THE EFFECT OF OMEGA-3 FATTY ACIDS ON EXPRESSION OF CONNEXIN-40
IN WISTAR RAT AORTA AFTER LIPOPOLYSACCHARIDE ADMINISTRATION
INTRODUCTION
Vascular gap junctions (GJs) are low-resistant transmembrane channels playing an important role in coordination and synchronization of physiological events of vascular wall cells to maintain the tissue homeostasis and vasomotion (1). GJs allow a direct exchange of ions, small metabolites and second messengers (<1 kDa) between cytoplasm of adjacent cells of endothelium and smooth muscle (EC-EC, SMC-SMC, EC-SMC) and ensure their mechanic and electrical coupling. Vascular GJs are composed of four Cx isoforms - Cx37, Cx40, Cx43 and Cx45 (1). Their expression is not uniform. It depends on a type of cells, size and type of blood vessels, age and species (2-4) as well as on pathophysiological conditions (1, 5, 6). Abnormal expression of Cxs has been shown to contribute to disturbances in endothelial homeostasis and permeability and contractility of smooth muscle cells as well, supporting GJs as an attractive therapeutic target for the treatment of vascular disorders.
Increasing evidence suggests relationship between Gram-negative bacterial lipopolysaccharide (LPS)-induced chronic low-grade inflammatory and development of cardiovascular diseases including atherosclerosis (7-10). LPS triggers a variety of proinflammatory responses including increased production of cytokines, chemokines, growth factors and reactive oxygen species. These via paracrine pathways can locally induce endothelial activation and/or dysfunction respectively, to increase vascular leak and subsequently the impairment of vascular wall function and its structural remodeling.
LPS-induced abnormal expression of Cx isoforms and GJ communication in the vascular wall cells cultured endothelial cells, leukocytes between EC and leukocytes (11-15) as well as in the cells of immune system (16) indicate that GJs could be involved in the development of inflammation-induced vascular wall injury. Mentioned changes were detected a few hours after LPS administration mimicking the acute phase of inflammatory process on Cxs expression. According to our knowledge, data concerning the effect of a systemic LPS-induced inflammation on Cxs expression in cardiovascular system are missing.
Regular chronic consumption of fish oil and/or isolated omega-3 polyunsaturated fatty acids (n-3 PUFA) respectively is well-known for improving of health. N-3 PUFA are considered to be powerful intracellular and intercellular mediators (17). They possess multifunctional beneficial effects (18, 19). They reduce lipid levels and blood pressure, attenuate thrombosis, improve NO production and suppress reactive species production. N-3 PUFA are also precursors for the synthesis of eicosanoids and modulators of the cell signaling network and cellular membrane fluidity that can lead to various effects on inflammatory processes (20). N-3 PUFA protected the renal Na,K-ATPase activity in endotoxemic rats (21) and HTG rats (22). Moreover, modulation of Cx43 expression in the rat heart and the aorta with chronic n-3 PUFA intake demonstrated in our previous studies (23-26) suggested a link of cardioprotective effects of n-3 PUFA with GJ communication. Whether shorter n-3 PUFA supplementation can have protective effects on vascular Cx isoforms expression during LPS-induced inflammation has not been yet studied.
From this perspective, the aim of the present work was to study the potential anti-inflammatory effect of 10-day n-3 PUFA diet on Cx40 expression in the rat aorta after a single dose of LPS-induced inflammatory process.
MATERIAL AND METHODS
Animal model
The procedures of animal experiments in this study were performed in the accordance with institutional guidelines and the protocols approved by the State Veterinary and Food Administration of the Slovak Republic.
The experiments were performed on adult normotensive male Wistar (W) rats (220–240 g of body weight). Prior to the experiments, the rats were acclimatized and housed in a temperature- and light-controlled room (23±1°C, 12 h light: 12 h dark cycle). All rats had ad libitum access to a standard food and drinking water before and after the beginning of the experiments. At the beginning of experiments, the rats were divided into four groups (n=6 per each group): 1) C - control healthy animals,
2) PUFA - healthy rats were daily treated with n-3 PUFA in food supplementation, 30 mg/kg/day for 10 days (57% eicosapentaenoic acid and 43% docosahexaenoic acid, commercial nutritional supplement MaxiCor, SVUS Pharma, Czech Republic), 3) LPS - animals injected with a single dose of LPS, i.p., 1 mg/kg of body weight, 4) PUFA+LPS - rats injected with LPS were supplemented with n-3 PUFA for 10 days, first dose of n-3 PUFA was applied on the day of vaccination. LPS (E. coli serotype 055:B5, Sigma Chemicals, Germany) was dissolved in sterile 0.9% NaCl solution. The groups 1) and 2) were injected with the same volume of sterile 0.9% NaCl solution. At the end of the experiments, the thoracic aorta was excised from anaesthetized animals, cleaned of adherent tissue and processed for further analysis.
Basic characteristics of experimental animals
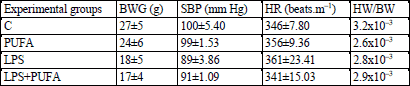
Blood pressure was measured at the beginning and at the end of the experiments according to Dlugosova et al. (23) using noninvasive plethysmography (Statham Pressure Transducer, AD Instruments, Germany). The body weight of the rats was measured at the same periods of the experiment. The weight of the whole heart and the left ventricle weight were measured at the end of the experiment only.
Blood samples were collected for plasma separation. After this process the aorta was isolated. Plasma was stored at –82°C until analysis of levels of high sensitive C-reactive protein (CRP) as a marker of acute inflammation and malondialdehyde (MDA) as an index of lipid peroxidation (27). The specific activity of N-acetyl-β-D-glucosaminidase (NAGA) as a marker of cellular injury and chronic phase of inflammation (28) was measured in plasma.
Hs-CRP levels were measured using commercial clinical Cholestech LDX System (California, USA). MDA concentration was measured spectrophotometrically with measurement of the color produced during the reaction of MDA with thiobarbituric acid according to Draper and Hadley (29). The activity of lysosomal NAGA was assayed according to standard methods as described previously in Navarova et al. (30).
