SLUGGISH GALLBLADDER EMPTYING AND GASTROINTESTINAL TRANSIT AFTER INTAKE OF COMMON ALCOHOLIC BEVERAGES
INTRODUCTION
Since the very remote past, ethanol inextricably accompanies the origin and development of the human civilizations anywhere on the Earth. One cannot neglect that also nowadays drinking alcoholic beverages is a ubiquitous and commonly accepted pattern of social behaviour (1).
Mouth and the digestive tract are in fact the first structures of the human organism which come into contact with ethanol contained in beverages of various proofs. Despite a common belief that “alcohol favours digestion” the immediate sequleae of this direct contact with ethanol of the digestive tract mucosa not infrequently appear to be deleterious. A support for this fairly disagreeable contention is provided by a study of Knoll et al. (2) who watched endoscopically an outbreak of haemorrhagic erosions on the mucosa of the stomach and the duodenum after local application of ethanol solutions. The most interestingly, less pronounced mucosal damage was observed if 100 ml of beer (4% vol), white wine (10.5% vol) or whisky (40% vol) was administered into the stomach than with aqueous ethanol solutions of matching concentrations (2).
Recently we described a strong inhibitory effect of alcoholic beverages upon the gastric emptying of a solid food and the meal-induced gallbladder emptying (3). The study design involved intake of alcoholic beverages or control fluids after ingestion of a mixed solid meal of 1485 kJ (355 kcal). It was revealed then that alcoholic beverages (beer, red wine, whisky) significantly slowed down the gastric evacuation of the solid meal, and delayed the meal-stimulated gallbladder emptying; the magnitude of those effects increased in the order: beer→red wine→whisky. Interestingly, alcoholic beverages produced only by fermentation (beer, red wine), but not their counterpartying aqueous ethanol solutions, did not elongate the orocaecal transit of the solid food. On the other hand, products of distillation - whisky and high proof ethanol solution - elicited a profound delay of the orocaecal transit of the solid test meal (3).
The results of that study have raised our interest in whether alcoholic beverages per se, i.e. taken on an empty stomach, would affect the above mentioned motor functions of the digestive tract and of the gallbladder. All the more so because a respective literature search performed within the Medline® and Scopus® databases has yielded very modest results, pointing out to scarcity of the current knowledge about the movement of alcoholic beverages along the human digestive tract. The only study on the effect of an alcohol beverage taken on an empty stomach upon the gastric myoelectrical activity was published by Levanon et al. (4). They found that that intake of an Australian 12.5% vol white wine had no effect on the dominant frequency of the gastric slow waves. Studies addressing gastric emptying of alcoholic beverages in humans are scarce. Moore et al. (5) examined scintigraphically the gastric emptying of 12% vol red Cabernet Sauvignon or a low-alcohol wine (1.7% vol) wherein the caloric “deficit” was supplemented with medium chain triglycerides. Both fluids were emptied from the stomach at a similar speed. More recently ultrasonographic measurements of the gastric evacuation of beer, red wine or diluted 1:2 whisky were reported. Nevertheless, at odds with a real life situation, the drinks were instilled into the stomach via a feeding tube (6).
Gallstones origin from the interplay of several pathologic conditions, such as hypersaturation of bile with cholesterol, imbalance between the bile crystallization promoters and protective factors, as well as impaired gallbladder motility and emptying (7). It seems quite interesting that epidemiological studies indicate that regular consumption of moderate amounts of alcohol reduces the risk of gallbladder stones (8-10). It seems tempting to suppose whether alcoholic beverages could somehow improve gallbladder motility and prevent bile stasis? Unfortunately thus far no study addressed the influence of intake of alcoholic beverages upon the gallbladder volume and emptying. Taking into account all the above, we decided to undertake a new study aimed at the determination of the gastric emptying kinetics and orocaecal transit of alcoholic beverages containing different amounts of alcohol, as well as their impact upon the gastric myoelectrical activity and gallbladder volume. We examined the same alcoholic beverages (beer, red wine, whisky) and control solutions as in the former work (3), but currently all the fluids were drunk in the interdigestive state - a condition uniquely characterized by the presence of the migrating myoelectric and motor complexes within the gastrointestinal tract (11, 12).
MATERIAL AND METHODS
The study was approved by the Bioethics Committee of the Medical University of Silesia (decision # NN-013-39/02) and was conducted in accordance with the Declaration of Helsinki, with written informed consent obtained from every subject.
In response to a public advertisement 24 volunteers aged between 20 and 29 years agreed to participate. Medical history and physical examination confirmed that they fulfilled the World Health Organization criteria of good health (13). According to the inclusion criteria of the study, the subjects had: (i) a negative result of a 13C-urea breath test for Helicobacter pylori infection (14), and (ii) an easily visible ultrasonographically, straight (not hook-shaped) gallbladder (15). Every volunteer denied excessive use of alcoholic beverages, which was defined as intake of more than two standard drinks a month. According to the National Institute of Alcohol Abuse and Alcoholism in Bethesda (USA), a standard drink is any drink that contains about 14 grams of pure alcohol (16).
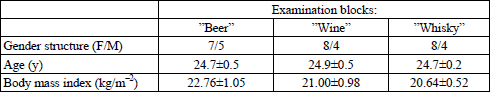
Exclusion criteria comprised: current use of any drugs, history of surgery affecting the anatomy of the digestive tract (except for an appendectomy), and pregnancy. The volunteers were allocated to three research groups of 12 subjects each (4 volunteers took part in two groups, whereas another 4 agreed to participate in all three groups), so that finally three comparable examination blocks were created, Table 1.
Supplies
The following alcoholic beverages were examined:
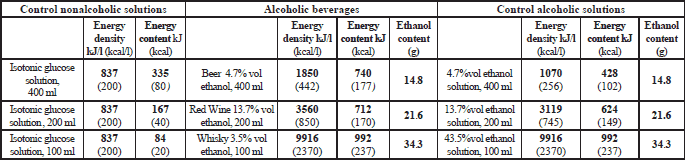
- lager beer Pilsner Urquell (Plzensky Prazdroj a.s., Czech Republic), 4.7% vol ethanol; 184 kJ (44 kcal), 0.5 g proteins and 4.0 g carbohydrates per 100 g;
- dry red wine (Chateau Salvanhiac appellation Saint Chinian controlee, vintage 2000, J.C. Rouanet, France), 13.7% vol ethanol; 356 kJ (85 kcal), 0.07 g proteins, 0 g fat, and 2.7 g carbohydrates per 100 g;
- blended scotch whisky (Johnnie Walker Red Label, John Walker&Sons, Kilmarnock, U.K.), 43.5% vol ethanol; 992 kJ (237 kcal), 0 g proteins, 0 g fat, 0 g carbohydrates per 100 g.
