LABELED [3H] - THYMIDINE INCORPORATION IN THE DNA OF 3T3-L1 PREADIPOCYTES DUE TO MT2- AND NOT MT3- MELATONIN RECEPTOR
INTRODUCTION
Obesity is a disorder of homeostasis resulting from the excessive supply of energy in relation to energy expenditure of the body. The development of the white adipose tissue constitutes an essential element of energetic homeostasis (1). Preadipocytes, which are the precursors of mature white adipose tissue cells, play an important role in preserving energetic homeostasis. Adipocytes differentiate from precursor fibroblastic cells, i.e., from preadipocytes, during the entire ontogenetic life can result in the development of obesity related to the increase in the number as well as the volume of adipocytes. It was observed that the number of adipocytes is 4-fold higher in extremely obese patients as compared to slim individuals (2).
In the murine 3T3-L1 preadipocyte cell line, which is a good experimental model used in the majority of studies on adipocytes, the process of differentiation is similar to the one in human cells (3).
Adipogenesis is subject to neurohormonal regulation due to the presence of numerous receptors within adipose tissue cells. There is an inverse relationship between the number of autonomic nervous system axons and the influence on preadipocyte proliferation (4). It was proven that preadipocyte proliferation is inhibited by noradrenaline in vitro (5) and intensified by 2 receptor stimulation (6). Preadipocyte proliferation is also stimulated by thyroid hormones, glucocorticosteroids, estradiol, progesterone and the growth hormone (7, 8). Insulin-like growth factor I (IGF-I), tumor necrosis factor (TNF-α) and macrophage colony-stimulating factor (M-CSF) are among important paracrine factors stimulating preadipocyte proliferation (7, 9).
The discovery of melatonin receptors on adipose tissue cells gives grounds for considering the possibility of melatonin acting as a factor influencing energy storage through the modulation of metabolism and adipocyte proliferation (10, 11).
Melatonin (N-acetyl-5-methoxytryptamine) is a biologically active indole derivative biosynthesized in enzymatic steps from tryptophan. Melatonin takes part in the regulation of the circadian and seasonal rhythms with its involvement in measuring the length of the day. The important role of melatonin as a modulator of sleep is well documented (12). Changes in melatonin levels have been reported with regard to circadian rhythm sleep disorders. Administration of exogenous melatonin has been reported to improve the sleep quality, increase its length, and decrease sleep latency and the number of wakeful episodes in elderly women with insomnia, as well as significantly improve the following-day functioning. Timed daily doses of melatonin in able-bodied people have been observed to reduce the sleep onset time, improve sleep maintenance, increase REM sleep, and correct circadian-based insomnias. Furthermore, melatonin administration may contribute to the management of jet lag (12).
Moreover, melatonin belongs to the endogenous antioxidants (13), exerts cytoprotective (14), analgesic (15), immunomodulative (16, 17) and oncostatic properties (16, 18). Melatonin is also used as an adjunct in the treatment of neoplasms, obesity, Alzheimer's disease and cardiovascular diseases (19).
To date, only a few studies on the influence of melatonin on preadipocytes have been published. What is more, their results are contradictory. The first study was the one reported by us and it confirmed that melatonin administered at supraphysiological concentrations results in the increase in the number of 3T3-L1 preadipocytes after 24 and 48 hours (20). In subsequent studies, Maldonado et al. reported a dose-dependent stimulative influence of melatonin on adipogenesis in a murine fibroblast cell line (21). Similarly, Gonzales et al. described the stimulative influence of melatonin at physiological and supraphysiological concentrations on 3T3-L1 preadipocyte differentiation and adipogenesis and also on the increase in the expression of adipogenic transcription factors: peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα) (22). Peroxisome proliferator activated receptors (PPARs) are transcriptional factors which are strongly involved in carbohydrate and lipid metabolism (23). Melatonin abolishes the inhibitory effect on 3T3-L1 preadipocyte differentiation induced by MCF-7 cells. Additionally, it reduces mRNA expression and the concentration of anti-adipogenic cytokines (TNF-α, IL-6 and IL-11) in 3T3-L1 cells in 3T3-L1 and MCF-7 cell co-culture (9).
On the other hand, however, Alonso-Vale et al. proved the inhibitory effect of melatonin at supraphysiological concentrations on adipocyte differentiation through the reduction in the expression of PPARγ and C/EBPα adipogenesis-regulating factors and late adipocyte differentiation markers, i.e., adiponectin, aP2, and perilipin (24).
The influence of melatonin on the proliferation depends on the cell type and concentrations. It was proven that melatonin at both physiological and pharmacological concentrations stimulates normal cell proliferation (25-31). Unlike normal cells, melatonin has an antiproliferative effect on cancer cells, mainly at pharmacological concentrations (18, 32-34).
There are three known types of membrane melatonin receptors, i.e., MT1, MT2 and MT3 (35). Both the MT1 and MT2 receptors bind melatonin at picomolar concentrations and the MT3 receptor - at nanomolar concentrations. Membrane melatonin receptors are widely distributed within cardiovascular, genitourinary, immune and central nervous systems as well as within the alimentary tract (15, 36-41). In light of studies describing the influence of melatonin on the adipose tissue metabolism and mammal body mass, the discovery of MT1 and MT2 receptors proved to be significant. These two types of receptors are located within human white and brown adipose tissues, on adipocyte surface of human PAZ6 cell line and rat isolated adipocytes (10, 39, 40) and the MT3 receptors are located on the surface of hamster adipocytes (41).
Due to lipid solubility, melatonin influences intracellular metabolism with the participation of calmodulin regardless of the participation of receptors (42).
