 30 min before ASA, and the increase in total hemoglobin output was all but totally inhibited by famotidine, the degree of inhibition even at 1 mg/kg being over 90%. Although ASA perfusion alone caused only few hemorrhagic lesions in the stomach under histamine-stimulated acid secretion, the additional treatment with clopidogrel caused multiple hemorrhagic lesions, the lesion score being 24.7ħ4.1 mm2 (Figs. 7A and 7B). The ulcerogenic response to ASA plus clopidogrel with acid secretion was also dose-dependently and significantly inhibited by famotidine, and this agent almost completely prevented the generation of hemorrhagic gastric lesions even at 1 mg/kg.
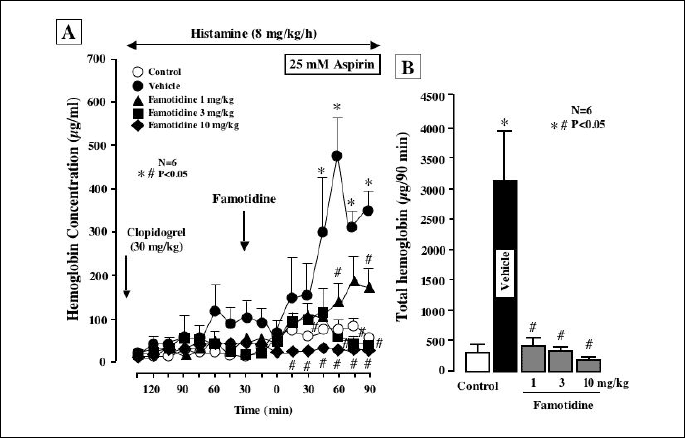 |
| Fig. 6. Effect of famotidine on gastric bleeding induced by luminal perfusion of ASA in the presence of clopidogrel under histamine-stimulated acid secretion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h) for 210 min. ASA (25 mM) was perfused in the stomach for the last 90 min of the experimental period. Clopidogrel (30 mg/kg) was given p.o. 24 h before the ASA perfusion. Famotidine (1-10 mg/kg) was given i.d. 30 min before ASA. Fig. 6A shows the time-course of change in the hemoglobin concentration in the luminal perfusate, and the data are presented as the mean±S.E. of values determined every 15 min from 6 rats. Fig. 6B shows total hemoglobin output in the perfusate for the last 90 min and the data are presented as the mean±S.E. for 6 rats. Significant difference at P<0.05; * from control (without clopidogrel); # from vehicle. |
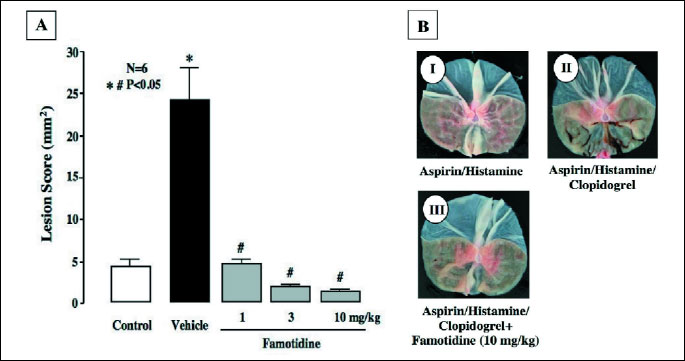 |
| Fig. 7. Effect of famotidine on gastric hemorrhagic lesions induced by luminal perfusion of ASA in the presence of clopidogrel under histamine-stimulated acid secretion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h) for 210 min. ASA (25 mM) was perfused in the stomach for the last 90 min of the experimental period. Clopidogrel (30 mg/kg) was given p.o. 24 h before the ASA perfusion. Famotidine (1-10 mg/kg) was given i.d. 30 min before ASA. Fig. 7A shows hemorrhagic lesions, and the data are presented as the mean±S.E. for 6 rats. Significant difference at P<0.05; * from control (without clopidogrel); # from vehicle. Fig. 7B shows the macroscopic appearance of hemorrhagic gastric lesions induced by luminal perfusion with ASA under various conditions; I: ASA perfusion plus histamine infusion (ASA/Histamine); II: ASA/Histamine plus clopidogrel pretreatment (ASA/Histamine/Clopidogrel); III: ASA/Histamine/Clopidogrel plus famotidine (10 mg/kg). Note that famotidine markedly prevented the generation of hemorrhagic lesions in the stomach induced by ASA/Histamine/Clopidogrel. |
Effect of various drugs on gastric bleeding and ulcerogenic responses to aspirin plus clopidogrel with stimulated acid secretion
Histamine (8 mg/kg/h) increased acid secretion in the stomach perfused with saline or 25 mM ASA, in the presence of clopidogrel (30 mg/kg) (Fig. 8). Although acid output was increased after the perfusion of ASA, omeprazole (30 mg/kg) significantly decreased the acid response to histamine before and after the ASA perfusion. As expected, neither rebamipide (30 mg/kg), irsogladine (10 mg/kg) nor teprenone (300 mg/kg) had any effect on the histamine-induced acid secretion before and after ASA perfusion in the presence of clopidogrel.