The reagents and materials comprised:
- 13C-Sodium acetate as a marker of gastric emptying of liquids (code INC639P, Euriso-Top, Saint Aubin, France; the manufacturer certifies ≥99% enrichment in 13C);
- crystalline lactulose as a marker of the orocaecal transit time (Duphalac® Dry, Solvay Pharma, Bruxelles, Belgium);
- aluminium covered plastic bags of 1.1 l capacity (Fischer Analysen Instrumente GmbH, Leipzig, Germany) equipped with a mouthpiece and an unidirectional valve for collection of expiratory air samples.
Study protocol
The subjects were invited to appear in the laboratory always in the morning, between 08:00 and 08:30 am, after a 12-hour overnight fast. This time regime was adopted so as to exclude any effect of the circadian rhythm of the gastrointestinal functions knowledgeably governed by the so-called gut clock (17). Following standard preparatory procedures described elsewhere (18), three Ag/AgCl electrodes (type Red Dot 2660, 3M, London, Ontario, Canada) were placed on the abdomen: the first active electrode was fixed in the midline, half way between the xyphoid process and umbiculus, the second active electrode was fixed at point lying 5 cm distant to A1 on a line leading up at a 45 degree angle towards the left costal margin, whereas the reference electrode was positioned on the right side of the abdomen below the right costal margin at its intersection with the right anterior axillary line.
At the beginning a 30-min electrogastrographic record of interdigestive gastric myoelectrical activity was accomplished in a supine position. Then a basal probe of expiratory air was taken for 13CO2 determination, breath H2 was measured, maximum longitudinal and transverse cross sections of the gallbladder were visualized ultrasonographically and stored on a hard disc, and a zero level of breath alcohol was verified with an alcotest.
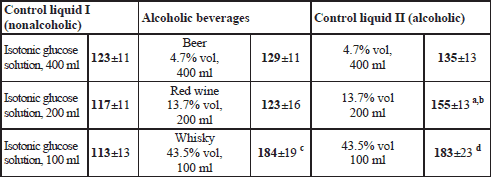
Within a given research block (“Beer”, “Wine”, “Whisky”) a volunteer attended three examination sessions held on separate days. On every of those days at time “0” the subject assumed temporarily a sitting position and drank within up to 4 minutes: an alcoholic beverage, or an aqueous ethanol solution of an identical proof, or a corresponding volume of isotonic glucose solution. Each of the fluids contained 40 mg 13C-sodium acetate and 10 g lactulose; the detailed data on the volumes of the fluids taken, and their composition is provided in Table 2.
The order of administration of the drinks within every examination block was randomized. For this purpose a numbered list of predefined sequences of administration of the fluids, comprising possible combinations of interventions, was prepared before implementation of the investigations. The laboratory staff ascribed consecutively a given sequence to subjects entering a research block.
After ingestion of the test fluid, an electrogastrogram was registered in a recumbent position for 120 min from time “0”. Simultaneously samples of expiratory air were collected for 13CO2 determination, breath H2 and alcohol concentrations were measured, and photographs of the gallbladder were taken (cf. the timetable depicted in Fig. 1.).
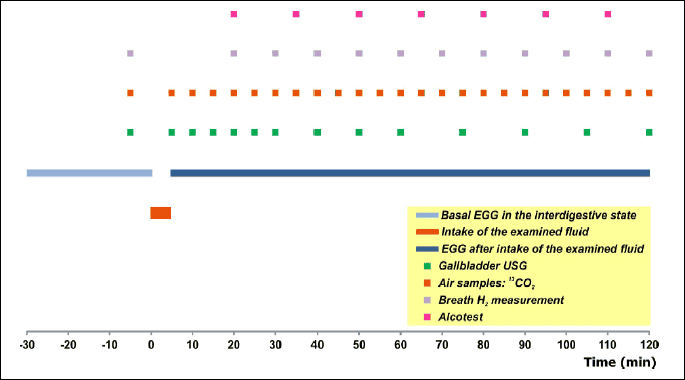
Later on, the subjects assumed a comfortable sitting position and breath samples for 13CO2 were collected every 10 min during the third hour, and at 15-min intervals during the fourth hour, breath alcohol was monitored at 15-min intervals until return of a zero reading, and, if necessary, breath H2 was measured until a consistent increment ≥20 ppm over the baseline occurred.
Measurement approaches and derivation of quantitative parameters
Electrogastrographic recordings were obtained with the use of PC Polygraf HR (Synectics/Medtronics, Denmark/USA) working at sampling frequency of 8 Hz. The digitized voltage sequences were stored on a hard disk of a computer for further analyses. The obtained tracings were analyzed off-line with the use of dedicated software (Multigram v. 6.40, Synectics Medical AB, Sweden) by a researcher blinded to the experimental conditions linked to a particular data set (K.J.). At first a visual inspection of the raw tracings was performed in order to identify and remove any fragments containing motion artefacts. Then a fast Fourier transform (FFT) was run on consecutive 256-s data sets with a 196-s overlap. Ultimately the analysis comprised derivation of: DF - dominant frequency, DDP - net post-ingestion change in dominant power, and relative time shares of normogastria (2.26–3.75 cycle per minute, cpm), bradygastria (0.5–2.25 cpm), and tachygastria (3.76–10 cpm) during a given epoch (18, 19).
13CO2 in breath samples was measured with non-dispersive isotope-selective infrared spectrometry (IRIS apparatus, Wagner Analysen Technik Vertriebs GmbH, Germany). Time to reach peak 13CO2 concentration (TtP) was taken as the measure of the liquid gastric emptying speed (20).
Hydrogen concentrations in expiratory air were measured with an EC-60 analyzer (Bedfont Scientific Ltd., U.K.). The orocaecal transit time (OCTT) was defined as the time elapsing from intake of lactulose-containing fluid until a sustained rise in breath hydrogen ≥20 ppm over the baseline was observed (21).