The best known nonselective antagonist of MT1 and MT2 receptors is luzindole, with a 25-fold higher affinity to the MT2 receptor. The application of luzindole abolishes proproliferative effects of melatonin on murine splenocytes in vitro as well as in vivo, inhibits the antiproliferative effect of melatonin on the Ishikawa cell line of human endometrial cancer and antagonizes the protective activity of endogenous melatonin in rat cerulein-induced pancreatitis and inhibits the expression of melatonin-stimulated receptors for IL-2 and IL-2 synthesis in human lymphocytes (14, 28, 43-46). In the murine fibroblast line, luzindole has an inhibitory effect on melatonin-induced lipogenesis (21). Luzindole abolishes the stimulative effect of melatonin on 3T3-L1 preadipocyte differentiation by inhibiting mRNA expression for C/EBPα and PPARγ. It also abolishes the inhibitory effect of melatonin on the activity and expression of mRNA for aromatase in 3T3-L1 cells (22) and on the expression of anti-adipogenic cytokines (TNF-α, IL-6 and IL-11) in 3T3-L1 and MCF-7 cell co-culture (9).
MT3 receptors were found on the surface of hamster adipocytes (41). There are both confirming and contradicting reports concerning the participation of the MT3 receptor and prazosin in the melatonin action mechanism (15, 47, 48). Prazosin, a selective adrenergic 1-receptor antagonist, is the only known selective MT3 receptor antagonist (35, 38, 49). On their surface, adipocytes of white and brown adipose tissues contain adrenergic α1, α2, β1, β2, β3 receptors (8, 50, 51).
It is suggested that melatonin treatment can result in an increase in adipose tissue mass (42, 52-54) and a reduction in body mass (55). The mechanism of melatonin participation in the body mass regulation has not yet been fully understood. According to some authors, it results from the stimulation of melatonin receptors in the central nervous system and the modulation of the autonomic nervous system activity and food intake modification (53), whereas others authors conclude that it is related to a direct action of melatonin on adipocytes (10, 40, 41). In the case of hibernating animals, an increase in adipose tissue mass is thought to be related to melatonin and the seasonal rhythm (52-54). A different effect of melatonin on autonomic nervous system activation is suggested, i.e., stimulative in the case of brown adipose tissue cells and, most probably, inhibitory in the case of white adipose tissue cells. The examination performed on adipocytes of the brown adipose tissue of the golden hamster showed that melatonin reduces adipose tissue mass, probably through the activation of thermogenesis (53). In humans and rats the amount of visceral adipose tissue increases with age whereas nocturnal melatonin concentrations decrease (56). Melatonin reduces the body mass in diet-induced obesity in rats (55).
Cell growth is inhibited by high concentrations of reactive oxygen species (ROS) and induced by low ROS concentrations due to the stimulation of the proliferation process (57). It was proven that ROS produced in mitochondria limit oxidative phosphorylation within the respiratory chain, which acts as a signal inhibiting the proliferation of 3T3-L1 preadipocytes. When ROS production is limited, e.g., after the application of antioxidants, an increased proliferation of these cells is obtained (58). Paradoxically, both oxidants, H2O2 and antioxidants, e.g., alpha-tocopherol may have similar effects on cell growth (59). The application of antioxidants such as vitamins E, C and A, or N-acetyl-L-cysteine inhibits cell proliferation and the application of butylated hydroxyanisole, trolox or propofol stimulates 3T3-L1 cell proliferation (60, 58).
The aim of the present study is to evaluate the influence of melatonin at physiological and supraphysiological concentrations on the proliferation of the 3T3-L1 murine preadipocyte after 3 and 24 hours of the experiment and to determine the participation of membrane melatonin MT2 receptors, and for the first time - MT3, in melatonin influence on cell proliferation in the 3T3-L1 murine preadipocyte culture during a 24-hour experiment.
MATERIALS AND METHODS
Cell culture
The 3T3-L1 murine preadipocyte cell line obtained from murine 3T3 fibroblasts is the precursor of mature adipose tissue cells. A suspension of the cells at a density of 1×106 was cultured in culture flasks (25 cm2) in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 4 mmol/L L-glutamine, 10% fetal bovine serum (FBS), 100 U/mL penicillin and 0.1 mg/mL streptomycin in standard conditions, i.e., 5% CO2 at 37°C.
Reagents
The 3T3-L1 cells were obtained from the American Type Culture Collection, Rockville, USA (ATCC). The following were used in the experiment: melatonin, luzindole, and prasosin - Sigma Chemical Co. (St. Louis, MO, USA) whereas trypsin-EDTA, Dulbecco's Modified Eagle's Medium (DMEM) supplemented with L-glutamine, fetal bovine serum (FBS), trypan blue were purchased from Bio-Whittaker (Verviers, Belgium). Penicillin and streptomycin were obtained from Gibco (Paisley, Scotland, UK) and [3H]-thymidine from Amersham (Aylesbury, UK). Melatonin was dissolved in ethanol 95% (0.001%) directly prior to the experiment and diluted in the medium to obtain a supraphysiological concentration (10-3 mol/L) and then a physiological concentration (10-9 mol/L). Melatonin solution was not added to the control group cells. Cells were only treated with 0.001% ethanol solution. Luzindole and prazosin were also dissolved in 0.2 mL of ethanol (95%) and diluted with the medium to the final concentration of 10-4 and 10-5 mol/L, respectively.
The concentrations of luzindole and prazosin given were selected according to the preliminary study and our previous experiments. The abovementioned concentrations have been chosen due to their optimal effects. Similar amounts were also used by in the studies conducted by previous authors.