Transmission electron microscopy
The aorta of the control rats and the LPS rats (n=4 for each groups) was cut into 3 mm rings. The specimens were immersion fixed in 2.5 % glutaraldehyde in 0.1 mol/l cacodylate buffer (pH 7.4) for 3 hour, then rinsed in buffer, postfixed in 1% OsO4, dehydrated in ethanol and embedded in Epon 812 (31). Thin sections after staining with uranyl acetate and lead citrate were examined with TESLA 500 electron microscope (Brno, Czech Republic).
Cx40 immunofluorescence
Indirect immunofluorescent detection of Cx40 was performed on frozen cross-sections of the non-fixed aortic rings of the rats of all experimental groups. Series of the sections were fixed in 4% paraformaldehyde, incubated in 0.3% Triton X-100, blocked by 10% goat serum and incubated with primary monoclonal antibody rabbit anti-Cx40 (1:500, Santa Cruz Biotechnology, Inc.) over night at 4°C. The sections were subsequently washed with phosphate-buffered saline (PBS) and then followed the application of secondary goat anti-rabbit conjugated with FITC-fluorescein isothiocyanate (1:500, Jackson ImmunoResearch Laboratory, Inc.). The primary antibody was omitted in negative controls to check the specificity of immunolabeling. After washing, the aortic sections were mounted into Vectashield mounting medium and viewed by confocal microscope Olympus FV1000 (Olympus, Japan).
Image densitometric analysis of Cx40
Both the density and the area of immunofluorescent Cx40 clusters were evaluated in endothelium and media using morphometric analysis system (Soft Imaging System, Germany) according to Dlugosova et al. (24) with a minor modification: after set of magnification, color of immunofluorescent signal of Cx40 was changed into gray scales. Endothelial layer and media were defined as a region of interest (ROI). Setting of threshold was used to determine minimal and maximal borders of a gray scale for immunoreaction. After the processes, we defined data for measurement of the density of Cx40 spots, their total area/mm2 of endothelium and media and the average size of one Cx40 spot. Signal was analyzed for each animal. The aorta from 6 animals in each group was cut to 6 rings, 3 to 8 sections per aortic ring were analyzed for each animal and the results were expressed in percentages.
Western blot of Cx40 and CD68
Frozen aortic tissue of the rats of all experimental groups was homogenized in SB20 solution (20% SDS, 10 mmol/l EDTA, 0.1 mol/l TRIS, pH 6.8) by sonificator UP 100H (Dr. Hielscher, Germany). An equal amount of the total protein in each sample was separated by 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane.
To analyze Cx40 expression the membrane was incubated with rabbit monoclonal antibody (Santa Cruz Biotechnology, Inc., 1:500) at a room temperature for 2 hours and followed by the incubation with secondary donkey anti-rabbit antibody coupled with horseradish peroxidase diluted 1:2000 (GE Healthcare).
To detect monocytes/macrophages in the aortic tissue, the membrane was incubated with primary mouse monoclonal antibody anti-CD68 diluted 1:1000 (Millipore) and followed by further incubation with secondary sheep anti-mouse antibody coupled with horseradish peroxidase 1:2000 (GE Healthcare).
The optical density of specific bands was detected by Kodak In-vivo Imaging System Fx Pro (USA) using “Carestream MI SE“software. The optical density of each band was verified in relation to the optical density of GAPDH.
Measurement of nitric oxide synthase activity and nitric oxide-dependent relaxation of aorta
The activity of NO synthase (NOS) was assayed in a homogenate of the aorta by determination of [3H]-L-citruline formation from [3H]-L-arginine (MP, Biomedicals) as described previously in Bernatova et al. (32).
The endothelium-dependent relaxation of the thoracic aorta was studied according to Sotnikova et al. (33). The rings of the aorta were mounted between two hooks in water-jacketed (37°C) chambers containing physiological saline solution (PSS) bubbled with a mixture of 95% O2 and 5% CO2 at pH 7.4. Special care was taken not to damage the endothelium. The composition of PSS was (in mmol.l–1): NaCl (118.0), KCl (4.7), KH2PO4 (1:2), MgSO4 (1.2), CaCl2 (2.5), NaHCO3 (25.0) and glucose (11.0). The preparations were connected to an isometric force transducer (Experimetria Hungary). After the equilibration period, vascular rings were precontracted with submaximal concentration of phenylephrine (10–6 mol.l–1). At the plateau of the contraction, the effect of acetylcholine in the cumulative concentrations of
10–8–10–5 mol.l–1 was tested to measure the endothelial function. The relaxation responses to acetylcholine are expressed in percentages of phenylephrine-induced contraction. Concentration-response curves of sodium nitropruside (10–10–10–7 mol/l) were performed in phenylephrine (10–6 mol/l)-precontracted preparations to measure endothelium-independent vasodilation of the aorta.
Statistical analysis
Obtained biochemical and functional data were statistically evaluated using ANOVA with Bonferroni multiple comparison test. Morphometric image analysis of Cx40 and Western blotting were evaluated using Student-Newman-Keuls method. The results were considered to be statistically significant when p values were less 0.05.
RESULTS
Basic characteristics
LPS significantly increased the levels of CRP in plasma (Table 1) when compared to controls. The 10 day n-3 PUFA supplementation markedly suppressed CRP concentration in the endotoxemic rats. Although CRP level was significantly lower in the LPS+PUFA group than in the LPS group, it is still significantly higher than in control rats. Treatment of healthy rats with n-3 PUFA had no effect on CRP levels.
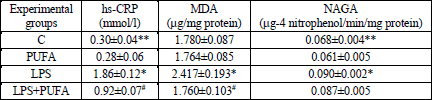
The plasma concentration of MDA (Table 1) was significantly higher in LPS group than in controls. N-3 PUFA administration to LPS rats suppressed MDA levels on the control level. In the healthy animals, n-3 PUFA supplementation had no effect on MDA production.
Measurement of the activity of NAGA in plasma (Table 1) demonstrated that LPS alone caused its significant increase. The 10-day n-3 PUFA treatment of the LPS rats did not significantly reduce the enzyme activity when compared to the LPS group, but it was still significantly higher than in the control group. Supplementation of the healthy rats with n-3 PUFA did not affect the activity of NAGA.