The gallbladder was viewed with the use of a Sonoline Prima apparatus (Siemens AG, Germany). In order to avoid an operator-dependent subjective error, the ultrasonographist left the examination room for the time a tested liquid was served to a subject. Gallbladder volumes were computed off-line in a blind manner from encoded series of photographs of the gallbladder according to a method of Dodds et al. (22). The kinetics of gallbladder emptying was characterized by a maximum ejection fraction (EFmax), time to reach it (TEFmax), and emptying speed, V_GBE=EFmax/TEFmax (15).
Ethanol concentrations in expiratory air were measured with Alcotest 7410Plus RS (Drager Sicherheitstechnik GmbH, Germany). According to the manufacturer’s recommendation, the first measurement was taken 15 minutes after ingestion of the alcohol containing fluid. The kinetic parameters comprised:
Cmax = peak concentration;
Tmax = time reach Cmax;
Telim = elimination time, i.e. until return of a zero reading;
AUC = area under the curve of ethanol concentrations.
Accuracy of the measurement methods and statistical analysis
Alcotest 7410Plus RS is a professional measurement device, equipped with an electrochemical sensor, ensuring a precision of ± 0.03 mg/L within the range from 0.00–0.50 mg/L. Its measurement results are considered to have evidential significance in the court provided that the device is subjected to a calibration procedure accomplished by the manufacturer every six months.
A within-subject study protocol involving 12 paired examinations renders the following smallest detectable differences (at p=0.05 level, two-tailed):
- 10.7% and 2.19 dB in the case of the relative time share of normogastria and the dominant power, respectively (19);
- 2.1 min for TtP (20);
- 18.8 min for OCTT of liquids (21);
The precision of the measurement of the gallbladder volume was established to amount to 1.0±0.4 cm3 within the volume range from 4.0–30.0 cm3 (23).
The statistical methods involved a repeated measures analysis of variance (ANOVA) followed by Tukey’s honest significant difference (HSD) test or Friedman’s ANOVA followed by a Wilcoxon signed rank test were applied where appropriate. Relationships between variables were tested with linear regression. Statistical significance was assumed at p<0.05 level, two-tailed. Results are presented as means ± S.E. or medians with interquartile ranges (24).
RESULTS
Participation
All the subjects completed the scheduled investigation sessions. Ingestion of the alcoholic beverages and the ethanol-containing and non-alcoholic control fluids was well tolerated and no adverse effects were observed. The participants were released from laboratory only after return of zero breath alcohol level.
Alcohol in breath air
The curves of ethanol concentration in expiratory air were characterized by a biphasic course with the presence of an ascending and a descending arm (Fig. 2). Data on the kinetics of breath ethanol concentration are assembled in Table 3.
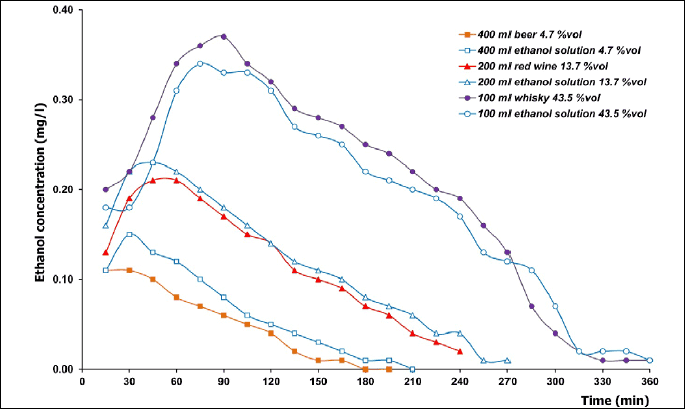
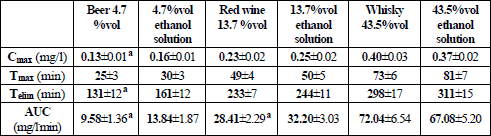
a statistically significant difference between an alcoholic beverage and an aqueous ethanol solution of a matching concentration.
Gastric myoelectrical activity
Ingestion of alcoholic beverages did not affect the DF or DP of the gastric slow waves with the exception of a borderline effect upon ΔDP disclosed within the “Whisky” block (ANOVA: F2;22=3.245, p=0.0582; Fx;y stands for the ratio of variance with the corresponding degrees of freedom: x and y). It was found that compared to intake of isotonic glucose, consumption of 100 ml whisky brought about a decrease in ΔDP from 2.87±0.73 to –0.37±1.14 dB (p=0.047) during the first half an hour (Table 4).
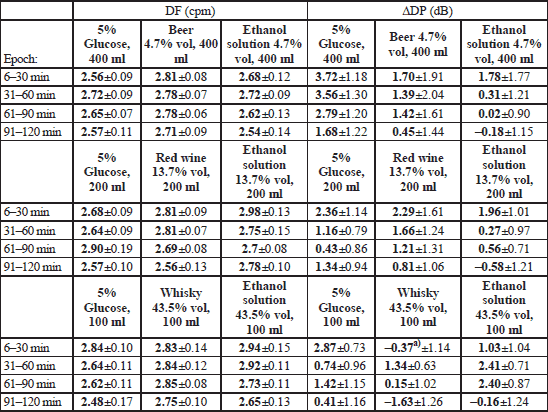
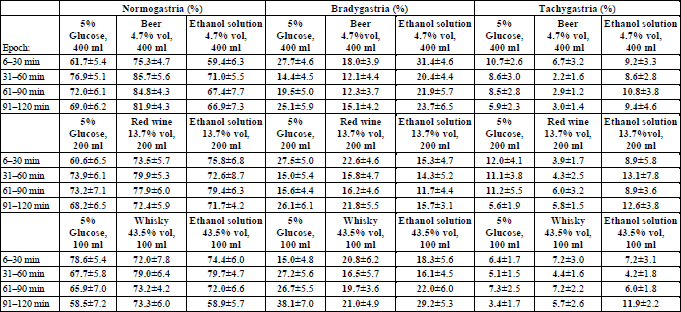
Within the “Beer” block ANOVA revealed a statistically significant main effect of the type of the drink upon the relative time share of either normogastria (F2;22=4.629, p=0.021) or bradygastria (F2;22=3.729, p=0.040). This effect was accounted for by a higher normogastria (81.8% vs. 66.1%, p=0.021), and a lower bradygastria (14.4% compared to 24.3%, p=0.038) time share after intake of beer than after ingestion of an aqueous ethanol solution of the same concentration. The effect mentioned could not be, however, discerned when normogastria or bradygastria time shares during particular 30-min observation epochs were analysed. Within either the “Wine” or the “Whisky” block there was no statistically significant effect of the type of ingested fluid upon the time share of particular rhythms of the gastric myoelectrical activity (Table 5).
