SALIVA AND OXIDATIVE STRESS IN ORAL
CAVITY
AND IN SOME SYSTEMIC DISORDERS
2Department of Conservative Dentistry, Medical University in Bialystok, Poland
INTRODUCTION
The oxidant-antioxidant balance of the organism can be disturbed by trauma, stress, exercise, nutrition, degenerative diseases, immune disturbances and hormonal imbalance; these factors can also accelerate the formation of free radicals (1-6). Oxidative stress occurs when the intracellular concentrations of reactive oxygen species increase over the physiological values (7). The cytotoxic effect of free radicals on cells is detrimental and leads to cell damage by affecting the peroxidation of double-chain fatty acids, protein and DNA as well as to the increase of oxidative stress (2, 3, 8, 9). These effects are possibly related to the development of cardiovascular diseases (10, 11), neurodegenerative diseases (12, 13), cancer (14, 15) and ageing processes (16-18). On the other hand, mammalian cells have developed antioxidant defense systems to prevent oxidative damage. The antioxidant balance of an individual is influenced by diet regime, physical activity and level of stress (2-5). Antioxidants are present in all body fluids and tissues and have a protective function against endogenously-formed free radicals, usually produced by a leakage in the electron transport system (2, 3, 8, 9). Antioxidant enzymes such as superoxide dismutase and glutathione peroxidase provide intracellular protection while low-molecular-weight scavenging antioxidants are present in the extracellular fluid. These include ascorbic acid, α-tocopherol and α-carotene. In addition, there are components such as uric acid, non-protein thiols and glutathione (15, 19-21).
Tryptophan, an exogenous amino acid, is metabolized in 96% through the kynurenine pathway by two different enzymes. In hepatic cells this process is accomplished by tryptophan 2,3-dioxygenase; in other cells (mainly macrophages and monocytes) it is converted by the enzyme - indoleamine 2,3-dioxygenase (IDO) (22). IDO is responsible for the oxidative metabolism of tryptophan and, with the exception of superoxide dismutase, it is the only enzyme known to use superoxide as a substrate. Therefore, IDO is a primary component of the antioxidant forces of the cell (23). We have previously shown that the saliva of diabetic patients is characterized by far higher IDO activity than that in healthy subjects (24).
Human saliva is a mixture of gingival cervicular fluid, whose composition is similar to that of serum, and the fluid produced in and secreted from salivary glands (22, 25). Due to rapidly developing analytic methods it became possible to carry out more advanced research into numerous compounds that are responsible for the physiochemical and biological properties of saliva (22). In addition to other functions, saliva may constitute the first line of defense against oxidative stress (26). Moreover, the presence of many of these substances may be indicative of pathophysiological changes in the oral cavity as well as of some systemic disorders.
Peroxidase is one of the most important salivary enzyme, secreted by the salivary glands, while uric acid the most abundant salivary antioxidant, non-enzymatic molecule, is plasma-born and its concentration is similar to that of serum (20). By contrast, the concentrations of other salivary antioxidants - such as ascorbic acid and albumin - are lower than that of serum (21). Nevertheless, this indicates an active secretion system for salivary antioxidants. Stimulated saliva is characterized by a lower concentration of antioxidants, but in terms of flow rates, antioxidant capacity is higher than in unstimulated saliva (21).
This review focuses mainly on the potential role of oxidative stress in saliva as an important factor determining the development of oral cavity diseases (Fig. 1).
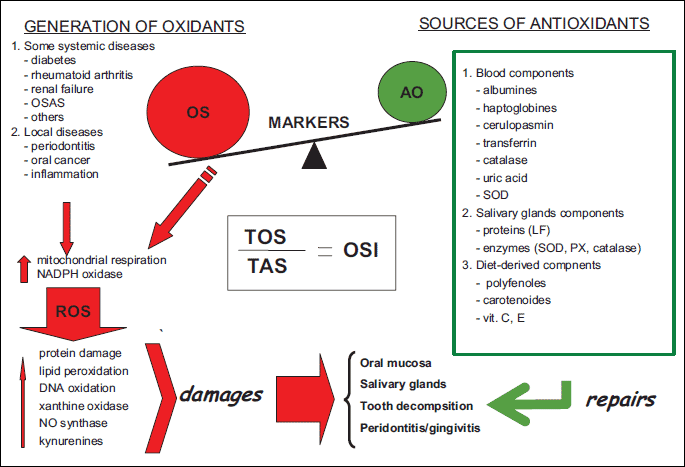
The composition of saliva varies in different local and systemic diseases and thus may reflect many pathophysiological states (1-3). Since saliva is easy to collect, it is recommended as a very sensitive and important non-invasive diagnostic tool in some systemic diseases. We also propose the estimation of kynurenines in saliva as a new biomarker of oxidative stress.