No significant reduction in the body weight gain was observed in the LPS group when compared to the controls (Table 2). N-3 PUFA feeding the LPS rats for 10 days did not affect the body weight gain comparing to the LPS group. N-3 PUFA had no effect on the body weight of the control rats. The evaluation of the body weight gain indicates a detail evidence for the LPS-induced changes independently of n-3 PUFA treatment. No relevant differences concerning systolic blood pressure, the heart rate and the relative heart weight were observed among the experimental groups (Table 2).
Transmission electronmicroscopy
Electronmicroscopy revealed a standard architecture of the endothelial cells of the aorta of the control animals (Fig. 1a). Focal irregularly extended tight junctions were rarely seen between adjacent endothelial cells. In the aorta of LPS rats (Fig. 1b-1d), the endothelium besides the healthy cells locally displayed slight to moderate ultrastructural abnormalities. They were manifested by edematous mitochondria (Fig. 1b), clumping of chromatin, increased presence of lysosomes and Weibel-Palade bodies (Fig. 1c) and increased amount of pinocytic vesicles (Fig. 1d). Cells with electronlucent cytoplasm (Figs. 1b, 1c) contained decreased amount of subcellular organelles (Fig. 1b) while increased amount of vacuoles. The inter-endothelial connections of the aorta of LPS rats, in contrast to the controls, frequently consisted of irregularly extended tight junctions (Fig. 1d). Overlapping of endothelial cells was frequently seen (Fig. 1b, 1c). Locally, monocyte contacting endothelial cell through gap junction was observed in subendothelial area (Fig. 1b). The subcellular alterations of endothelium represent the structural substrate of the endothelial activation/dysfunction.
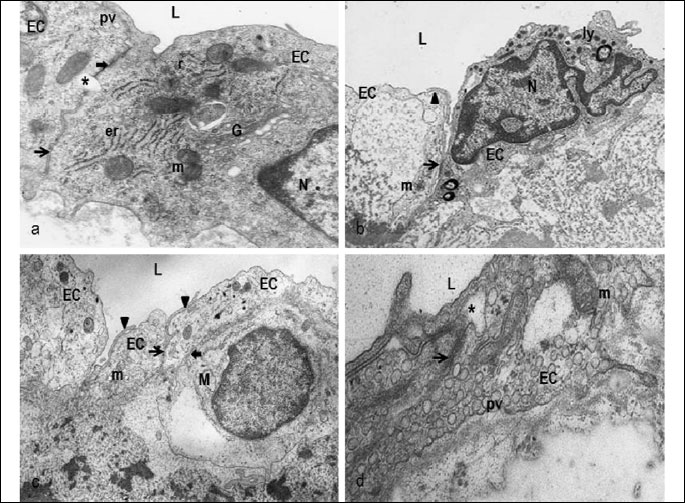
L - lumen, EC - endothelial cells, N - nucleus, r - ribosomes, er - rough endoplasmic reticulum, m - mitochondria, G - Golgi complex, pv - pinocytic vesicles, ly - lysosomes, M - monocyte, thick arrow - gap junction, arrow - tight junction, arrowhead - overlapping of endothelial cells, asterisk - extended tight junctions. Original magnification: a) 14,000×, b) 10,000×, c) 8,000×, d) 14,000×.
Cx40 distribution and spatial expression in the aortic wall
The immunolabeling of Cx40 was found in the sections of the rat aortic wall of all experimental groups (Fig. 2a): it was relatively regularly distributed within the endothelium, while heterogeneously scattered Cx40 punctuated pattern was observed throughout media.
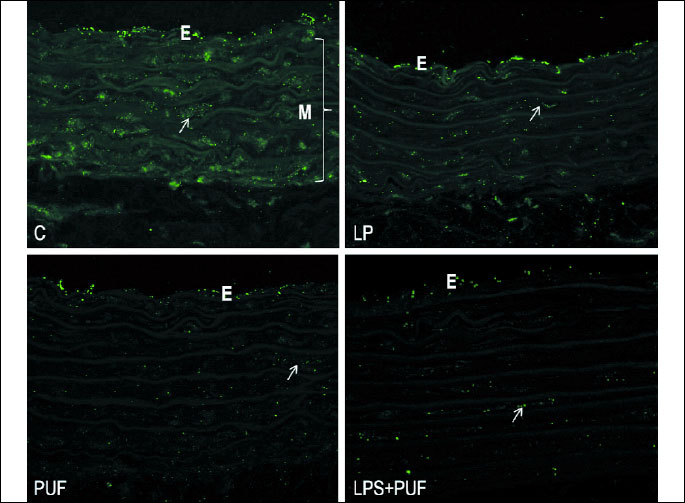
Morphometric analysis quantifies the total immunofluorescent area of Cx40 clusters per unit area of the individual aortic layers (endothelium and media) indicating Cx40 pattern expression. Considerably higher Cx40 expression pattern was demonstrated in the endothelium than in media of the aorta of all groups (Fig. 2b). Endothelial Cx40 expression was significantly higher in LPS group than in the control one. The administration of n-3 PUFA to the endotoxemic rats markedly reduced the Cx40 immunolabeling in the endothelium only when compared to LPS rats. In media, there were no differences among the groups.
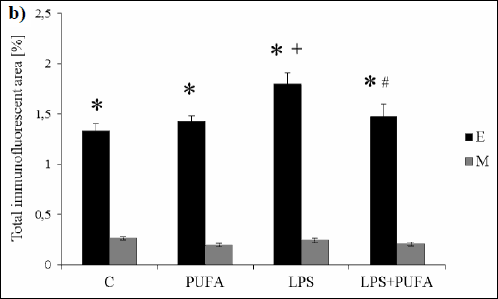 |
Fig. 2. b) Densitometric analysis of Cx40 spatial pattern in endothelium (E) and media (M) of the aorta of the control group (C), the treated control group (PUFA), the inflammatory group (LPS) and the treated inflammatory group (LPS + PUFA). (n=6 animals per group). Results are mean ± S.E.M. * p<0.05 versus media, + p<0.05 versus Controls, # p<0.05 versus LPS group. |
LPS injection resulted in the significant increase in Cx40 density clusters (Fig. 2c) in endothelium, while not in media comparing with the healthy animals. In media, there was a tendency of Cx40 cluster density to be increase. Daily n-3 PUFA supplementation of LPS rats for 10 days suppressed Cx40 density in endothelium when compared to LPS animals. No significant differences were observed between media of the treated and the untreated endotoxemic rats. N-3 PUFA diet did not change Cx40 density in endothelium and media of the control rats.