Gastric emptying
ANOVA revealed a statistically significant effect of the type of a drink upon the gastric emptying within every of the three research blocks: “Beer” - F2;22=7.486, p=0.0033; “Wine” - F2;22=27.606; p=1.005.10–6, and “Whisky” - F2;22=31.216, p=3.76.10–7.
Beer was emptied from the stomach significantly slower than the two control solutions, whereas low-grade ethanol and isotonic glucose were emptied at the same speed (Table 6).
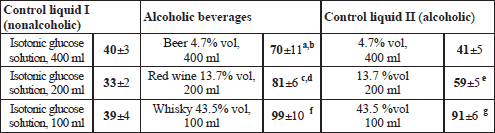
block "Beer": a p=0.00678 in comparison to TtP of 400 ml of isotonic glucose and b p=0.00932 in comparison to 4.7 %vol ethanol solution;
block "Wine": c p=0.000136 in comparison to TtP of 200 ml of isotonic glucose and d p=0.00772 in comparison to 13.7% vol ethanol solution, as well as e p=0.00157 in comparison to TtP of 200 ml of isotonic glucose;
block "Whisky": f p=0.000136 and g p=0.000140 in comparison to TtP of 100 ml of isotonic glucose.
An almost two and a half times slower was the gastric evacuation of red wine than of isotonic glucose. Significantly slower than 5% glucose was also emptied the 13.7% vol ethanol but the difference in TtP length appeared to be less than in the former case. Ultimately, the gastric evacuation of red wine was statistically significantly slower than of the aqueous ethanol solution of matching concentration (Table 6).
Whisky and an aqueous solution of ethanol of the same concentration were emptied from the stomach at a similar speed, but both those fluids left the stomach much slower than isotonic glucose did (Table 6).
Orocaecal transit
Within the “Beer” block OCTT did not depend on the type of the ingested fluid. A significant effect of the type of a drink upon the OCTT was disclosed in two other blocks: “Wine” F2;22=6.414, p=0.0064, and “Whisky” F2;22=6.856, p=0.0048.
The OCTT of red wine and of isotonic glucose was quite similar, whereas the OCTT of 13.7% vol ethanol was statistically significantly longer in comparison to either of the two other fluids (Table 7).
The OCTT of both high grade liquids - whisky and the aqueous ethanol solution - appeared to be over one hour longer than the OCTT of isotonic glucose (Table 7).
block ”Wine”: a p=0.0178 in comparison to OCTT of 200 ml of isotonic glucose and b p=0.0443 in comparison to OCTT of red wine;
block ”Whisky”: c p=0.0101 and d p=0.0120 in comparison to OCTT of 100 ml of isotonic glucose.
Gallbladder emptying
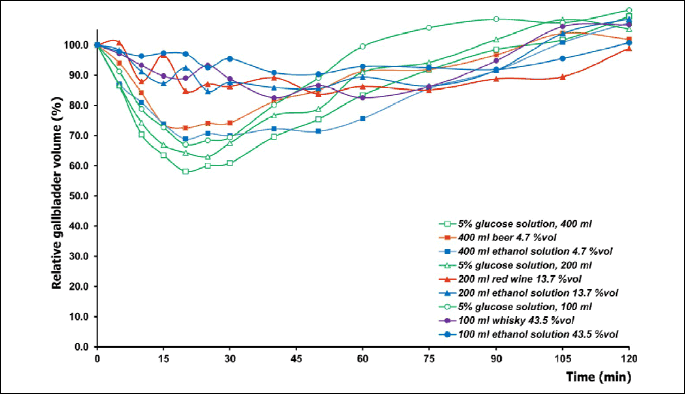
As shown in Fig. 3, intake in the fasted state of various volumes of isotonic glucose, beer or low-grade aqueous ethanol solution brought about a rapid decrease of the gallbladder volume followed by a refilling phase. Noticeably smaller was the gallbladder emptying after intake of red wine, whisky, and 13.7% vol or 43.5% vol ethanol.
A statistically significant effect of the type of drink on the EFmax was disclosed within the research block “Wine” (F2;22=5.558, p=0.011) and “Whisky” (F2;22=8.290, p=0.0021). Accordingly, EFmax was significantly lower after intake of red wine, whisky, as well as medium and high grade aqueous ethanol solutions than after ingestion of isotonic glucose (Table 8).
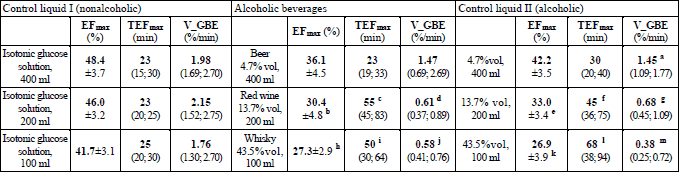
Within the particular examination blocks the following differences were found to be statistically significant:
block „Beer”: a p=0.0229 in comparison to V_GBE after intake of 400 ml of 5% glucose solution;
block „Wine”: b p=0.0136 and e p=0.0426 in comparison to EFmax, c p=0.0109 and f p=0.0178 in comparison to TEFmax, as well as d p=0.00222 and g p=0.0281 in comparison to V_GBE after intake of 200 ml of 5% glucose solution;
block „Whisky”: h p=0.00592 and k p=0.00474 in comparison to EFmax, I p=0.0121 and i p=0.00474 in comparison to TEFmax, as well as j p=0.00370 and m p=0.00474 in comparison to V_GBE after intake of 100 ml of 5% glucose solution.
Friedman’s ANOVA detected an impact of the type of fluid upon the TEFmax: χ2=7.400, p=0.025 and χ2=10.956, p=0.0042 in the case of the “Wine” and “Whisky” block, respectively. Similarly, an influence of the drink type was disclosed with regard to the V_GBE: “Beer” χ2=6.167, p=0.046; “Wine” χ2=15.500, p=0.00043; “Whisky” χ2=10.500, p=0.0052. The results of subsequent post hoc are displayed in Table 8.