Experimental protocol
The cells were cultured with or without melatonin at 10-3 and 10-9 mol/L, with or without melatonin antagonists, i.e., luzindole (10-4 mol/L) and prazosin (10-5 mol/L) for 3 and 24 hours without any change of medium. Luzindole (10-4 mol/L) and prazosin (10-5 mol/L) were added 30 min before subsequent exposure of the cells to melatonin. After the experiment, the medium was collected, centrifuged and frozen for examination. Cells were trypsinized (2–3 mL of 0.25% Trypsin-EDTA solution) and the number of cells was estimated by direct counting in a Bürker chamber. The results were presented as cell count/mL. Cell viability was evaluated by means of trypan blue staining performed for 15 min. The proportion of dead cells was shown as percentage. Cell proliferation was determined after 3 and 24 hours of incubation with melatonin and melatonin antagonist receptors. The experiment was repeated five times in each group.
[3H]-thymidine incorporation
Cell proliferation was determined by means of labeled [3H] thymidine incorporation in the DNA of the cell. After counting in a Bürker chamber, preadipocytes were treated with trypsin solution (0.25%) and then rinsed twice with the culture medium. 0.2 mL [3H]-thymidine (1.0 µCi/mL (2.5 µmol/L)) was applied for one hour. The cells were rinsed and suspended in 0.5 mL medium and then placed in plastic scintillation vessels, filled with 10 mL of Insta-gel scintillation fluid and placed in the scintillation counter chamber for 4 hours. The samples were examined three times after 1 min. The background value was determined with the results expressed as the number of radioactive disintegrations per minute/sample (dpm/sample) per million cells, based on the readings for standards. From the obtained results, the mean value of the taken measurements was calculated. The reading of the radioactive disintegrations in the scintillation counter was performed at the Unit and the Department of Histology and Embryology of the Medical University of Silesia in Zabrze, Poland.
Statistical analysis
The data were collected in the Microsoft Excel spreadsheet. After the preliminary calculation, the data were transferred to a database of StatSoft STATISTICA 7.0, by means of which the analyses were conducted. The conformity of the distribution of continuous variables to the Gaussian distribution was verified with the Kolmogorov-Smirnov test. The evaluation of the significance of statistical difference between the means was performed with parametric Student's t-test and non-parametric tests, i.e., the Mann-Whitney U test for unrelated variables and the Wilcoxon signed-rank test for related variables, depending on the conformity of the results to the Gaussian distribution. The results were given as arithmetic means ± standard deviation. Statistical significance was defined as p<0.05.
RESULTS
The influence of melatonin on cell count
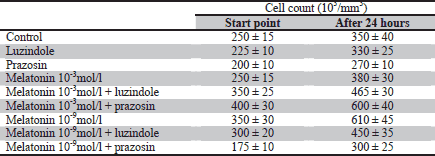
Melatonin at both the physiological and supraphysiological concentration has a stimulative effect on the number of 3T3-L1 preadipocytes. An increase by 12% and 34% in cell count was observed compared to the controls at 10-3 mol/L and 10-9 mol/L concentrations (p<0.05), respectively. The application of luzindole inhibits the above effect of melatonin both at the 10-3 mol/L and
10-9 mol/L concentration (p<0.05). The presence of prazosin does not have a statistically significant influence on the effects of melatonin action (Table 1, Fig. 1). The number of dead cells determined by means of blue trypan staining did not exceed 3%.
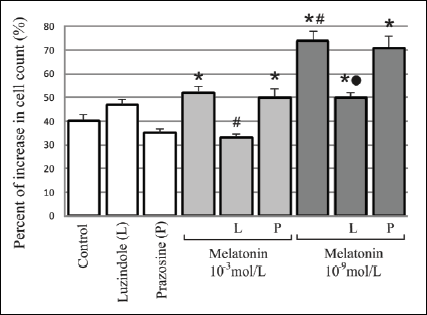 |
Fig. 1. Influence of a 24-hour incubation with melatonin (10-3mol/L and 10-9mol/L) and melatonin receptors antagonists - luzindole (10-4 mol/L) and prazosin (10-5mol/L) on 3T3-L1 preadipocyte cell count. The values are presented as percent of increase in cell count. The initial number of cells in particular groups were claimed as 100%. n=5; * p<0.05 vs. control; # p<0.05 vs. melatonin 10-3 mol/L; " p<0.05 vs melatonin 10-9 mol/L. |
The influence of melatonin on [3H]-labeled-thymidine incorporation in the cells
A stimulative effect of melatonin on [3H]-thymidine incorporation in 3T3-L1 preadipocytes was observed at both concentrations and time intervals as compared to the control. It has to be noted that the 10-9 mol/L concentration has a stronger effect compared to 10-3 mol/L concentration both after 3 hours (133% vs. 117%, respectively; p<0.05) and 24 hours (209% vs. 105%; p<0.05). The stimulative effect of melatonin on [3H]-thymidine incorporation in the cells at 10-9 mol/L concentration is expressed much more strongly during a 24-hour incubation compared to a 3-hour incubation (209% vs. 133%; p<0.05) (Fig. 2).
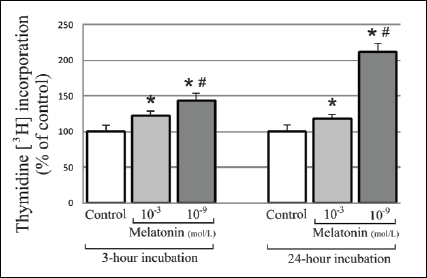 |
Fig. 2. Influence of 10-3mol/L and physiological concentration (10-9mol/L) of melatonin on [3H]-thymidine incorporation in 3T3-L1 preadipocytes, during 3-hour and 24-hour incubation. The values are presented as percents ± S.D. The initial number of cells in particular groups were claimed as 100%. n=5; * p<0.05 vs. control; # p<0.05 vs. melatonin 10-3 mol/L. |
The increase in labeled [3H]-thymidine incorporation in the DNA of the cells compared to the control was observed after the application of melatonin at 10-3 mol/L concentration after 24 hours (4.9% increase; p<0.05). The application of luzindole with melatonin resulted in the inhibition of cell proliferation by 7% (p<0.05) compared to the application of melatonin independently. The presence of prazosin did not have a statistically significant influence on the effects of melatonin action (Fig. 3).