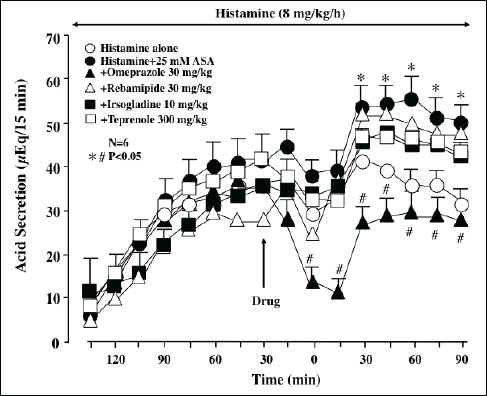 |
Fig. 8. Effect of various antiulcer drugs on histamine-stimulated acid secretion in the absence and presence of luminal ASA perfusion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h). ASA (25 mM) was perfused in the stomach for the last 90 min of the experimental period. Clopidogrel (30 mg/kg) was given p.o. 24 h before the ASA perfusion. Omeprazole (30 mg/kg), rebamipide (30 mg/kg), irsogladine (10 mg/kg) or teprenone (300 mg/kg) was given i.d. 30 min before ASA. Data are presented as the mean±S.E. of values determined every 15 min from 6 rats. Significant difference at P<0.05; * from histamine alone (without ASA perfusion); # from vehicle. |
Gastric bleeding induced by ASA plus clopidogrel in the presence of histamine was completely prevented by omeprazole. Perfusion of the stomach with ASA in the presence of clopidogrel caused gastric bleeding when acid secretion was stimulated by histamine, the increase in the hemoglobin concentration being prominent from 45 min later (Fig. 9A), and the total amount of hemoglobin reached about 10 times the values for control animals without clopidogrel pretreatment (Fig. 9B). Prior administration of omeprazole (30 mg/kg) almost completely prevented the gastric bleeding in response to ASA under such conditions and markedly inhibited total hemoglobin output in the stomach during the entire experiment. Likewise, other antiulcer drugs such as rebamipide (30 mg/kg), irsogladine (10 mg/kg) and teprenone (300 mg/kg) also significantly prevented gastric bleeding in response to ASA perfusion with acid secretion in the presence of clopidogrel. Total hemoglobin output was also significantly reduced by these drugs, by 75%, 87.5% and 81.2%, respectively. In addition, the gastric ulcerogenic response to luminal perfusion of ASA plus clopidogrel pretreatment under acid secretion was almost completely attenuated by the proton pump inhibitor omeprazole (30 mg/kg), the inhibition being 92.3% (Fig. 9C). Likewise, the severity of gastric lesions was also significantly reduced by the mucosal protective drugs, such as rebamipide (30 mg/kg), irsogladine (10 mg/kg) and teprenone (30 mg/kg), by 52.0%, 56.4% and 40.3%, respectively, although their effects were significantly less pronounced than that of omeprazole.