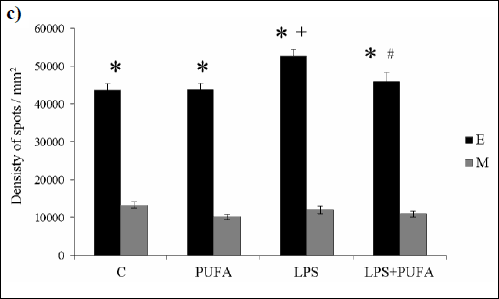 |
Fig. 2. c) Morphometric analysis of Cx40 density in endothelium (E) and media (M) of the rat aorta of the control group (C), the treated control group (PUFA), the inflammatory group (LPS) and the treated inflammatory group (LPS + PUFA). (n=6 animals per group). Results are mean ± S.E.M. *p<0.05 versus media, +p<0.05 versus Controls, #p<0.05 versus LPS group. |
Quantification of the average area of Cx40 clusters (Fig. 2d) demonstrated that LPS injection caused their enlargement in endothelium when compared to the controls, while no changes were seen in media between both groups. N-3 PUFA treatment of LPS rats significantly decreased the cluster area in the endothelium only when compared to LPS rats. N-3 PUFA supplementation of the healthy rats augmented the cluster area in the endothelium only when compared to healthy rats.
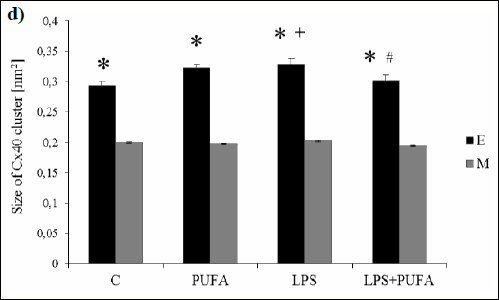 |
Fig. 2. d) Average size of single Cx40 clusters in endothelium (E) and media (M) of the rat aorta of the control group (C), the treated control group (PUFA), the inflammatory group (LPS) and the treated inflammatory group (LPS + PUFA). (n=6 animals per group). Results are mean ± S.E.M. *p<0.05 versus media, +p<0.05 versus controls, #p<0.05 versus LPS group. |
Western blotting of Cx40 and CD68
Western blotting analysis of Cx40 expression in the tissue of aorta showed significant differences among the experimental groups (Fig. 3a, 3b): alone, LPS significantly up-regulated Cx40 expression when compared to controls. PUFA diet of the endotoxemic rats induced marked Cx40 down-regulation when compared to the LPS group and the control group. PUFA supplementation of the control rats did not significantly affect Cx40 expression.
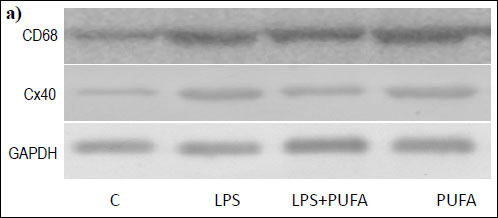 |
Fig. 3. a) Representative Western blot of CD68, Cx40 and GAPDH expression in the rat aorta of the control group (C), the inflammatory group (LPS), the treated inflammatory group (LPS + PUFA) and the treated control group (PUFA). (n=6 animals per group). |
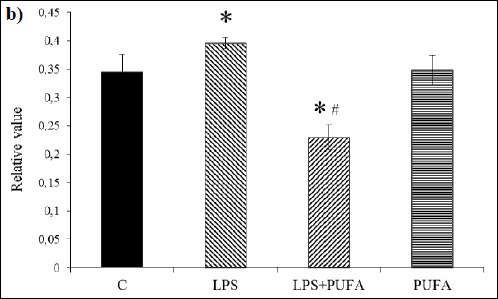 |
Fig. 3. b) Expression of Cx40 in the rat aorta of the control group (C), the inflammatory group (LPS), the treated inflammatory group (LPS + PUFA) and the treated control group (PUFA) normalized to GAPDH. (n=6 animals per group) Results are mean ± S E M. *p<0.05 versus Controls, #p<0.05 versus LPS group. |
Western blotting of CD68 as the macrophage/monocyte receptor marker in the aortic tissue revealed its expression in the aorta of the control rats (Fig. 3a). Up-regulation of CD68 was found in the aorta of endotoxemic rats when compared to the controls (Fig. 3c). N-3 PUFA treatment of LPS rats significantly suppressed Cx40 expression when compared to LPS group. Treatment of the healthy rats with n-3 PUFA did not significantly change CD68 expression.
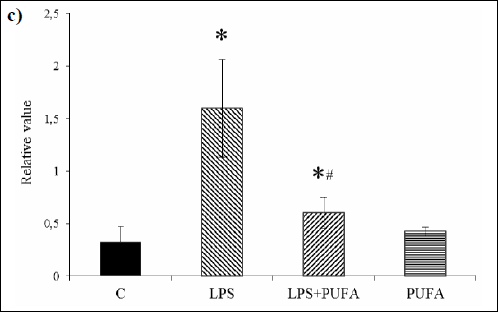 |
Fig. 3. c) Expression of CD68 in the rat aorta of the control group (C), the inflammatory group (LPS), the treated inflammatory group (LPS + PUFA) and the treated control group (PUFA) normalized to GAPDH. (n=6 animals per group) Results are mean ± S.E.M. *p<0.05 versus Controls, #p<0.05 versus LPS group. |
The nitric oxide-dependent relaxation of the aorta and the nitric oxide synthase activity
As is demonstrated on Fig. 4, LPS injection impaired the NO-dependent relaxation of the aorta when compared to the healthy animals. N-3 PUFA administration to the LPS group did not protect the relaxation neither had influence on the aortic relaxation in the control group. Endothelium-independent relaxation of the aorta induced by sodium nitroprusside did not differ among the experimental groups (not shown).