Correlation analysis
Relationships among the changes of parameters characterizing the gastric myoelectrical activity, gastric emptying, OCTT, and gallbladder emptying observed after intake of alcoholic drinks, and the ethanol concentration therein, as well as the breath ethanol kinetic parameters displays Table 9.
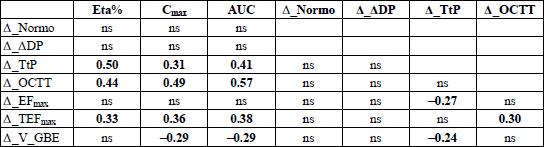
Eta% = ethanol concentration within an alcoholic beverage or aqueous solution; Cmax = maximum concentration, and AUC = area under the curve of ethanol concentration in expiratory air; Normo = relative time share of normogastria, DDP = net change in dominant power after intake of a fluid in relation to the fasted state, TtP = time to reach maximum breath 13CO2 elimination, reflecting the speed of gastric evacuation of liquids, OCCT = orocaecal transit time, EFmax = gallbladder maximum ejection fraction and TEFmax = time of its occurrence, V_GBE = gallbladder emptying speed
DISCUSSION
Background methodological considerations
In our former work we outlined in detail the methodological challenges which have to be faced when designing a study on the effects of alcoholic beverages upon the digestive tract (3). Since this item is vital for a proper interpretation of the results of the current study, we shall briefly provide the necessary background knowledge.
Ethanol is a compound of low molecular weight, what means that a theoretical osmolality of aqueous solutions would reach extremely high values, namely 845 mmol/kg, 2,711 mmol/kg and 13,186 mmol/kg in the case of 4.7% vol, 13.7% vol, and 43.5% vol, respectively. Moreover, the molecule of ethanol is very small (2.6 A° ) and because of it passes easily through biological membranes, such as the mucosa of the stomach or the small intestine, behaving like its solutions had a zero osmolality coefficient. Therefore there is no need to consider the theoretical osmolality of ethanol solutions in a study wherein ethanol solutions are introduced into the gastrointestinal tract (25). Besides, an attempt to create a control solution of osmolality equalling the theoretical osmolality of an aqueous ethanol solution would end up quite absurd - for example NaCl at a concentration of 27 g/L H20 osmolality reaches osmolality amounting to barely 827 mmol/kg. Knowledgeably ingestion of such a hyperosmotic NaCl solution elicits a profound disruption of the gastric myoelectrical activity (26).
In addition one should take into consideration that ethanol is a compound of high-energy, providing 29.7 kJ/g (7.1 kcal/g). For this reason a glucose solution of the same energy density as the red wine used in the present study would need to have a concentration of 186 g/L H20, and thus would be highly hyperosmotic (1034 mmol/kg). Former research indicated that already a less hyperosmotic glucose solution (150 g/L H20, osmolality 836 mmol/kg) adversely affected the gastric myoelectrical activity, bringing about a characteristic increase in tachygastria share within the electrogastrogram (26).
Distilled water was suggested once as a reference fluid for examinations on the effects of alcohol on the digestive system (25). A recent study indicates, however, that intake of a zero-osmotic fluid does not provide an ideal electrogastrogram in healthy subjects. An optimum record of gastric myoelectric activity is achieved after ingestion of an isotonic glucose solution because stimulation of chemoreceptors sensitive to glucose results in a positive chronotropic effect and stabilization of the gastric pacemaker activity (26). Therefore we chose isotonic glucose solution as the reference non-alcoholic fluid providing an optimum referential pattern of gastric myoelectrical activity in terms of the relative time share of normogastria, and the post-ingestion increase in the dominant frequency and power (26).
Due to characteristic organoleptic properties (taste, smell, and in some instances also colour) of alcoholic beverages and aqueous ethanol solutions (27) it is impossible to blind the volunteers to the intervention if a non-invasive principle of the examination has to be secured (an intragastric instillation of alcoholic beverages definitely would be at odds with real life situation). On the other hand, such outcome measures like breath ethanol concentration, electrogastrographic parameters, as well as results of 13CO2 and H2 breath tests are quantitative, operator-independent indices. Serial ultrasonographic visualisation of the gallbladder, which is needed to obtain data for construction of a gallbladder emptying curve, may be operator dependent. Therefore the ultrasonographist was blinded with regard to the fluid administered to subjects and the volume measurements were accomplished off-line on coded series of photographs.
Finally, it should be emphasized that recent studies provided evidence of validity of the measurement of gastric emptying of liquids with the use of the 13C-acetate breath test. In particular it was proven that 13C-acetate absorption and metabolism is independent of the volume and caloric delivery of test meals, nor is affected by stimulated gastric secretion, and that kinetics of 13CO2 breath elimination reflects the dynamics of meal or caloric emptying from the stomach (28, 29).
Gastric myoelectrical activity after intake of alcoholic beverages
Our study shows that the gastric pacesetter appeared to be “resistant” to the exposure of the stomach to alcoholic beverages. Beer, red wine, whisky, as well as aqueous ethanol solutions at matched concentrations did not exert a chronotropic effect on the gastric pacemaker. The same negative finding pertains to the inotropic effect, with the exception of whisky the ingestion of which brought about a decrease in the DP of the gastric slow waves. The observed decreased ΔDP can be interpreted as a suppressive effect of this beverage upon the amplitude of the gastric slow waves (30). This reasoning is supported by a study accomplished by Linda Knight et al. (31) in vivo in the dog. With the use of ultrafast dynamic scintigraphy they found that ethanol solutions diminished the amplitude of antral contractions, whereas their frequency remained unchanged.
An open question remains, why the aqueous 43.5% vol ethanol solution did not affect the dominant power of the gastric slow waves.