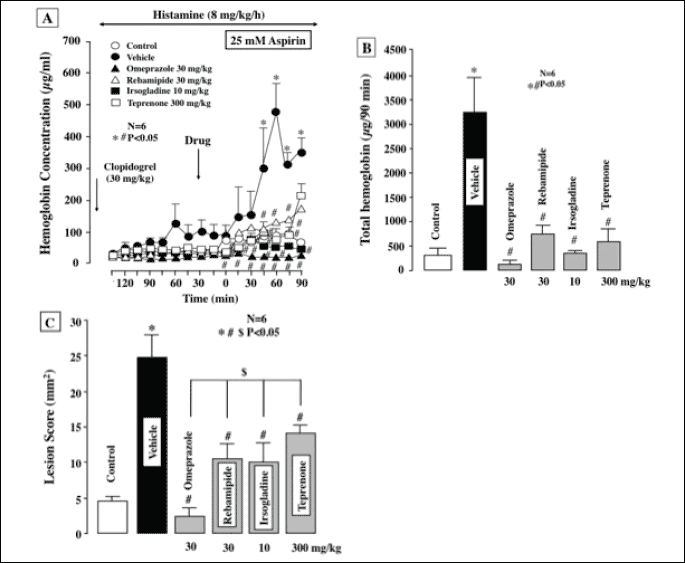 |
| Fig. 9. Effect of various antiulcer drugs on gastric bleeding and hemorrhagic lesions induced by luminal perfusion of ASA in the presence of clopidogrel under histamine-stimulated acid secretion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h) for 210 min. ASA (25 mM) was perfused in the stomach for the last 90 min of the experimental period. Clopidogrel (30 mg/kg) was given p.o. 24 h before the ASA perfusion. Omeprazole (30 mg/kg), rebamipide (30 mg/kg), irsogladine (10 mg/kg) or teprenone (300 mg/kg) was given i.d. 30 min before ASA. Fig. 9A shows the time-course of change in the hemoglobin concentration in the luminal perfusate, and the data are presented as the mean±S.E. of values determined every 15 min from 6 rats. Fig. 9B shows total hemoglobin output in the perfusate for the last 90 min, while Fig. 9C shows hemorrhagic lesions, and these data are presented as the mean±S.E. for 6 rats. Significant difference at P<0.05; * from control (without clopidogrel); # from vehicle; $ from omeprazole in Fig. 9C. |
Effect of various drugs on changes in gastric mucosal myeloperoxidase activity induced by aspirin plus clopidogrel with stimulated acid secretion
The MPO activity in the normal gastric mucosa was 0.006ħ0.0001 µmol H2O2/min/mg protein and increased markedly after luminal perfusion of 25 mM ASA in the presence of clopidogrel (30 mg/kg) under histamine (8 mg/kg/h)-stimulated acid secretion, reaching 0.068ħ0.003 µmol H2O2/min/mg protein, approximately 7 times greater than the control value (Fig. 10). The increased MPO activity induced by ASA under such conditions was strongly suppressed by pretreatment with the antisecretory drugs, famotidine (10 mg/kg) and omeprazole (30 mg/kg), with over 95% inhibition in both cases. Likewise, the mucosal protective agents, rebamipide (30 mg/kg), irsogladine (10 mg/kg) and teprenone (300 mg/kg), also significantly attenuated the increase in MPO activity, although less so than famotidine or omeprazole.