PERIODONTAL DISEASE (PD)
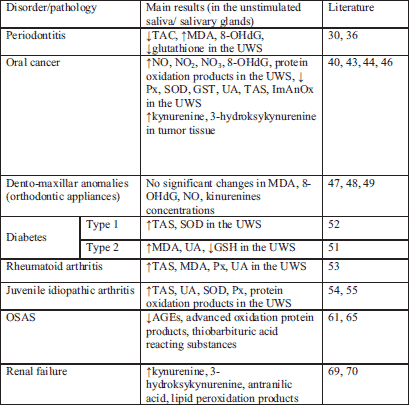
PD is initiated by the colonization of gums by bacterial pathogens (27, 28). This process results in the host tissue damage due to free radical production enhanced in individuals with PD because of the lack of adequate antioxidant defense (29). Chapple et al. (30) have reported a reduced total salivary antioxidant capacity in PD patients. PD was 4.5 times as likely to develop in individuals with the lowest total antioxidant activity flow. In 2003 Sculley et al. (31) decided to investigate whether PD is associated with impaired salivary antioxidant status and increased oxidative injury; they have demonstrated that PD is associated with reduced salivary antioxidant status and increased oxidative damage in the oral cavity. It is certain that lower antioxidant concentrations in the gingival cervicular fluid will contribute to increased damage to the gingivae and surrounding structures by activated neutrophils (32). These authors have also suggested that whole saliva may contain easily measurable indicators of oxidative process and may provide a tool for the development and monitoring of new PD treatment strategies (33). Unfortunately, so far there has been no evidenced progress in the development of such strategies of treating periodontitis via the modification of oxidative stress. Moreover, the total oxidant status (TAS) and oxidative stress index (OSI) in the same biological fluids were significantly higher in patients with chronic periodontitis compared to periodontally healthy controls (34). Results published by Trivedi et al. (35) also confirmed an important role of oxidative stress in both diabetes type 2 and PD. These authors observed augmented MDA levels in saliva and blood in 30 patients with periodontitis and in 30 patients with diabetes type 2 and periodontitis in respect to 60 periodontally healthy individuals (30 patients with diabetes type 2 and 30 systemically healthy patients). Between individuals with chronic PD and the healthy groups there was a highly significant decrease for glutathione reductase (GR) and catalase (CAT) activities except SOD in blood. Only salivary SOD and GR activities were significantly lower in periodontitis and periodontitis-type 2 diabetes groups. It is suggested that the compensatory mechanism of the body is partially collapsed as a result of excessive production of free radicals in PD and is not able to cope with increased generation of free radicals attributable to diabetes mellitus, hereby worsening the situation (35).
The association between salivary levels of 8-hydroxydeoxy-guanosine (8-OHdG), an oxidative stress marker was also studied (36). It was found in groups of PD subjects with augmented concentration of 8-OHdG in saliva and with the presence of periodontally-involved teeth of a hopeless prognosis. The authors propose to use this marker for the identification of patients with teeth of hopeless prognosis due to advanced periodontal destruction resulting from PD. Recent studies have demonstrated higher levels of 8-OHdG and MDA in the chronic periodontitis group compared to controls, whereas salivary activities for uric acid, TAC and glutathione peroxidase (GPx) were decreased (37). Total antioxidant capacity (TAC) and superoxide dismutase (SOD) enzyme concentration decreased both in serum and gingival crevicular fluid in subjects with chronic PD (38).
The accumulative data indicate that PD subjects develop all-encompassing changes in the total oxido-reductive potential, which is manifested in a change in the number of numerous markers; however, based on these outcomes it is not possible to undoubtedly determine the most selective markers for periodontitis.