We further measured the NOS activ
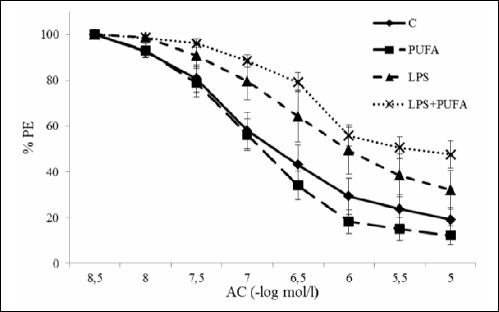 |
Fig. 4. NO-dependent relaxation of the rat aorta of the control group (C), the inflammatory group (LPS), the treated inflammatory group (LPS + PUFA) and the treated control group (PUFA). (n=6 animals per group) Results are mean ± S.E.M. *p<0.05 versus controls. |
ity in the aorta to correlate functional data with biochemical one (Fig. 5). LPS caused marked increase in the NOS activity when compared to the controls. Interestingly, n-3 PUFA supplementation of the endotoxemic rats did not reduce the enzyme activity it even increased more than twice when compared to the LPS group. N-3 PUFA significantly stimulated the NOS activity in the aorta of the healthy rats as well.
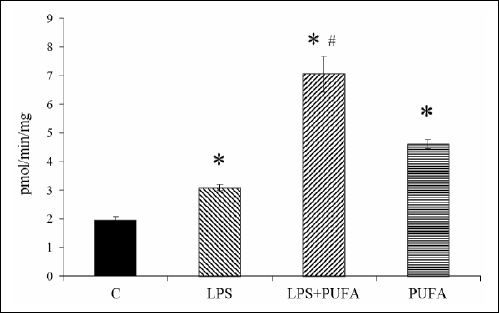 |
Fig. 5. NO-synthase activity measured in the rat aorta of the control group (C), the inflammatory group (LPS), the treated inflammatory group (LPS + PUFA) and the treated control group (PUFA). (n=6 animals per group) Results are mean ± S.E.M. Results are mean ± S.E.M. *p<0.05 versus Controls, #p<0.05 versus LPS. |
DISCUSSION
Experimental inflammation induced by various bacterial LPS has been used as a common model of infection and chronic inflammation which as it is mentioned above is recognized as one of risk factors for cardiovascular diseases. In 10-day experiment, we injected the adult normotensive healthy rats with a single dose of LPS (1 mg/kg b.w.). The used concentration when compared to higher one (4–10 mg/kg) (34) did not affect the body weight of the rats, neither heart rate nor systolic blood pressure. The dose was low enough not to induce sepsis and the mortality, as rodents are much less sensitive to LPS when compared to human (35). However, it was high enough to elicit more than six times increased level of CRP the circulating marker of acute inflammation and more that 100% elevation of the NAGA activity the marker of chronic inflammation and lysosomal injury of cells when compared to controls. This was associated with significantly increased levels of MDA indicating elevated generation of oxygen free radicals playing an important role in the progression of inflammatory disorders (36). Under inflammation, the endothelium is a major target of proinflammatory mediators and oxygen free radicals which are cause the endothelial dysfunction, affect intercellular connections and increase endothelial permeability (36, 37), resulting in changes in vascular tone. Ten days after LPS administration we demonstrated reduced the NO-dependent relaxation of the aorta while no changes in NO-independent vasodilation associated with the increased NOS activity suggesting changes in endothelial function. Our results are consistent with numerous literature data (38-40) demonstrating that the LPS-related increase in the NOS activity can be attributed to iNOS isoform. The subcellular aortic endothelial abnormalities including the intercellular connections which we observed in LPS rats correlate with the increased NAGA activity and can contribute to the further progression of the endothelial dysfunction and impaired endothelial barrier integrity. The latter allows increased passage of circulating inflammatory cells into the aorta the presence of which we demonstrated by the upregulation of CD68. Inflammation-induced production of superoxide by adhered neutrophils in the vicinity of endothelial and smooth muscle cells and its interaction with NO result in endothelial damage, decrease of the endothelium-dependent relaxation and can induce also vascular contraction (41). Detrimental effects of a single dose of LPS on the Na,K-ATPase activity regulating Na+ homeostasis in the body was demonstrated in rat kidney in our recent study (21). The results together demonstrate that a single dose of LPS injected to the healthy rats resulted in the moderate inflammatory response associated with endothelium-dependent aortic wall disease. However, it was reported that the progression of a disease can depend on the individual sensitivity to LPS and various genetic predispositions (42-44).
Vascular cell-to-cell coupling plays an important role in the propagation of electrical signals along blood vessels that contribute to regulation of vasomotor responses. We observed that LPS administration resulted in upregulation of Cx40 expression in the aortic tissue associated with the significant increase in endothelial Cx40 spatial expression only. The latter could represent interendothelial Cx40-channels as well as LPS-induced activation and stimulation of Cx40 hemmichannels (45, 46). Both, channels and hemmichannels are known to optimize cellular responses to actual (patho)physiological conditions and/or they to cause progress of disturbances in cellular function respectively. In media we observed a tendency of Cx40 expression to be increased. It seems to be important in relationship to upregulation of CD68 demonstrating increased infiltration of inflammatory cells into aortic tissue.
Surprisingly, we observed in contrast to others (2) Cx40 distribution in media of the aorta of healthy rats, too. This might indicate rat strain differences (47) or inflammatory cells such as macrophages and monocytes demonstrated by CD68. The latter are also known to express Cx40 (14, 15). The suggestion needs to be clarified in future studies. The local infiltration of inflammatory cells into the aortic wall of control group can occur due to the focal age-dependent abnormalities of endothelial monolayer structure. Because the aorta is an electrically quiescent tissue and the coordination of the responses of smooth muscle cells to diverse neural and endothelial signals particularly depends on gap junctional communications (48), the Cx40 immunolabeling in both aortic layers supports the role of Cx40-gap junction communication in maintaining of the aortic function. However, the function of Cx40 in media is not well-known. Therefore, we suggest that abnormal endothelial Cx40 expression caused by LPS might contribute to endothelial dysfunction and impaired aortic function.