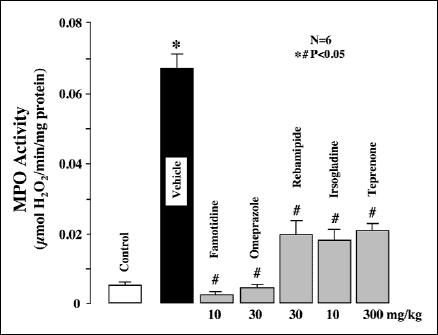 |
Fig. 10. Effect of various antiulcer drugs on the increased MPO activity caused by luminal perfusion of ASA in the presence of clopidogrel under histamine-stimulated acid secretion in urethane-anesthetized rats. Acid secretion was stimulated by the i.v. infusion of histamine (8 mg/kg/h) for 210 min. ASA (25 mM) was perfused in the stomach for the last 90 min of the experimental period. Clopidogrel (30 mg/kg) was given p.o. 24 h before the ASA perfusion. Famotidine (10 mg/kg), omeprazole (30 mg/kg), rebamipide (30 mg/kg), irsogladine (10 mg/kg) or teprenone (300 mg/kg) was given i.d. 30 min before ASA. Data are presented as the mean±S.E. for 6 rats. Significant difference at p<0.05; * from control (without clopidogrel); # from vehicle. |
We set up a new model of gastric bleeding in response to low-dose ASA with histamine-stimulated acid secretion, and demonstrated that the response was markedly aggravated by clopidogrel, a P2Y12 receptor antagonist, confirming the increased risk of bleeding in the upper GI tract on the concomitant use of antiplatelet drugs with low-dose ASA (4, 5). In addition, the increase in gastric bleeding was markedly prevented by the H2RA famotidine and the PPI omeprazole, supporting clinical findings that inhibiting gastric acid secretion is effective in the treatment of gastric bleeding and peptic ulcer hemorrhage (6-8, 17, 18). We also observed that the bleeding and hemorrhagic lesions caused by co-treatment with ASA and clopidogrel when acid secretion was stimulated by histamine was significantly mitigated by mucosal protective drugs such as rebamipide, irsogladine, and teprenone.
Antiplatelet therapy is effective in reducing the incidence of cerebrovascular accidents, myocardial infarction and death from vascular causes in individuals with symptomatic atherothrombic diseases (1). Low-dose ASA is most commonly used for the secondary prevention of vascular events. Furthermore, most clinical trials have established the superiority of combined antiplatelet treatment over ASA alone in preventing thrombotic outcomes (19). However, the risk of bleeding in the upper GI tract is increased on the concomitant use of antiplatelet drugs with NSAIDs or low-dose ASA (2-5). We have confirmed in rats that clopidogrel aggravated the severity of NSAID-generated antral ulcers, converting non-hemorrhagic lesions into hemorrhagic ones (10), and that gastric bleeding caused by the luminal perfusion of acidified ASA was markedly increased by pretreatment with clopidogrel (11, 12). The present study further showed that clopidogrel increased gastric bleeding in response to ASA when acid secretion was stimulated by histamine, without the addition of exogenous acid.
In the present experimental system, the perfusion of ASA alone caused neither gastric bleeding nor hemorrhagic damage in the stomach. Even in the presence of histamine (to stimulate acid secretion), this treatment only caused a small amount of bleeding and minor hemorrhagic damage. However, when the animals were pretreated with clopidogrel, ASA perfusion under histamine-stimulated acid secretion provoked marked bleeding and damage, depending on the dose of histamine. These results confirmed the importance of luminal acid in the pathogenic mechanism of ASA-induced gastric damage (16) and the increased risk of gastric bleeding on the concomitant use of ASA and clopidogrel. Ritchie et al. (20) proposed three factors essential for the generation of gastric lesions; 1) gastric barrier disruption, 2) luminal acid, and 3) pharmacologically induced gastric mucosal ischemia. Many studies have demonstrated that ASA disrupts the gastric mucosal barrier and damages the stomach in the presence of acid (16, 21). Gastric mucosal blood flow is increased in response to barrier disruption, mediated by endogenous prostaglandins (PGs), to prevent local acidosis due to acid back-diffusion (22). However, ASA attenuates this gastric hyperemic response by suppressing PG production through inhibition of cyclooxygenase activity (23). Thus, the present model which employed ASA perfusion, stimulation of acid secretion, and inhibition of PG production by ASA, seems to fulfill the conditions necessary to generate damage in the stomach. As expected, pretreatment of the animals with clopidogrel aggravated the gastric bleeding in response to ASA when acid secretion was stimulated, and eventually increased the severity of the ASA-induced hemorrhagic damage in the stomach.