Gastric emptying of alcoholic beverages
Our study confirmed former observations of a slowed gastric evacuation of beer and red wine, consumed on an empty stomach (6). A completely novel finding is that whisky and a high proof alcohol solution leave the stomach at an extremely slow rate. The effects mentioned cannot be accounted for by interference with gastric myoelectrical function because no definite inhibitory effect thereon of alcoholic beverages could be demonstrated. Thus a hypothesis arises that ethanol could exert a direct inhibitory effect on the stomach musculature. In vitro studies performed on gastric smooth muscle preparations of the cat and the dog showed that ethanol induces a strong inhibition of contractile activity (32, 33). In the present study the net slowing of gastric emptying taken as a difference in TtP length after ingestion of alcoholic fluids and after intake of isotonic glucose correlated closely with ethanol concentration in them. A less tight, but still statistically significant correlation was found between the TtP elongation and Cmax or AUC - the parameters reflecting the body ethanol concentration. Therefore, the concentration of ethanol within the fluid contacting with the stomach wall seems to be decisive for a slowed gastric evacuation of alcoholic beverages. Indeed, Linda Knight et al. (31) showed in dogs a stronger inhibitory effect on gastric emptying of ingested alcohol than when ethanol solution was administered intravenously. Izbeki et al. (34) demonstrated that capsaicin-sensitive afferents of the vagus nerve are involved in slowing of gastric evacuation and intestinal transit caused by local contact of the mucosa with ethanol.
Two findings deserve, however, some additional reflection.
Firstly, we found that beer was emptied from the stomach slower than the aqueous solution of ethanol of identical concentration. On one hand this difference could be accounted for by a higher energy density of beer. On the other hand, one should recall that, apart from alcohol, beer contains protein and carbohydrates, as well as dozens of chemical compounds potentially biologically active (35-37). The cholecystokinin receptor A (CCK-A) was hypothesized to be involved in the mechanism of slowing of gastric emptying by ethanol (38). Could thus cholecystokinin (CCK), a hormone known to inhibit gastric emptying (39), be responsible for the slow gastric evacuation of beer? The answer seems to be negative because intragastric application of beer admittedly stimulates the release of CCK but to the extent not exceeding that observed after administration of glucose solutions (40, 41). On the other hand, a CCK-A receptor-mediated inhibition of gastric emptying may be elicited by other chemical compounds present in beer, for example by N-methyltyramine (42-44).
Secondly, red wine appeared to leave the stomach much slower than its counterpart aqueous ethanol solution - the TTP difference in relation to isotonic glucose amounted to 48 min in the former case, whereas to 26 min in the latter. The difference in energy density between red wine and the aqueous ethanol solution of identical concentration appears to be too small (red wine 3560 kJ/L, ethanol 3119 kJ/L) to account for that phenomenon. Therefore attention should be directed towards participation of non-alcoholic components of red wine. A direct relaxatory effect of resveratrol, the most important antioxidant contained in red wine, as well as of a flavonoid - quercetin - upon the gastric smooth muscle can be hypothesized (45, 46).
Orocaecal transit of alcoholic beverages
The study supplies new facts with regard to the OCTT of alcoholic beverages drank on an empty stomach. In the case of whisky, as well as high (43.5% vol) and medium (13.7% vol) proof aqueous ethanol solutions their OCTT was significantly slower than the OCTT of corresponding volumes of isotonic glucose. Interestingly, the OCTT of beer or red wine did not differ from the OCTT of 5% glucose solution. Thus the OCTT of beer and red wine did not undergo any elongation, despite the fact that the gastric emptying of those two beverages was considerably delayed. This is an intriguing finding because according to the principle of the measurement of OCTT by means of the hydrogen breath test with per oral administration of lactulose, the OCTT values obtained summarize the oesophageal transit (short enough to be omitted), gastric emptying, and passage along the small intestine until the head of the chyme containing lactulose will have reached the caecum. Correlation analysis indicated, however, that the gastric emptying and OCTT shifts elicited by alcoholic beverages were unrelated. A hypothesis arises therefore that intestinal motility could be stimulated by compounds present in these two beverages. This supposition is supported in part by findings of Japanese researchers who isolated from beer and identified two compounds - aperidine and hordatine A - which both exert in vitro a motility stimulating effect upon the longitudinal muscle of guinea pig ileum (47-49).
Gallbladder emptying in response to intake of alcoholic beverages
New information was obtained in the current study on the impact of alcoholic beverages taken on an empty stomach upon the gallbladder volume. One should briefly remind that gallbladder emptying is initiated and controlled by a number of neural and humoral mechanisms, and under physiological conditions three phases: cephalic, gastric and intestinal of this process are distinguished (50). Intake of water provokes CCK-independent gallbladder emptying, with an EFmax of approximately 25% (51). In our laboratory we established that ingestion of 300 ml isotonic saline results in gallbladder emptying with EFmax amounting to 24.7±2.1% at TEFmax of 17.5±3.7 min (52). In the present study an average gallbladder EFmax of 48.4%, 46.0% and 41.7% was observed after intake of 400, 200, and 100 ml of isotonic glucose, respectively. The profounder gallbladder emptying in response to isotonic glucose than after ingestion of water or isotonic saline would be explained by release of CCK in response to stimulation of glucoreceptors (53).
It was shown previously that beer (40, 41), white wine (40), and whisky (54) applied intragastrically or orally are stimulators of CCK release. Therefore theoretically their intake should stimulate gallbladder emptying. Quite an opposite finding was obtained in this study, because after intake of alcoholic drinks gallbladder emptying appeared to be smaller than after isotonic glucose. It should be pointed out that while analysing a result of an experimental intervention upon the gallbladder emptying one should distinguish between a delay and an inhibition. In the first case a decreased EFmax will be accompanied by an unchanged TEFmax, whereas a reverse will be characteristic for the latter case. Obviously, a mixed result, an inhibited and delayed gallbladder emptying, is also possible. Results of this study imply that gallbladder emptying after drinking red wine, whisky, and aqueous solutions of ethanol was inhibited and delayed.
The changes of EFmax (expressed as the differences between its values after drinking an alcoholic fluid and after intake of isotonic glucose) did not depend on the Cmax and AUC - the parameters reflecting indirectly the concentration of ethanol in the body. On the other hand, the changes in EFmax were inversely proportional to the differences in TtP, which implies that EFmax was the less, the longer was the retention of an alcoholic fluid in the stomach. The TEFmax was proportional to Cmax and AUC, and the elongation of OCTT. The third parameter describing the kinetics of gallbladder emptying, which combines the information conveyed by EFmax and TEFmax, is the gallbladder emptying speed, V_GBE. The net changes in V_GBE were linked by an inversely proportional relationship to Cmax and AUC, and net changes in TtP. The relationships outlined suggest that the suppressing effect of alcoholic beverages upon gallbladder emptying depended partly on the concentration of ethanol within the body tissues, and in some degree it was also secondary to slower gastric emptying of alcoholic liquids.