Modulation of Cx isoforms during LPS-mediated response was reported in several studies. However, the results are diverse. LPS intake induced the decrease in Cx40 and Cx37 expression in endothelium of mouse aorta (49), microvascular endothelium (50) and HUVEC cells (51) as well. LPS-induced downregulation of Cx40 was also demonstrated in mouse lung (52). On the other hand, upregulation of Cx40 expression was found in the endothelium of Wistar rat aorta during sepsis (12). In all above mentioned studies LPS was administered only for a few hours to mimic acute-phase of inflammatory response. In our work, we demonstrated upregulation of Cx40 at the day 10 after LPS administration that in view of the life span of rat mimicked rather subchronic and/or chronic phase of inflammation respectively and could be relatively sufficient to cause/progress the vascular wall disease. We correlated our results with the works of Wang et al. (53) and Kwak et al. (54) who studied Cx40 expression in the atherosclerotic aorta of rabbit and mouse two weeks after high cholesterol diet which is associated with inflammation as well. Wang et al. observed Cx40 upregulation, while Kwak et al. found no changes. The effect of chronic LPS application alone induced upregulation of Cx32 and Cx30, while downregulation of Cx43 in rat hippocampus (55, 56). Data together suggest selective modulation of Cxs due to species-dependent variability, duration of inflammation as well as a dose and a route of LPS application (35, 57).
Cx40 expression in the aorta of endotoxemic rats might be modulated with high concentration of oxygen and nitrogen free radical (38, 58) as well as by proinflammatory cytokines (59). The NO pathway represents further potent modulator of Cx expression and their specific interactions are considered as key mechanisms in the control of a vascular function (60, 61). Alonso et al. (62) observed association of reduced eNOS activity with downregulation of Cx40 in the aorta of Cx40–/– mice. Modulation of Cx43 expression with NOS activity in the rat hearts was observed in our previous study (25). The present study demonstrated LPS-induced the increased NOS activity associated with upregulation of Cx40 expression in the rat aorta. The impaired NO-dependent relaxation of the aorta in correlation with literature data indicating the decreased eNOS activity and LPS-related elevation of the NOS activity suggests the increased activity of iNOS isoform and NO overproduction. Our results correlate with data showing that de novo formation of Cx40 was associated with the overproduction of NO in cultured endothelial cell (63). Our results thus indicate that LPS-induced Cx40 upregulation might be related to increased iNOS activity. The issue needs more detail study.
The chronic consumption of n-3 PUFA represents a known powerful tool aimed on the reduction of cardiovascular disease and supporting good health. Despite the extensive studies, the exact mechanisms involved in their protective effects are still not completely known. The improved Cx43 expression in the aorta and the heart of hypertensive and HTG rats after chronic supplementation with n-3 PUFA (23-26) indicated the involvement of GJs as one of the mechanisms through which n-3 PUFA can protect the function of cardiovascular system. In the present study we demonstrated that 10-day n-3 PUFA supplementation of LPS rats reduced Cx40 expression of the aorta supporting the involvement of Cx40-GJ as the target for n-3 PUFA. Cx40 downregulation could result from n-3 PUFA-related reduction of inflammation and oxidative stress as the levels of inflammatory mediators CRP, MDA, CD68 expression and the NAGA activity were decreased. The anti-inflammatory and anti-oxidative effects of n-3 PUFA are consistent with further studies (64). The results also indicate NO-independent modulation of Cx40 expression as well. N-3 PUFA are a part of cellular membrane and affect its absorption and transport properties. Moreover, they are known to modulate lipid metabolism (65). During the n-3 PUFA diet, these can be incorporated into membrane to influence lipid microdomains which are rich in Cx proteins (66). Cxs are dynamic transmembrane proteins therefore the modulation of Cx40 with n-3 PUFA incorporation seems very likely.
Our results indicated that 10-day n-3 PUFA intake could protect the function of the aorta. Unawares, n-3 PUFA administration to the LPS rats did not improve the NO-dependent relaxation response of the aorta, nor protected the NOS activity, which even enhanced. The results do not allow the elucidation of this situation. However, n-3 PUFA are known with their diverse (healthy and toxic) effects and their co-existence could occur (67, 68). It was reported that the administration of n-3 PUFA prior a pathological insult for chronic period ameliorated the symptoms of inflammation and protected against the effects of endotoxin in some animal models (69). We applied n-3 PUFA at the same day as a pathological insult, LPS in our case. At the end of experiment we observed reduced inflammation, but the levels of CRP and the activity of NAGA in LPS+PUFA group were still higher than in controls suggesting the persistence of a lower inflammation. Because n-3 PUFA are highly unsaturated and highly susceptible to the oxidation to form toxic oxidation products (70-72), we suggest that the suppression of inflammatory process could relatively be low enough to decrease the expression of Cx40 but it might still affect n-3 PUFA to produce oxidative form products and contribute to the deterioration of endothelium-dependent vasomotor response. Our work thus indicates the importance of the timing of n-3 PUFA administration. The results could also be dependent on the dose and the period of n-3 PUFA supplementation (73, 74) as well as on the type of the used artery and the age of experimental animals (23, 75, 76). Moreover, the farnesoid X receptors due to their anti-inflammatory properties in atherosclerosis and lipid homeostasis may represent further potential mechanism of n-3 PUFA involved in the modulation of NO-dependent vascular reactivity observed in the thoracic aorta (77). However, the exact mechanisms underlying the effects of n-3 PUFA on the aortic function and Cx40 expression remain to be elucidated.
Our results demonstrated that a single dose of LPS resulted in upregulation of Cx40 expression in the aorta associated with the moderate inflammation. We also observed that 10-day n-3 PUFA diet in vivo normalized Cx40 expression in the aorta of LPS rat and had a partial antiinflamatory effects. The results indicate Cx40 as a potential target of n-3 PUFA diet to protect the aortic function against LPS-induced inflammation.
Acknowledgements: This study was supported by VEGA grants No. 2/0108/10 and 2/0065/13. We thank V. Mocsonokyova and V. Dytrichova for their technical help.
Conflict of interests: None declared.
REFERENCES
- Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid Redox Signal 2009; 11: 267-282.
- Van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol 1999; 112: 479-486.
- Yeh HI, Chang HM, Lu WW, et al. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J Histochem Cytochem 2000; 48: 1377-1389.
- Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol 2002; 29: 620-625.
- Hou CJ, Tsai CH, Yeh HI. Endothelial connexins are down-regulated by atherogenic factors. Front Biosci 2008; 13: 3549-3557.
- Burnier L, Fontana P, Angelillo-Scherrer A, Kwak BR. Intercellular communication in atherosclerosis. Physiology (Bethesda) 2009; 24: 36-44.