Secondly, it was found that famotidine, a H2RA, dose-dependently prevented gastric ulcerogenic and bleeding responses to ASA plus clopidogrel under conditions of histamine-stimulated acid secretion. Since this agent inhibited the acid secretion by blocking histamine H2 receptors and since ASA alone without the histamine infusion did not provoke gastric bleeding and damage even in the presence of clopidogrel, the prophylactic effect of famotidine is assumed to be brought about by inhibition of acid secretion. This interpretation is supported by the finding that another antisecretory drug, omeprazole, also strongly inhibited gastric bleeding and ulcerogenic responses to ASA plus clopidogrel with acid secretion. Certainly, we cannot totally exclude the possibility that famotidine exerts a protective effect against gastric lesions caused by ASA through a mechanism independent of acid inhibition. Indeed, Guth et al. (24) reported that cimetidine, another H2RA, protected against gastric mucosal injury by topical ASA plus HCl by some mechanism other than inhibition of acid and pepsin secretion. We have previously reported that famotidine prevented the aggravation of NSAID-induced antral lesions by clopidogrel in rats (10).
Although ASA-clopidogrel antiplatelet dual therapy is widely prescribed worldwide, with PPIs frequently used to prevent gastrointestinal bleeding (6, 7), some studies recommend not adding a PPI to the dual therapy without formal indications (3, 25, 26). Clopidogrel is a prodrug and requires several biotrans-formational steps, mediated mainly by cytochrome P-450 isoenzymes, to generate an active metabolite. It exerts its antiplatelet effect by forming an inactivating disulfide bond with the platelet ADP receptor P2Y12 (9). Several functional polymorphisms have been found in genes encoding cytochrome P-450 isoforms involved in the metabolic activation of clopidogrel upstream of P2Y12 (27). The isoenzyme CYP2C19 seems to be one of the determinants of the pharmacodynamic response to clopidogrel and is also involved in the metabolism of PPIs, such as omeprazole (28-30). Thus, it is assumed that PPIs reduce the biological action of clopidogrel, probably through competitive metabolic effects on CYP2C19. In the present study, we did not examine whether the antiplatelet activity of clopidogrel is negatively affected by omeprazole. However, since clopidogrel was administered 24 h before the onset of ASA perfusion, and since omeprazole was given 30 min before ASA perfusion, it is unlikely that the activation of clopidogrel is influenced by omeprazole. Nevertheless, famotidine would be preferable to PPIs for preventing gastric bleeding caused by ASA-clopidogrel antiplatelet therapy, in as much as this agent, unlike PPIs, has little influence on CYP2C19 (31).
Many studies have reported the effect of antisecretory drugs on the bleeding in the upper GI tract associated with low-dose ASA and clopidogrel (6, 7), however, little is known about the effect of gastroprotective agents with the increased risk of gastric bleeding from this dual therapy. The present study showed that gastric protective drugs were also effective against gastric bleeding in response to ASA plus clopidogrel on stimulation of acid secretion. The mucosal protective drugs employed in this study, rebamipide, teprenone and irsogladine, are used to treat gastritis and gastric ulcers in Japan. Certainly, none of these agents had a significant effect on histamine-stimulated acid secretion, irrespective of whether they were administered before or after the histamine infusion. Among them, rebamipide suppresses inflammatory cell infiltration and the generation of free radicals, exhibits radical- scavenging action, and exerts a potent anti-inflammatory effect (32-34). Irsogladine is also reported to protect the gastric mucosa by enhancing the mucosal integrity of the stomach through the facilitation of gap junctional intracellular communication and by increasing the intracellular levels of 3,5-cyclic adenosine monophosphate (35, 36). On the other hand, teprenone exhibits a protective effect in various models by stimulating the secretion of mucus and the expression of heat shock proteins (37, 38). We have previously reported that rebamipide, irsogladine and teprenone significantly reduced the severity of hemorrhagic gastric lesions produced by NSAID plus clopidogrel, together with the suppression of increased MPO activity (10). Likewise, the present study also confirmed that these agents significantly reduced the severity of gastric bleeding and damage as well as the increase in MPO activity, although the effects were less pronounced than that of famotidine or omeprazole. At present, the precise mechanisms by which these drugs reduced the gastric ulcerogenic response to ASA plus clopidogrel when acid secretion was stimulated by histamine remain unknown. Moreover, the clinical effectiveness of gastroprotective agents on gastric bleeding associated with dual anti-platelet therapy, using low-dose ASA plus clopidogrel, remains still unproven. Thus, further animal and clinical studies are needed to clarify these points.