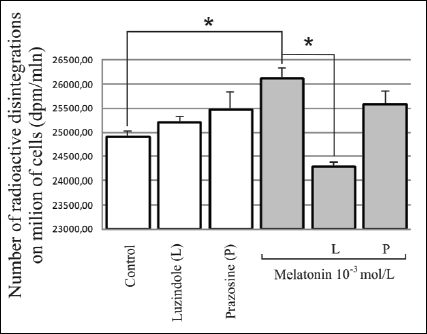 |
Fig. 3. Influence of melatonin at 10-3mol/L concentration and melatonin receptors antagonists i.e. MT2 - luzindole (10-4mol/L) and MT3 - prazosin (10-5 mol/L) on [3H]-thymidine incorporation in 3T3-L1 preadipocytes, during a 24-hour experiment. The results are presented as the number of radioactive disintegrations per million cells (mean values ± S.D.). n=5; * p<0.05. |
There was a significant, over two-fold increase in the labeled [3H]-thymidine incorporation in the DNA of the cells compared to the control at 10-9 mol/L concentration (109.4% increase; p<0.05) during a 24-hour exposure. The above melatonin action was partially inhibited by about 34% due to the application of luzindole (p<0.05). However, the presence of prazosin did not have a statistically significant influence on the effects of melatonin action (Fig. 4).
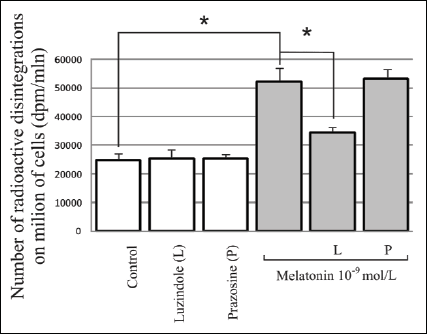 |
Fig. 4. Influence of melatonin at the physiological concentration (10-9mol/L) and melatonin receptor antagonists MT2 - luzindole (10-4mol/L) and MT3 - prazosin (10-5 mol/L) on [3H]-thymidine incorporation in 3T3-L1 preadipocytes, during a 24-hour experiment. The results are presented as the number of radioactive disintegrations per million cells (mean values ± S.D.) n=5; * p<0.05. |
The combined application of both a melatonin antagonist receptors together with melatonin resulted in contradictory results which were difficult to interpret (the results have not been presented).
In summary, the application of melatonin at both concentrations stimulated the 3T3-L1 cell proliferation. It has to be noted that the physiological concentration had a stronger effect compared to supraphysiological concentrations.
DISCUSSION
In the conducted experiment, melatonin at physiological (10-9 mol/L) and supraphysiological (10-3 mol/L) concentrations had a stimulative effect on the 3T3-L1 cell proliferation during 3- and 24-hour incubation. The maximum proliferation increase was obtained during long exposure using the physiological concentration. Membrane receptors, mainly MT2, participate in the above melatonin action.
Melatonin at 10-3 mol/L and 10-9 mol/L concentrations had a stimulative effect on the number of 3T3-L1 preadipocytes. The highest increase in cell count by 34% compared to the control was observed at the physiological concentration. Comparing the 3- and 24-hour proproliferative effect of melatonin it was observed that physiological concentrations of melatonin resulted in over a two-fold increase in [3H]-thymidine incorporation in the cells after 24 hours and only a 33% increase after 3 hours. However, in the instance of supraphysiological concentrations an increase by 4.8% and 17% was observed, respectively. The above results may be related to the fact that a full cell cycle of 3T3-L1 preadipocytes lasts about 18 hours and in the initial phase of the preadipocyte cell cycle - between G1 and S phases - due to a cell-to-cell contact, their growth stops and one or two cell divisions occur (the phenomenon known as clonal expansion) (2).
In our previous study on the influence of melatonin on 3T3-L1 preadipocytes, it was proven that melatonin at high, i.e., 10-6 mol/L and 10-3 mol/L concentrations caused a significant increase in the number of cells, which was directly proportional to the exposure time and inversely proportional to the dose (20). The increase was from 6 to 50% as assessed by the direct method.
To date it, has been shown that melatonin at physiological and pharmacological concentrations stimulates normal cell proliferation, i.e., human osteoblasts (25), keratinocytes (26), dermal fibroblasts (27), murine splenocytes (28), rat hippocampal dentate gyrus cells (29), human mesenchymal stem cells (30), and neural stem cells (31). On the other hand, there are reports on an antiproliferative effect of melatonin on cells from rat thymus, brain, lung and kidney homogenates (61).
Adipocytes are produced from pluripotential mesenchymal stem cells. Mesenchymal cells differentiate into adipoblasts, preadipocytes, and then adipocytes. Numerous transcription factors participate in the differentiation into mature adipocytes. These are the following factors: CCAAT/enhancer-binding protein β(C/EBPb), CCAAT/enhancer-binding protein δ(C/EBPδ), (PPARγ), (C/EBPα) and cAMP response element-binding protein (CREB) (62).