- De Boer OJ, van der Wal AC, Becker AE. Atherosclerosis, inflammation and infection. J Pathol 2000; 190: 237-243.
- Al-Bannawi A, Al-Wesebai K, Taha S, Bakhiet M. Chlamydia pneumonia induces chemokine expression by platelets in patients with atherosclerosis. Med Princ Pract 2011; 20: 438-443.
- Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflamatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 2004; 24: 2227-2236.
- Smith BJ, Lightfoot SA, Lerner MR, et al. Induction of cardiovascular pathology in a novel model of low-grade chronic inflammation. Cardiovasc Pathol 200; 18: 1-10.
- Simon AM, McWhorter AR, Chen HD, Jackson CL, Ouellette Y. Decreased intercellular communication and connexin expression in mouse aortic endothelium during lipopolysaccharide-induced inflammation. J Vasc Res 2004; 41: 323-333.
- Rignault S, Haefliger JA, Gasser D, et al. Sepsis up-regulates the expression of connexin 40 in rat aortic endothelium. Crit Care Med 2005; 33: 1302-1310.
- Oviedo-Orta E, Errington RJ, Evans WH. Gap junction intercellular communication during lymphocyte transendothelial migration. Cell Biol Int 2002; 26: 253-263.
- Wong CW, Christen T, Kwak BR. Connexins in leukocytes: shuttling messages? Cardiovasc Res 2004; 62: 357-367.
- Zahler S, Hoffmann A, Gloe T, Pohl U. Gap junctional coupling between neutrophils and endothelial cells: a novel modulator of transendothelial migration. J Leukoc Biol 2003; 73: 118-126.
- Neijssen J, Pang B, Neefjes J. Gap junction-mediated intercellular communication in the immune system. Prog Biophys Mol Biol 2007; 94: 207-218.
- Ambrozova G, Pekarova M, Lojek A. Effect of polyunsaturated fatty acids in the reactive oxygen and nitrogen species production by raw 254.7 macrophages. Eur J Nutr 2010; 49: 133-139.
- Demaison L, Moreau D. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell Mol Life Sci 2002; 59: 463-477.
- Luostarinen R, Saldeen T. Dietary fish oil decreases superoxide generation by human neutrophils: relation to cyclooxygenase pathway and lysosomal enzyme release. Prostaglandins Leukot Essent Fatty Acids 1996; 55: 167-172.
- Das UN. Infection, inflammation, and polyunsaturated fatty acids. Nutrition 2011; 27: 1080-1084.
- Mezesova L, Jendruchova-Javorkova V, Vlkovicova J, et al. Supplementation with n-3 polyunsaturated fatty acids to lipopolysaccharide-induced rats improved inflammation and functional properties of renal Na,K-ATPase. Nutr Res 2013; 33: 772-779.
- Vrbjar N, Mezesova L, Javorkova V, et al. Gender specific influence of fish oil or atorvastatin on functional properties of renal Na,K-ATPase in healthy Wistar and hypertriglyceridemic rats. Physiol Res 2011; 60: 887-897.
- Dlugosova K, Okruhlicova L, Mitasikova M, et al. Modulation of connexin-43 by omega-3 fatty acids in the aorta of old spontaneously hypertensive rats. J Physiol Pharmacol 2009; 60: 63-69.
- Dlugosova K, Weismann P, Bernatova I, Sotnikova R, Slezak J, Okruhlicova L. Omega-3 fatty acids and atorvastatin affect connexin-43 expression in the aorta of hereditary hypertriglyceridemic rats. Can J Physiol Pharmacol 2009; 87: 1074-1082.
- Radosinska J, Bacova B, Bernatova I, et al. Myocardial NOS activity and connexin-43 expression in untreated and omega-3 fatty acids-treated spontaneously hypertensive and hereditary hypertriglyceridemic rats. Mol Cell Biochem 2011; 347: 163-173.
- Bacova B, Radosinska J, Knezl V, et al. Omega-3 fatty acids and atorvastatin suppress ventricular fibrillation inducibility in hypertriglyceridemic rat hearts: implication of intracellular coupling protein, conexin-43. J Physiol Pharmacol 2010; 61: 717-723.
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 1997; 43: 1209-1214.
- Kramer JH, Murthi SB, Wise RM, Mak IT, Weglicki WB. Antioxidant and lysosomotropic properties of acute D-propranolol underlies its cardioprotection of postischemic hearts from moderate iron-overloaded rats. Exp Biol Med (Maywood) 2006; 231: 473-484.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186: 421-431.
- Navarova J, Nosalova V. Effect of H2-receptor antagonists on indomethacin-induced lysosomal enzyme release from rat gastric mucosa. Methods Find Exp Clin Pharmacol 1994; 16: 119-124.
- Sotnikova R, Okruhlicova L, Noskovic P. Endothelial protective effect of stobadine on ischemia/reperfusion-induced injury. Gen Physiol Biophys 1998; 17: 253-264.
- Bernatova I, Pechanova O, Babal P, et al. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am J Physiol 2002; 282: H942-H948.
- Sotnikova R, Okruhlicova L, Navarova J, et al. Toxic effect of hyperglycemia on rat aortic function and structure. Effect of L-arginine. Biologia 2005; 5: 107-111.
- Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 2007; 18: 1072-1083.
- Morris M, Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch Immunol Ther Exp (Warsz) 2012; 60: 13-18.
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2013; Oct 22 (epub ahead of print).
- Lum H, Roebuck KA. Oxidant stress and endothelial dysfunction. Am J Physiol Cell Physiol 2001; 280: C719-C741.
- Kang WS, Tamarkin FJ, Wheeler MA, Weiss RM. Rapid up-regulation of endothelial nitric-oxide synthase in a mouse model of Escherichia coli lipopolysaccharide-induced bladder inflammation. J Pharmacol Exp Ther 2004; 310: 452-458.
- Muller B, Kleschyov AL, Gyorgy K, Stoclet JC. Inducible NO synthase activity in blood vessels and heart: new insight into cell origin and consequences. Physiol Res 2000; 49: 19-26.
- Chauhan SD, Seggara G, Vo PA, Macallister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J 2003; 17: 773-775.
- Bauer V, Sotnikova R, Drabikova K. Effects of activated neutrophils on isolated rings of rat thoracic aorta. J Physiol Pharmacol 2011; 62: 513-520.