Given the present findings, it is concluded that clopidogrel increases gastric bleeding induced by low-dose ASA on stimulation of acid secretion, and both famotidine and omeprazole are highly effective in preventing gastric bleeding under such conditions. In addition, the mucosal protective drugs, such as rebamipide, teprenone and irsogladine, are also effective against gastric bleeding and ulceration after dual antiplatelet therapy, although less so than antisecretory drugs. It is assumed that antisecretory drugs, especially H2RAs including famotidine which is likely not to interfere the biotransformation of clopidogrel, can be used as a prophylactic for preventing gastric bleeding during ASA-clopidogrel therapy. Finally, the present model may be useful for the screening of drugs that protect against gastric bleeding induced by the co-administration of low-dose ASA and antiplatelet drugs.
Conflict of interest: None declared.
- Collaborative overview of randomised trials of antiplatelet therapy-I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists Collaboration. Br Med J 1994; 308: 81-106.
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N Engl J Med 1999; 340: 1888-1899.
- Ng FH, Wong SY, Chang CM, et al. High incidence of clopidogrel-associated gastrointestinal bleeding in patients with previous peptic ulcer disease. Aliment Pharmacol Ther 2003; 18: 443-449.
- Ibanez L, Vidal X, Vendrell L, Moretti U, Laporte JR. Spanish-Italian collaborative group for the epidemiology of gastrointestinal bleeding: upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment Pharmacol Ther 2006; 23: 235-242.
- Hallas J, Dall M, Andries A, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. Br Med J 2006; 333: 726.
- Yasuda H, Yamada M, Sawada S, et al. Upper gastrointestinal bleeding in patients receiving dual antiplatelet therapy after coronary stenting. Intern Med 2009; 48: 1725-1730.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363: 1909-1917.
- Gill JM, Player MS, Metz DC. Balancing the risks and benefits of proton pump inhibitors. Ann Fam Med 2011; 9: 200-202.
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 2004; 113: 340-345.
- Yamasaki K, Izuhara C, Honda M, Nishio H, Takeuchi K. Aggravation by clopidogrel, an antiplatelet drug, of antral ulcers induced by NSAID in rats: a prophylactic effect of famotidine (abstract in English). Pharma Medica 2009; 27: 106-115.
- Izuhara C, Yamasaki K, Honda M, Nishio H, Takeuchi K. Aggravation by clopidogrel, an anti-platelet drug, of HCl/aspirin-induced gastric bleeding and lesions in rats: prophylactic effect of rebamipide (abstract in English). Jap Pharmacol Ther 2010; 38: 333-345.
- Izuhara C, Takayama S, Yamada N, Kimura M, Takeuchi K. Aggravation by clopidogrel, an antiplatelet drug, of HCl/aspirin-induced gastric hemorrhagic lesions in rats: prophylactic effect of irsogladine (abstract in English). Jpn Pharmacol Ther 2011; 39: 421-435.
- Konturek SJ. Gastric secretion-from Pavlovs nervism to Popielskis histamine as direct secretagogue of oxyntic glands. J Physiol Pharmacol 2003; 54(Suppl 3): 43-68.
- Holzer P, Pabst MA, Lippe I.Th. Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology 1986; 96: 1425-1433.