The majority of the mentioned studies indicate proproliferative effects of melatonin on normal cells, including 3T3-L1 preadipocytes, which is consistent with our results. Maldonado et al. described a dose-dependent stimulating effect of melatonin on adipogenesis in the murine fibroblast line (21). Interesting studies were reported by Gonzalez et al. and Alvarez-Garcia et al., where 3T3-L1 cells are presented as a model of fibroblasts of adipose tissue surrounding breast carcinoma. It was shown that melatonin at physiological and supraphysiological concentrations stimulates the 3T3-L1 preadipocyte differentiation and adipogenesis, as well as the expression of C/EBPα and PPARγ transcription factors. Melatonin abolishes the inhibitory influence on 3T3-L1 preadipocyte differentiation induced by MCF-7 cells in 3T3-L1 and MCF-7 cell co-culture. Additionally, it reduces the expression of mRNA and the concentration of anti-adipogenic cytokines (TNF-α, IL-6 and IL-11) in 3T3-L1 and MCF-7 cell co-culture (9).
On the other hand, Alonso-Vale et al. (24) demonstrated the inhibitory effect of melatonin at supraphysiological concentrations on adipocyte differentiation by the reduction in the expression of adipogenic transcription factors (PPARγ and C/EBPα) and late adipocyte differentiation markers, i.e., adiponectin and perilipin.
Based on the reports in the literature on the presence of membrane melatonin receptors on the surface of white adipose tissue adipocytes, a melatonin receptor antagonist - luzindole - was used in the present experiment. In the conducted study, melatonin influences preadipocyte proliferation partly by MT1 and MT2 membrane receptors, which was proven by the ability of luzindole to inhibit the effects of melatonin, particularly by the MT2 receptor. This is due to the fact that luzindole, which is a nonselective antagonist of MT1 and MT2 melatonin receptors, has the highest, i.e., about 25-fold affinity to the MT2 receptor. Luzindole antagonizes the influence of melatonin at physiological concentrations on 3T3-L1 cell proliferation most powerfully, i.e., by about 34%. However, it does not abolish its action entirely, which may show that other melatonin receptors, e.g., MT1 membrane receptor or a nuclear receptor participate in this process.
Similarly, Gonzalez et al. (22) showed that luzindole abolishes the influence of melatonin on 3T3-L1 preadipocytes by the inhibition of mRNA expression for PPARγ and C/EBPα increased by melatonin to the level present in the control group. Additionally, luzindole abolished the inhibitory effect of melatonin on the activity and expression of mRNA for aromatase in 3T3-L1 cells. The above action proves that MT2 receptors participate in the mechanism of melatonin action on such cells (22).
Luzindole abolishes the inhibitory effect of melatonin on the expression of anti-adipogenic cytokines (TNF-α, IL-6 and IL-11) in 3T3-L1 and MCF-7 cell co-culture (9).
Numerous studies have proven that luzindole inhibits the effects of melatonin action on other cells (28, 44-46). Our previous experiment showed that luzindole partly abolishes the stimulative effect of melatonin at pharmacological concentrations on the increase in the 3T3-L1 cell count, estimated by the direct counting (20). The authors of the above studies agree that luzindole at the concentrations similar to the above has an inhibitory effect on proproliferative melatonin action with the participation of MT2 receptor.
To date, in the available literature there have not been any studies on the presence of the MT3 receptor in white adipose tissue cells. In the present study, prazosin, which is a selective MT3 receptor antagonist, was used. It is also a well-known selective antagonist of 1adrenergic receptors. The MT3 membrane receptor, a 26 kDa protein, is a homolog of flavoenzyme - human quinone reductase 2 (QR2, EC 1.6.99.2). The receptor does not act through G proteins and is characterized by a low affinity to melatonin. The role of the MT3 receptor in intracellular transmission is to stimulate phosphatidylinositol (63). The receptor is present in the liver, kidney, brain, heart, adipose tissue, skeletal muscles and lungs of the hamsters and the mice (37, 52, 63), and on the surface of hamster adipocytes (42). The only known selective MT3 receptor antagonist is prazosin - a selective antagonist of the a1 adrenergic receptors (35, 38, 49). White and brown adipose tissue adipocytes have adrenergic receptors, i.e., α1, α2, β1, β2, β3 on their surface (8, 51, 52). There are both confirming and contradictory reports on the participation of the MT3 receptor and prazosin in melatonin action mechanism (15, 47, 48).
In the present study, the influence of prazosin on proproliferative effect of melatonin action has not been observed. Few in vivo and in vitro studies show an inhibitory effect or lack of the effect of prazosin and the participation of MT3 receptor on melatonin action (47, 64, 65). Podda et al. showed that melatonin inhibits the neural activity within rat central vestibular nucleus in vitro and that the application of prazosin did not antagonize this effect (64). On the other hand, Souze et al. (47) proved that prazosin suppresses stimulative effects of melatonin on the cell growth and tyrosinase activity in the human melanoma cell line (47). Similarly, Santagostino-Barbone et al. showed that prazosin at the 0.3 µmol/L concentration inhibits the melatonin-induced contraction of colonic smooth muscle isolated from guinea pigs by about 30% compared to the maximum melatonin effect (65). The absence of prazosin action in the present study may be explained by a potential absence of MT3 receptor on the surface of 3T3-L1 preadipocytes.
The discovery of melatonin receptors on adipose tissue cells gives grounds for considering the possibility of melatonin acting as a factor influencing energy storage through the modulation of metabolism and adipocyte proliferation (10). Melatonin inhibits, rather than stimulates, the influence of the autonomic nervous system on white adipose tissue cells. Therefore, it can stimulate not only lipid storage, by the inhibition of lipolysis, but also adipocyte proliferation (53).