- Michel O, Dentener M, Corazza F, Buurman W, Rylander R. Healthy subjects express differences in clinical responses to inhaled lipopolysaccharide that are related with inflammation and with atopy. J Allergy Clin Immunol 2001; 107: 797-804.
- Zollbrecht C, Grassl M, Fenk S, et al. Expression pattern in human macrophages dependent on 9p21.3 coronary artery disease risk locus. Atherosclerosis 2013; 227: 244-249.
- Fries S, Grosser T, Price TS, et al. Marked interindividual variability in the response to selective inhibitors of cyclooxygenase-2. Gastroenterology 2006; 130: 55-64.
- Ramachandran S, Xie LH, John SA, Subramaniam S, Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS One 2007; 8: e712.
- De Vuyst E, Decrock E, De Bock M, et al. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell 2007; 18: 34-46.
- Klaunig JE, Ruch RJ. Strain and species effects on the inhibition of hepatocytes intercellular communication by liver tumor promoters. Cancer Lett 1987; 36: 161-168.
- Haefliger JA, Meda P. Chronic hypertension alters the expression of Cx43 in cardiovascular muscle cells. Braz J Med Biol Res 2000; 33: 431-438.
- Simon AM, McWhorter AR, Chen H, Jackson CL, Ouellette Y. Decreased intercellular communication and expression in mouse aortic endothelium during lipopolysaccharide-induced inflammation. J Vasc Res 2004; 41: 323-333.
- Bolon ML, Kidder GM, Simon AM, Tyml K. Lipopolysaccharide reduces electrical coupling in microvascular endothelial cells by targeting connexin40 in tyrosine-, ERK1/2-, PKA-, and PKC-dependent manner. J Cell Physiol 2007; 21: 159-166.
- Van Rijen HV, Van Kempen MJ, Postma S, Jongsma HJ. Tumor necrosis factor alpha alters the expression of connexin43, connexin40 and connexin37 in human umbilical vein endothelial cells. Cytokine 1998; 10: 258-264.
- Rignault S, Haefliger JA, Waeber B, Liaudet L, Feihl F. Acute inflammation decreases the expression of connexin40 in mouse lung. Shock 2007; 28: 78-85.
- Wang LH, Chen JZ, Sun YL, et al. Statins reduce connexin40 and connexin43 expression in atherosclerotic aorta of rabbits. Int J Cardiol 2005; 100: 467-475.
- Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerosis plaque. Arterioscler Thromb Vasc Biol 2002; 22: 225-230.
- Abbasian M, Sayyah M, Babapur V, Mahdian R, Choopani S, Kaviani B. Upregulation of connexin30 and 32 gap junctions in rat hippocampus at transcription level by chronic ventral injection of lipopolysaccharide. Iran Biomed J 2012; 16: 127-132.
- Sayyah M, Kaviani B, Khoshkholgh-Sima B, et al. Effect of chronic intracerebroventricular administration of lipopolysaccharide on connexin43 protein expression in rat hippocampus. Iran Biomed J 2012; 16: 25-32.
- Heremans H, Van Damme J, Dillen C, Dijkmans R, Billiau A. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Schwartzman-like shock reactions in mice. J Exp Med 1990; 171: 1853-1869.
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J 2001; 15: 10-12.
- Hu J, Cotgreave IA. Differential regulation of gap junctions by proinflammatory mediators in vitro. J Clin Invest 1997; 99: 2312-2316.
- Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochim Biophys Acta 2012; 1818: 1895-1902.
- Kameritsch P, Hoffmann A, Pohl U. Opposite effects of nitric oxide on different connexins expressed in the vascular system. Cell Commun Adhes 2003; 10: 305-309.
- Alonso F, Boittin FX, Beny JL, Haefliger JA. Loss of connexin40 is associated with decreased endothelium-dependent relaxation and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol 2010; 299: H1365-H1373.
- Hoffmann A, Gloe T, Pohl U, Zahler S. Nitric oxide enhances de novo formation of endothelial gap junctions. Cardiovasc Res 2003; 60: 421-430.
- Kralova Lesna I, Suchanek P, Brabcova E, Kovar J, Malinska H, Poledne R. Effect of different types of dietary fatty acids on subclinical inflammation in humans. Physiol Res 2013; 62: 145-152.
- Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 2008; 197: 12-24.
- Tong J, Briggs MM, Mlaver D, Vidal A, McIntosh TJ. Sorting of lens aquaporins and connexins into raft and nonraft bilayers: role of protein in homo-oligomerization. Biophys J 2009; 97: 2493-2502.
- Di Nunzio M, Valli V, Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot Ess Fatty Acids 2011; 85: 121-127.
- Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem Res Toxicol 2011; 24: 2093-20103.
- Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc 2002; 61: 345-358.
- Esterbauer H. Cytotoxicity and genotoxicity of lipid oxidation products. Am J Clin Nutr 1993; 57 (Suppl. 5): 779-785.
- Halvorsen BL, Blomhoff R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr Res 2011; 55: doi: 10.3402/fnr.v55i0.5792
- Awada M, Soulage CO, Meynier A, et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J Lipid Res 2012; 53: 2069-2080.
- de Lima TM, de Sa Lima L, Scavone C, Curi R. Fatty acids control of nitric oxide production by macrophages. FEBS Lett 2006; 580: 3287-3295.
- Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem 2010; 21: 444-450.
- Gourdin MJ, Ponchau O, Jamart J, De Kock M. Ageing influences the effect of pre-hypoxic administration of clonidine, an alpha2-adrenoceptor agonist, on post-hypoxic vasomotricity. J Physiol Pharmacol 2012; 63: 165-171.
- Sotnikova R, Okruhlicova L, Dlugosova K, et al. Effect of n-3 PUFA on endothelium-dependent relaxation of the superior mesenteric artery. In: Proceedings of Experimental Approaches in Basic Research and Diagnostic of Diseases: Tailoring the Treatment. Tribulova N, Okruhlicova L, Slezak J. (eds.) VEDA Bratislava 2008, pp.127-134.
- Zhang R, Ran HH, Zhang YX, et al. Farnesoid X receptor regulates vascular reactivity through nitric oxide mechanism. J Physiol Pharmacol 2012; 63: 367-372.
A c c e p t e d : January 28, 2014