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984; 87: 1344-1350.
- Okabe S, Takeuchi K, Nakamura K, Takagi K. Pathogenesis of gastric lesions induced by aspirin in the pylorus-ligated rats. Jpn J Pharmacol 1974; 24: 363-371.
- Ghassemi KA, Kovacs TO, Jensen DM. Gastric acid inhibition in the treatment of peptic ulcer hemorrhage. Curr Gastroenterol Rep 2009; 11: 462-469.
- van Rensburg C, Barkun AN, Racz I, et al. Clinical trial: intravenous pantoprazole vs. ranitidine for the prevention of peptic ulcer rebleeding: a multicentre, multinational, randomized trial. Aliment Pharmacol Ther 2009; 229: 497-507.
- Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for he prevention of atherothrombosis. N Engl J Med 2005; 353: 2373-2383.
- Ritchie WP. Acute gastric mucosal damage induced by bile salts, acid, and ischemia. Gastroenterology 1975; 68: 699-707.
- Davenport HW. Gastric mucosal hemorrhage in dogs. Effects of acid, aspirin, and alcohol. Gastroenterology 1969; 56: 439-449.
- Takeuchi K, Ueki S, Tanaka H. Endogenous prostaglandins in gastric alkaline response in the rat stomach after damage. Am J Physiol 1986; 250: G842-G849.
- Mashita Y, Taniguchi M, Yokota A, Tanaka A, Takeuchi K. Oral but not parenteral aspirin up-regulates COX-2 expression in rat stomachs: a relationship between COX-2 expression and PG deficiency. Digestion 2006; 73: 124-132.
- Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesions in the rat: cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology 1979; 76: 88-93.
- Lin KJ, Hernadez-Diaz S, Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology 2011; 141: 71-79.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301: 937-944.
- Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 2003; 108: 989-995.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors: emphasis on rabeprazole. Aliment Pharmacol Ther 1999; 13(Suppl 3): 27-36.
- Gilard M, Arnaud B, Le Gal G, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated to aspirin J Thromb Haemost 2006; 4: 2508-2509.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101: 714-719.
- Sambol NC, Upton RA, Chremos AN, Lin ET, Williams RL. A comparison of the influence of famotidine and cimetidine on phenytoin elimination and hepatic blood flow. Br J Clin Pharmacol 1989; 27: 83-87.
- Ogino K, Hobara T, Ishiyama H, Yamasaki K, Kobayashi H, Izumi Y, Oka S. Antiulcer mechanism of action of rebamipide, a novel antiulcer compound, on diethyldithiocarbamate-induced antral gastric ulcers in rats. Eur J Pharmacol 1992; 212: 9-13.
- Sakurai K, Osaka T, Yamasaki K. Rebamipide reduces recurrence of experimental gastric ulcers: role of free radicals and neutrophils. Dig Dis Sci 2005; 50: S90-S96.
- Ohashi Y, Aihara E, Takasuka H, Takahashi K, Takeuchi K. Antral ulcers induced by alendronate, a nitrogen-containing bisphosphonate, in rat stomachs; prophylactic effect of rebamipide. J Physiol Pharmacol 2009; 60: 85-93.
- Ueda F, Ban K, Ishima T. Irsogladine activates gap-junctional intercellular communication through M1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther 1995; 274: 815-819.
- Kyoi T, Noda K, Oka M, Ukai Y. Irsogladine, an anti-ulcer drug, suppresses superoxide production by inhibiting phosphodiesterase type 4 in human neutrophils. Life Sci 2004; 76: 71-83.
- Terano A, Hiraishi H, Ota S, Sugimoto T. Geranylgeranylacetone, a novel anti-ulcer drug, stimulates mucus synthesis and secretion in rat gastric cultured cells. Digestion 1986; 33: 206-210.
- Suemasu S, Tanaka K, Namba T, et al. A Role for HSP70 in protecting against indomethacin-induced gastric lesions. J Biol Chem 2009; 284: 19705-19715.