Conclusions
The combination of the results of this study and of that one published formerly (3) shows that intake of alcoholic beverages not solely hampers the postprandial functionality of the digestive system - an effect which was reflected by a slowed gastric emptying and orocaecal transit of a solid food and meal-induced gallbladder emptying. We demonstrated now that if taken on an empty stomach, alcoholic beverages affect also the interdigestive gastrointestinal and gallbladder functionality. Specifically, on the basis of the results obtained the following conclusions can be drawn: (i) alcoholic beverages taken on an empty stomach exert a suppressive effect upon the transport function of the digestive tract and gallbladder emptying, and (ii) the extent of this action depends on the type of a beverage (whether it is obtained from fermentation only, or fermentation followed by distillation) and ethanol concentration therein.
Acknowledgments: Preliminary conceptual work was supported by a grant from the Medical University of Silesia (contract NN-1-088/01). The proper research was financed from a research grant 3P05B16722 obtained from the State Committee For Scientific Research (currently: The Ministry of Science and Higher Education) of the Republic of Poland, contract # 0450/P05/2002/22. Crystalline lactulose was a gift from Solvay Pharma, Bruxelles, Belgium. The alcoholic beverages were funded by AKJ.
Conflict of interests: None declared.
REFERENCES
- Kasicka-Jonderko A. Alkohol a uklad trawienny - czy zawsze trzeba o nim mowic zle? (Alcohol and the digestive system - should it always be blamed?) Prz Gastroenterol 2012; 7: 264-275.
- Knoll MR, Kolbel CB, Teyssen S, Singer MV. Action of pure ethanol and some alcoholic beverages on the gastric mucosa in healthy humans: a descriptive endoscopic study. Endoscopy 1998; 30: 293-301.
- Kasicka-Jonderko A, Jonderko K, Bozek M, Kaminska M, Mglosiek P. Potent inhibitory effect of alcoholic beverages upon gastrointestinal passage of food and gallbladder emptying. J Gastroenterol 2013; 48: 1311-1323.
- Levanon D, Goss B, Chen JD. Inhibitory effect of white wine on gastric myoelectrical activity and the role of vagal tone. Dig Dis Sci 2002; 47: 2500-2505.
- Moore JG, Christian PE, Datz FL, Coleman RE. Effect of wine on gastric emptying in humans. Gastroenterology 1981; 81: 1072-1075.
- Franke A, Teyssen S, Harder H, Singer MV. Effect of ethanol and some alcoholic beverages on gastric emptying in humans. Scand J Gastroenterol 2004; 39: 638-644.
- Venneman NG, van Erpecum KJ. Pathogenesis of gallstones. Gastroenterol Clin North Am 2010; 39: 171-183.
- La Vecchia C, Negri E, D’Avanzo B, Franceschi S, Boyle P. Risk factors for gallstone disease requiring surgery. Int J Epidemiol 1991; 20: 209-215.
- Leitzmann MF, Giovannucci EL, Stampfer MJ, et al. Prospective study of alcohol consumption patterns in relation to symptomatic gallstone disease in men. Alcohol Clin Exp Res 1999; 23: 835-841.
- Buchner AM, Sonnenberg A. Factors influencing the prevalence of gallstones in liver disease: the beneficial and harmful influences of alcohol. Am J Gastroenterol 2002; 97: 905-909.
- Geldof H, van der Schee EJ, Grashuis JL. Electrogastrographic characteristics of interdigestive migrating complex in humans. Am J Physiol 1986; 250: G165-G171.
- Borycka-Kiciak K, Kiciak A, Zabielski R, Romanowicz-Barcikowska K, Tarnowski W, Bielecki K. Does different approach during pancreatoduodenectomy influence intestinal migrating myoelectrical complex recovery? Study in experimental pig model. J Physiol Pharmacol 2013; 64: 341-351.
- Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference, New York, 19 June - 22 July 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948.
- Waluga M, Jonderko K, Krusiec-Swidergol B, Kaminska M, Kasicka-Jonderko A, Lesinska M. Effect of Helicobacter pylori eradication on gastric emptying and symptoms in patients with dyspepsia. Prz Gastroenterol 2011; 6: 118-124.
- Jonderko K, Nowak A, Kasicka-Jonderko A, Blaszczynska M. Effect of cigarette smoking on gallbladder emptying and filling in man. Am J Gastroenterol 1994; 89: 67-71.
- National Institute on Alcohol Abuse and Alcoholism. What Is a Standard Drink? Accessed 18 September 2013 from: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide2.htlm
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 2011; 62: 139-150.
- Jonderko K, Kasicka-Jonderko A, Blonska-Fajfrowska B. Does body posture affect the parameters of a cutaneous electrogastrogram? J Smooth Muscle Res 2005; 41: 133-140.
- Jonderko K, Kasicka-Jonderko A, Krusiec-Swidergol B, et al. How reproducible is cutaneous electrogastrography? An in-depth evidence-based study. Neurogastroenterol Motil 2005; 17: 800-809.
- Kasicka-Jonderko A, Szymszal M, Jonderko K, et al. Reproducibility of liquid gastric emptying measurement with the use of an ultralow dose of 13C-sodium acetate and isotope-selective nondispersive infrared spectrometry. Ann Acad Med Silesiensis 2005; 59: 144-152.
- Jonderko K, Krusiec-Swidergol B, Kasicka-Jonderko A, Blonska-Fajfrowska B. Effect of lactulose dose on reproducibility of the gastrocaecal transit time measurement. Diagn Lab 2003; 39: 283-293.
- Dodds WJ, Groh WJ, Darweesh RM, Lawson TL, Kishk SM, Kern MK. Sonographic measurement of gallbladder volume. AJR Am J Roentgenol 1985; 145: 1009-1011.
- Jonderko K, Bueno L. Validation in vivo of an ultrasonographic method of measuring gallbladder volume. Ultrason Pol 1994; 4: 53-60.
- Statistics Textbook. StatSoft, Inc. Tulsa, StatSoft 2013. Accessed 17 September 2013 from: http://www.statsoft.com/textbook/ http://www.statsoft.com/textbook/stathome.html.