ORAL CANCER
Oral cancer is the sixth commonest malignancy in the world (39). The incidence is particularly high in the countries with high rates of alcohol and tobacco consumption (major risk factors). Free radicals such as ROS and reactive nitrogen species (RNS) that induce oxidative and nitrosative stress are main contributors to oral carcinogenesis (32, 40-42). Bahar et al. (40) analyzed salivary RNS and found increased concentrations of NO, NO2 and NO3, whereas all salivary concentration of antioxidants were reduced in patients with squamous cell carcinoma. In the same patients, the 8-OHdG-indicator of DNA oxidation and salivary carbonylation levels were also increased. In 2010, Goku et al. (43) evaluated the oxidant-antioxidant status of blood samples and tumor tissue in patients with oral squamous cell carcinoma and reported that antioxidant levels were significantly reduced in tissue samples from these patients compared with the control group. Recently, lower total antioxidant capacity, uric acid level, salivary peroxidase and superoxide dismutase activity in saliva have also been observed in patients with oral cavity cancer compared to healthy subjects (44). A synergistic deleterious interaction between cigarette smoke (CS) and saliva may result with a rapid destruction of biological macromolecules such as enzymes and proteins, thus giving saliva a pivotal role in the pathogenesis of CS-induced OP (oropharyngeal) cancers. This lethal synergistic effect of CS and saliva is probably based on the reaction between redox-active metals in saliva and low-reacting free radicals in CS. Nagler et al. (25) suggest that when exposed to CS, salivary behavior is reversed and saliva loses its antioxidant capacity, becoming a potent prooxidant milieu. Also Hershkovich et al. (45) have found a significantly reduced total value of salivary antioxidant capacity in elderly persons, increased oxidative stress, and increased salivary concentrations and total values of reactive nitrogen species, all contributing to increased DNA oxidation of oral epithelial cells, which may explain the higher prevalence of oral cancer in the elderly population. In patients with oral squamous-cell carcinoma we observed increased concentration of kynurenine and 3-hydroxykynurenine in cancer tissue comparing to normal tissue (46). It cannot be excluded that kynurenines, due to their ability to generate reactive oxygen species may be responsible for the changes observed in saliva of patients with oral cancer as well as for the development and progress of oral carcinogenesis.
In conclusion, saliva as a non-invasive alternative to serum testing for the diagnosis and for prognosis prediction of oral cancer, as well as for the monitoring of post-therapy status by measuring - among other things - the oxidative/anti-oxidative status of oral cavity. Salivary analysis has also been postulated as a useful diagnostic tool for distant malignancies such as breast cancer. At present, however, the accumulative data indicate that salivary analysis is mainly used for screening which can be helpful in the future (41).
ORTHODONTIC APPLIANCES
Orthodontic appliances used in the treatment of various dento-maxillar anomalies most frequently presume the application of high intensity forces which can induce an inflammatory process localized around the tooth. The presence of this process will produce an increased synthesis of free radicals secondary followed by the oxidative stress. In the literature, there are a limited number of human studies on the oxidative stress and oxidative damage that may occur as a result of aseptic inflammation in tissues with orthodontic tooth movements. In 2009 Olteanu et al. (47) determined and compared the levels of some oxidative stress markers in the saliva of patients with orthodontic appliances, before and after 1 hour, 24 hours and 7 days after the initiation of the treatment (47). The variation in the concentrations of the saliva markers of the oxidative stress reached a maximum at 24 hours for ceruloplasmine and MDA, and at one hour for the hydrogen donors respectively, at the 7 days from the installation of an appliance the concentrations of studied markers were close to the initial values. These authors conclude that the observed changes in the levels of the saliva markers of oxidative stress do not determine the appearance of certain pathological processes at the level of the oral cavity in patients with orthodontic appliances. Recently Ozcan et al. (48) have decided to evaluate changes in the some oxidative stress markers in saliva for the determining of oxidative stress damage that may occur in the process of remodeling, including periodontal tissue dysfunction caused by orthodontic tooth movement. The unstimulated saliva samples of patients with fixed orthodontic appliances were investigated to detect IL-1β, TNF-α and 8-OHdG levels using ELISA method and NO and MDA levels using spectrophotometric method, before treatment, at the 1st month of treatment and at the 6th month of treatment. The authors did not show any significant change in all biochemical parameters detected in the saliva at any measurement period; it was also in our study that no significant differences were found in kynurenine concentration in the unstimulated saliva of individuals with fixed orthodontic appliances compared to the control group (49). To conclude, the presented data indicate that orthodontic tooth movement and orthodontic materials used in orthodontic treatment do not cause oxidative damage in the oral cavity, at least at the first six month of the treatment.
DIABETES
Oxidative stress plays an important role in the pathogenesis and complications of diabetes. There are multiple sources of oxidative stress in diabetes. In non-enzymatic pathway hyperglycaemia can directly cause increased ROS generating hydroxyl ●OH- radicals and enhanced production of superoxides (*O2-). Another sources of non-enzymatic generation of reactive species is the mitochondrial respiratory chain. At the mitochondrial level hyperglycaemia inducing generation of *O2- becomes the initial trigger of oxidative stress in diabetes (21, 50). Enzymatic sources of enhanced generation of reactive species include nitrous oxide species, NAD(P)H oxidase and xanthine oxidase.