Few study results on the relationship between melatonin and adipocyte metabolism are contradictory. On the one hand, Brydon et al. proved that the exposure of human PAZ6 adipocytes to melatonin resulted in the decrease in the amount of mRNA, GLUT-4 receptor protein count, and glucose uptake in these cells (10). Conversely, Alonso-Vale et al. (11) found that melatonin results in the increase in adipocyte sensitivity to insulin. Moreover, melatonin at physiological concentrations (10-9 mol/L) resulted in an over two-fold increase in glucose uptake in the murine C2C12 skeletal muscle cell line, accelerated phosphorylation of insulin receptor substrate (1 IRS-1), and resulted in the increase in phosphoinositide 3-kinase (PI3-kinase) through the participation in glucose homeostasis (66). Additionally, it is suggested that there exists a relationship between the development of diabetes and the decrease in melatonin concentration in elderly individuals and in individuals exposed to light at night (67).
It has been noticed that changes in adipose tissue mass in hibernating animals are most likely related to the regulation of seasonal rhythms, for which melatonin is responsible. The increase in body mass in these animals starts from July when the day length and the amount of light begin to decrease and melatonin secretion increases so that the maximum body mass is gained in September (41). In rats and humans the amount of visceral adipose tissue increases with age, whereas nocturnal melatonin concentrations decrease (56). It was proven that in shift workers who experience disturbances of day-night and sleep-wakefulness rhythms, which are related to melatonin production disturbances, there is a co-occurrence of disturbances in the body mass regulation, along with hypersomnia and the excessive demand for sweets (67).
The amount of melatonin produced in the circadian rhythm is also age-dependent. The highest nocturnal melatonin concentration is noted in children, and the lowest in the elderly. Human brown adipose tissue is most developed in children, together with the highest melatonin concentrations; the amount of the tissue decreases with age and along with an accompanying decrease in melatonin secretion (68).
Since melatonin was found to reduce body mass in diet-induced rat obesity (55), and considering the role of melatonin in maintaining energetic balance of the body, attention has been paid to the relationship between melatonin and leptin. Melatonin at physiological concentrations was observed to increase mRNA expression for leptin in murine adipocytes during simultaneous exposure to insulin, acting through the MT1 membrane receptor (39). Also, a simultaneous decrease in both melatonin and leptin concentrations in night shift workers was observed (69). The increase in leptin concentration with a simultaneous decrease in melatonin concentration in middle-aged rats seems to be interesting and can be connected to the pathogenesis of age-related obesity, where leptin concentrations correlate positively with the amount of the adipose tissue (70).
The above mentioned controversial literature reports on varying influence of melatonin on the adipose tissue metabolism may partly result from a disparate behavior of the hormone in vivo and in vitro. In vitro conditions cannot precisely correspond to in vivo conditions, in which melatonin is inactivated in the liver within an hour from its application with the omission of significant neuroendocrine systems.
The present study has shown that melatonin is capable of stimulating preadipocyte proliferation. A direct relationship between the influence of produced ROS and cell proliferation has so far been observed in endothelial, smooth muscle and prostate cells, different neoplastic cell lines, fibroblasts and macrophages (59, 60, 71, 72). Paradoxically, both oxidants and antioxidants can exert similar effects on cell growth. High ROS concentrations have an inhibitory effect on cell proliferation, whereas low ones induce cell growth by the stimulation of proliferation (57).
The action to reduce the ROS production by the application of antioxidants such as butylated hydroxyanisole (BHA), trolox or propofol, with or without H2O2, results in an increase in the 3T3-L1 preadipocyte proliferation. However, the application of rotenone and/or oligomycin - chemical substances capable of producing ROS - resulted in a reversible inhibition of the preadipocyte proliferation. Mitochondrion-produced ROS limit oxidative phosphorylation within the respiratory chain, which acts as a signal inhibiting the proliferation of 3T3-L1 preadipocytes (58).
The present study has proven that the application of melatonin, an antioxidant, resulted in the stimulation of 3T3-L1 cell proliferation at physiological and supraphysiological concentrations both during short- and long-lasting exposure. These effects were accompanied by the stimulation of antioxidative enzymes, mainly mitochondrial (MnSOD) and peroxisomal (CAT), which was shown in our previous study (48). The same effects with the application of oxidants and antioxidants were produced by an experiment on a human preadipocyte cell culture from the subcutaneous adipose tissue of slim individuals (58).
The application of antioxidants alone to a human fibroblast culture without factors triggering the ROS production resulted in a dose-dependent inhibition of tritium-labeled thymidine incorporation (57). Similarly, it was shown that the inhibition of the ROS production by the application of such antioxidants as vitamins E, C and A, or N-acetyl-L-cysteine reduced cell proliferation (60). These results, which are not consistent with our observations, may be related to the differences connected with the usage of different antioxidants, different cell lines, experimental models and doses. These results can also be related to the fact that depending on their concentrations, antioxidants can act as oxidants and have similar effects on cell proliferation.
Thus, the above studies indicate a relationship between oxygen metabolism and preadipocyte number along with the amount of the adipose tissue, which is closely related to the change in body mass occurring in obesity. Moreover, we have proven in our previous work that melatonin precursor, serotonin, influences the cardiovascular system and modulates sympathetic activity (73). Based on these observations, melatonin can be considered to be among the factors capable of influencing the adipose tissue mass regulation.
Our observations from the present study confirm that melatonin at physiological and supraphysiological concentrations stimulates the 3T3-L1 preadipocyte proliferation. It has to be noted that physiological concentrations of melatonin are more effective. The present experiment has, for the first time, assessed the MT3 receptor participation through the application of prazosin. The action of melatonin has not been confirmed in this mechanism. It has been proven that melatonin has a proproliferative effect on 3T3-L1 preadipocytes, partially by MT2 receptors, and that prazosin has no effect on the proliferation. This proves that melatonin receptors, primarily MT2, participate in the proproliferative melatonin effect.