- Chari ST, Teyssen S, Singer MV. What controls should be used in studies of acute effects of alcohol and alcoholic beverages on the stomach and the pancreas? Scand J Gastroenterol 1993; 28: 289-295.
- Syrkiewicz-Trepiak D, Jonderko K, Kasicka-Jonderko A. Effect of osmolality of caloric and acaloric liquids on gastric myoelectrical activity in humans. Med Sci Monit 2010; 16: CR252-CR259.
- Kuyumcu S, Goetze O, Menne D, et al. Gastric secretion does not affect the reliability of the 13C-acetate breath test: A validation of the 13C-acetate breath test by magnetic resonance imaging. Neurogastroenterol Motil 2013; 25: 176-e87.
- Waluga M, Jonderko K, Buschhaus M. Pragmatically on the sense of taste - a short treatise based on culinary art. Prz Gastroenterol 2013; 8: 338-346.
- Goetze O, Fox M, Kwiatek MA, et al. Effects of postgastric 13C-acetate processing on measurement of gastric emptying: a systematic investigation in health. Neurogastroenterol Motil 2009; 21: 1047-e85.
- Koch KL, Stern RM. Handbook of Electrogastrography. Oxford, New York, Oxford University Press, 2004.
- Knight LC, Maurer AH, Wikander R, Krevsky B, Malmud LS, Fisher RS. Effect of ethyl alcohol on motor function in canine stomach. Am J Physiol 1992; 262: G223-G230.
- Sanders KM, Berry RG. Effects of ethyl alcohol on phasic and tonic contractions of the proximal stomach. J Pharmacol Exp Ther 1985; 235: 858-863.
- Sim SS, Choi JC, Min DS, et al. Effect of ethanol on spontaneous phasic contractions of cat gastric smooth muscle. Scand J Gastroenterol 2002; 37: 23-27.
- Izbeki F, Wittmann T, Jancso G, Csati S, Lonovics J. Inhibition of gastric emptying and small intestinal transit by ethanol is mediated by capsaicin-sensitive afferent nerves. Naunyn Schmiedebergs Arch Pharmacol 2002; 365: 17-21.
- Missiaen J, Saison D, Delvaux FR. Contribution of monophenols to beer flavour based on flavour thresholds, interactions and recombination experiments. Food Chem 2011; 126: 1679-1685.
- Sterckx FL, Saison D, Delvaux FR. Determination of volatile monophenols in beer using acetylation and headspace solid-phase microextraction in combination with gas chromatography and mass spectrometry. Anal Chim Acta 2010; 676: 53-59.
- USDA Database for the Flavonoid Content of Selected Foods, Release 2.1 Beltsville (Maryland): United States Department of Agriculture, Agricultural Research Service; 2007. http://www.ars.usda.gov/Services/docs.htm?docid=6231.
- Izbeki F, Wittmann T, Csati S, Lonovics J. The mechanisms of the inhibitory effect of ethanol on gastric emptying involve type A CCK receptors. Regul Pept 2004; 117: 101-105.
- Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. The role of gastric motility in the control of food intake. Review article. Aliment Pharmacol Ther 2011; 33: 880-894.
- Hajnal F, Flores MC, Valenzuela JE. Effect of alcohol and alcoholic beverages on nonstimulated pancreatic secretion in humans. Pancreas 1989; 4: 486-491.
- Chari ST, Harder H, Teyssen S, Knodel C, Riepl RL, Singer MV. Effect of beer, yeast-fermented glucose, and ethanol on pancreatic enzyme secretion in healthy human subjects. Dig Dis Sci 1996; 41: 1216-1224.
- Tsutsumi E, Kanai S, Ohta M, Suwa Y, Miyasaka K. Stimulatory effect of N-methyltyramine, a congener of beer, on pancreatic secretion in conscious rats. Alcohol Clin Exp Res 2010; 34 (Suppl. 1): S14-S17.
- Gerloff A, Singer MV, Feick P. Beer but not wine, hard liquors, or pure ethanol stimulates amylase secretion of rat pancreatic acinar cells in vitro. Alcohol Clin Exp Res 2009; 33: 1545-1554.
- Gerloff A, Singer MV, Feick P. Beer-induced pancreatic enzyme secretion: characterization of some signaling pathways and of the responsible nonalcoholic compounds. Alcohol Clin Exp Res 2009; 33: 1638-1645.
- Huang WF, Ouyang S, Lin YF, Zhang H. Effect of quercetin on gastrointestinal motility and its mechanism. World Chin J Digestol 2009; 18: 1815-1820.
- Wang LD, Qiu XQ, Tian ZF, Zhang YF, Li HF. Inhibitory effects of genistein and resveratrol on guinea pig gallbladder contractility in vitro. World J Gastroenterol 2008; 14: 4955-4960.
- Fujii W, Hori H, Yokoo Y, Suwa Y, Nukaya H, Taniyama K. Beer congener stimulates gastrointestinal motility via the muscarinic acetylcholine receptors. Alcohol Clin Exp Res 2002; 26: 677-681.
- Yamaji N, Yokoo Y, Iwashita T, et al. Structural determination of two active compounds that bind to the muscarinic M3 receptor in beer. Alcohol Clin Exp Res 2007; 31(Suppl. 1): S9-S14.
- Yokoo Y, Fujii W, Hori H, et al. Isolation of stimulants of gastrointestinal motility in beer. Alcohol Clin Exp Res 2004; 28(Suppl. 8): 129S0-130S.
- Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008; 47: 2112-2126.
- Yamamura T, Takahashi T, Kusunoki M, Kantoh M, Seino Y, Utsunomiya J. Gallbladder dynamics and plasma cholecystokinin responses after meals, oral water, or sham feeding in healthy subjects. Am J Med Sci 1988; 295: 102-107.
- Jonderko K, Bula M, Blonska-Fajfrowska B. Effect of somatovisceral stimulation on gallbladder emptying in humans depends on the caloric content of a meal. Acta Physiol Scand 2000; 170: A82-A83.
- Little TJ, Doran S, Meyer JH, et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab 2006; 291: E647-E655.
- Manabe T, Sawai H, Okada Y, et al. Effects of whisky on plasma gastrin and cholecystokinin in young adult men. J Int Med Res 2003; 31: 201-214.
A c c e p t e d : January 2, 2014