Conflict of interests: None declared.
REFERENCES
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796-1808.
- Schaffler A, Muller-Ladner U, Scholmerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev 2006; 27: 449-467.
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. Factors affecting the adipose conversion. Cell 1975; 5: 19-27.
- DiGirolamo M, Fine JB, Tagra K, Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am J Physiol 1998; 274: R1460-R1467.
- Jones DD, Ramsay TG, Hausman GJ, Martin RJ. Norepinephrine inhibits rat preadipocyte proliferation. Int J Obes Relat Metab Disord 1992; 16: 349-354.
- Valet P, Pages C, Jeanneton O, et al. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest 1998; 101: 1431-1438.
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev 2001; 2: 239-254.
- Monjo M, Pujol E, Roca P. Alpha2- to beta3-adrenoceptor switch in 3T3 L1 preadipocytes and adipocytes: modulation by testosterone, 17beta-estradiol, and progesterone. Am J Physiol Endocrinol Metab 2005; 289: E145-E150.
- Alvarez-Garcia V, Gonzalez A, Alonso-Gonzalez C, Martinez-Campa C, Cos S. Melatonin interferes in the desmoplastic reaction in breast cancer by regulating cytokine production. J Pineal Res 2012; 52: 282-290.
- Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology 2001; 142: 4264-4271.
- Alonso-Vale MI, Andreotti S, Peres SB, et al. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Endocrinol Metab 2005; 288: E805-E812.
- Kostoglou-Athanassiou I. Therapeutic applications of melatonin. Ther Adv Endocrinol Metab 2013; 4: 13-24.
- Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol 2003; 50: 1129-1146.
- Jaworek J, Konturek SJ, Leja-Szpak A, et al. Role of endogenous melatonin and its MT2 receptor in the modulation of caerulein-induced pancreatitis in the rat. J Physiol Pharmacol 2002; 53: 791-804.
- Mantovani M, Kaster MP, Pertile R, Calixto JB, Rodrigues AL, Santos AR. Mechanisms involved in the antinociception caused by melatonin in mice. J Pineal Res 2006; 41: 382-389.
- Vijayalaxmi B, Thomas CR, Reiter RJ, Herman T. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol 2002; 20: 2575-2601.
- Carrillo-Vico A, Garcia-Perganeda A, Naji L, Calvo JR, Romero MP, Guerrero JM. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci 2003; 60: 2272-2278.
- Cini G, Neri B, Pacini A, et al. Antiproliferative activity of melatonin by transcriptional inhibition of cyclin D1 expression: a molecular basis for melatonin-induced oncostatic effects. J Pineal Res 2005; 39: 12-20.
- Tozawa T, Mishima K, Satoh K, Echizenya M, Shimizu T, Hishikawa Y. Stability of sleep timing against the melatonin secretion rhythm with advancing age: clinical implications. J Clin Endocrinol Metab 2003; 88: 4689-4695.
- Zwirska-Korczala K, Jochem J, Adamczyk-Sowa M, et al. Influence of melatonin on cell proliferation, antioxidative enzyme activities and lipid peroxidation in 3T3-L1 preadipocytes - an in vitro study. J Physiol Pharmacol 2005; 56(Suppl. 6): 91-99.
- Maldonado MD, Siu AW, Sanchez-Hidalgo M, Acuna-Castroviejo D, Escames G.. Melatonin and lipid uptake by murine fibroblasts: clinical implications. Neuro Endocrinol Lett 2006; 27: 601-608.
- Gonzalez A, Alvarez-Garcia V, Martinez-Campa C, Alonso-Gonzalez C, Cos S. Melatonin promotes differentiation of 3T3-L1 fibroblasts. J Pineal Res 2012; 52: 12-20.
- Bogacka I, Bogacki M, Gaglewska M, Kurzynska A, Wasielak M. in vitro effect of peroxisome proliferator activated receptor (PPAR) ligands on prostaglandin E2 synthesis and secretion by porcine endometrium during the estrous cycle and early pregnancy. J Physiol Pharmacol 2013; 64: 47-54.
- Alonso-Vale MI, Peres SB, Vernochet C, Farmer SR, Lima FB. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBPbeta transcriptional activity. J Pineal Res 2009; 47: 221-227.
- Nakade O, Koyama H, Ariji H, Yajima A, Kaku T. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J Pineal Res 1999; 27: 106-110.
- Hipler UC, Fischer TW, Elsner P. HaCaT cell proliferation influenced by melatonin. Skin Pharmacol Appl Skin Physiol 2003: 16: 379-385.
- Carossino AM, Lombardi A, Matucci-Cerinic M, Pignone A, Cagnoni M. Effect of melatonin on normal and sclerodermic skin fibroblast proliferation. Clin Exp Rheumatol 1996; 14: 493-498.
- Drazen DL, Bilu D, Bilbo SD, Nelson RJ. Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 2001; 280: R1476-R1482.
- Kim MJ, Kim HK, Kim BS, Yim SV. Melatonin increases cell proliferation in the dentate gyrus of maternally separated rats. J Pineal Res 2004; 37: 193-197.
- Zhang L, Su P, Xu C, et al. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARc expression and enhancing Runx2 expression. J Pineal Res 2010; 49: 364-372.
- Moriya Y, Horie N, Mitome M, Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J Pineal Res 2007; 42: 411-418.
- Casado-Zapico S, Rodriguez-Blanco J, Garcia-Santos G, et al. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res 2010; 48: 72-80.
- Park SY, Jang WJ, Yi EY, et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1α stabilization under hypoxia. J Pineal Res 2010; 48: 178-184.
- Cabrera J, Negrin G, Estevez F, Loro J, Reiter RJ, Quintana J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res 2010; 49: 45-54.
- Dubocovich ML, Cardinali DP, Delagrange P, et al. Melatonin receptors. In: The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media. London, 2000, pp. 270-277.
- Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 2006; 60: 97-108.
- Mailliet F, Ferry G, Vella F, et al. Characterization of the melatoninergic MT3 binding site on the NRH: quinone oxidoreductase 2 enzyme. Biochem Pharmacol 2005; 71: 74-88.
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 2003; 8: d1093-d1108.
- Alonso-Vale MI, Andreotti S, Peres SB, et al. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Endocrinol Metab 2005; 288: E805-E812.
- Zalatan F, Krause JA, Blask DE. Inhibition of isoproterenol-induced lipolysis in rat inguinal adipocytes in vitro by physiological melatonin via a receptor-mediated mechanism. Endocrinology 2001; 142: 3783-3790.
- Le Gouic S, Atgie C, Viguerie-Bascands N, et al. Characterization of a melatonin binding site in Siberian hamster brown adipose tissue. Eur J Pharmacol 1997; 339: 271-278.
- Soto-Vega E, Meza I, Ramirez-Rodriguez G, Benitez-King G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. J Pineal Res 2004; 37: 98-106.
- Carrillo-Vico A, Lardone PJ, Fernandez-Santos JM et al. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2 receptor system. J Clin Endocrinol Metab 2005; 90: 992-1000.
- Dubocovich ML. Luzindole (N-0774): a novel melatonin receptor antagonist. J Pharmacol Exp Ther 1988; 246: 902-910.
- Kobayashi Y, Itoh MT, Kondo H, et al. Melatonin binding sites in estrogen receptor-positive cells derived from human endometrial cancer. J Pineal Res 2003; 35: 71-76.
- Rivera-Bermudez MA, Masana MI, Brown GM, Earnest DJ, Dubocovich MI. Immortalized cells from the rat suprachiasmatic nucleus express functional melatonin receptors. Brain Res 2004; 1002: 21-27.
- Souza AV, Visconti MA, Castrucci AM. Melatonin biological activity and binding sites in human melanoma cells. J Pineal Res 2003; 34: 242-248.
- Adamczyk-Sowa M, Sowa P, Zwirska-Korczala K, Pierzchala K, Bartosz G, Sadowska-Bartosz I. Role of melatonin receptor MT(2) and quinone reductase II in the regulation of the redox status of 3T3-L1 preadipocytes in vitro. Cell Biol Int 2013; 37: 835-842.
- Alexander SP, Mathie A, Peters JA. 7TM Receptors. Br J Pharmacol 2007; 150(Suppl. 1): S4-S81.
- Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J Biol Chem 2000; 275: 33059-33067.
- Lindquist JM, Fredriksson JM, Rehnmark S, Cannon B, Nedergaard J. Beta 3- and alpha1-adrenergic Erk1/2 activation is Src- but not Gi-mediated in brown adipocytes. J Biol Chem 2000; 275: 22670-22677.
- Delagrange P, Atkinson J, Boutin A, et al. Therapeutic perspectives for melatonin agonists and antagonists. J Neuroendocrinol 2003; 15: 442-448.
- Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med (Maywood) 2002; 227: 363-376.
- Mustonen AM, Nieminen P, Hyvarinen H. Melatonin and the wintering strategy of the tundra vole, Microtus oeconomus. Zoolog Sci 2002; 19: 683-687.
- Prunet-Marcassus B, Desbazeille M, Bros A, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 2003; 144: 5347-5352.
- Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest 1998; 101: 1353-1361.
- Murrel GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 1990; 265: 659-665.
- Carriere A, Fernandez Y, Rigoulet M, Penicaud L, Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett 2003; 550: 163-167.
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 1995; 18: 775-794.
- Havens CG, Ho A, Yoshioka N, Dowdy SF. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol Cell Biol 2006; 26: 4701-4711.
- Sainz RM, Mayo JC, Kotler M, Uria H, Antolin I, Rodriguez C. Melatonin decreases mRNA for histone H4 in thymus of young rats. Life Sci 1998; 63: 1109-1117.
- Zhang JW, Klemm DJ, Vinson C, Lane MD. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J Biol Chem 2004; 279: 4471-4478.
- Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem 2000; 275: 31311-31317.
- Podda MV, Deriu F, Giaconi E, Milia M, Tolu E. Melatonin inhibits rat medial vestibular nucleus neuron activity in vitro. Neurosci Lett 2003; 341: 209-212.
- Santagostino-Barbone MG, Masoero E, Spelta V, Lucchelli A. 2-Phenylmelatonin: a partial agonist at enteric melatonin receptors. Pharmacol Toxicol 2000; 87: 156-160.
- Ha E, Yim SV, Chung JH, et al. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res 2006; 41: 67-72.
- Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 2001; 58: 747-752.
- Arendt J. The pineal gland: basic physiology and clinical implications. In: Endocrinology, LJ De Groot, JL Jameson (eds). Philadelphia, Elsevier Saunders, 2006, pp. 557-575.
- Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 2003; 73: 2467-2475.
- Wolden-Hanson T, Mitton DR, McCants RL, et al. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology 2000; 141: 487-497.
- Hu Y, Rosen DG, Zhou Y, et al. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer. role in cell proliferation and response to oxidative stress. J Biol Chem 2005; 280: 39485-39492.
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol 2013; 64: 409-421.
- Sowa P, Adamczyk-Sowa M, Zwirska-Korczala K, Namyslowski G, Misiolek M, Pierzchala K. The role of serotonergic 5-HT1A receptors in central cardiovascular regulation in haemorrhagic shock in rats. J Physiol Pharmacol 2013; 64: 219-229.
A c c e p t e d : January 9, 2014