The increased serum and salivary production of free oxygen radicals and augmented levels of antioxidants were reported in patients with type 1 and 2 diabetes mellitus (50, 51).
In the study by Reznick et al. (52) there participated twenty patients aged 13–19 years with type 1 diabetes. They were divided into two groups containing those with the controlled and the uncontrolled diabetes mellitus. The glutathione peroxidase, superoxide dismutase and total antioxidant status were estimated. A significant correlation was found between the severity of diabetes (haemoglobin A1c values) and the increase in both unstimulated whole saliva and serum estimated antioxidants.
Also Al-Rawi (51) has demonstrated that high concentration of lipid fractions in the saliva of type-2 diabetics usually follows that recorded in serum. Salivary MDA levels, a product of lipid peroxidation, were significantly increased in these patients along with elevated uric acid concentrations. These results are in line with the recent study by Trivedi et al. (35). They have found higher MDA levels in the unstimulated whole saliva of 30 patients with type 2 diabetes mellitus in comparison to 30 systemically healthy voluntiers. This effect was more pronounced when diabetes was accompanied by periodontits. Interestingly, there was no correlation between the results obtained in saliva and in plasma in respect to SOD activities (35) and uric acid concentration (52). These results, however, indicate joint general increase in plasma and saliva antioxidants in diabetes type 1 and 2; this may also occasionally reflect some specific local responses manifested by increased salivary antioxidant factors. It seems that the evaluation of lipid peroxidation, antioxidant parameters and TAS in the saliva of diabetic patients may have an important prognostic value in the assessment of disease activity and severity (37). We have also found that patients with type-2 diabetes and hypertension demonstrate elevated concentrations of kynurenine and kynurenic acid in saliva compared to healthy volunteers (24).
Based on the present findings it can be speculated that in diabetic patients, in the case of imbalance between the production of free radicals and the free radical neutralizing salivary antioxidant capacity, oxidative stress may be involved in the development of oral disease, particularly when diabetes is paired with periodontitis.
RHEUMATOID ARTHRITIS (RA)
Although salivary gland involvement in rheumatoid arthritis has been known for a long time, it did not attract much attention. Past studies have shown that, besides the immune response, there is another important biological mechanism underlying the pathogenesis of RA that is based on the harmful effects of free radicals activity.
Oxidative stress is increasingly recognized as one of the major factors contributing to the chronic inflammatory process within the RA-inflamed joint.
In the studies by Nagler et al. (53) thirty four consenting rheumatoid arthritis patients, matched in age and gender, participated in the investigation. The mean age of the RA group was 51±2 years old. The mean duration of the disease since primary diagnosis was 6.28±1.6 years for these patients. The severity of RA was assessed with the Health Assessment Questionnaire grading criteria. The patients were divided into three subgroups: mildly affected, moderately affected and severely affected. In the RA patients the mean values of TAS, MDA, peroxidase and uric acid (UA) were higher both in plasma and in the unstimulated saliva in comparison to healthy controls. The increase of mean values of peroxidase, TAS and UA in the unstimulated saliva were correlated with RA severity. They concluded that the demonstrated correlation between the altered salivary parameters and the severity of the disease may indicate that the evaluation of salivary status in RA patients may be of great importance for further elucidation of the role of free radicals in the pathogenesis, diagnosis and evaluation of RA.
The other data demonstrate also a significant enhancement of the salivary antioxidant system in juvenile idiopathic arthritis (JIA) patients (54, 55). This was demonstrated by various analyzed parameters (55). Thirty five children with JIA of mean age of 12.1±3.9 years were studied, of whom 26 had active disease and 14 were receiving anti TNF therapy (53). The total antioxidant status, the total antioxidant capacity, UA, SOD, salivary peroxidase and the salivary protein oxidation levels (carbonyls) were significantly higher in the saliva of all JIA patients, whether treated or not treated with anti-TNF agents. The TAS in the unstimulated saliva of the active patients was nearly two time higher than that of non-active patients. Besides the diagnostic significance of these results, they may have possible implications for future therapy and it is suggested that local and systemic antioxidant therapy should be included in the treatment of juvenile idiopathic arthritic patients (55, 56).
OBSTRUCTIVE SLEEP APNEA SYNDROME (OSAS)
OSAS tends to cause episodic hypoxia - reoxygenation (57). Due to this, numerous studies have reported that OSAS is associated with reactive oxidative stress (ROS). OSAS patients have been found to demonstrate increased blood levels of ROS (58-61); recently Sales et al. (62) have observed decreased blood levels of anti-oxidants in these patients. This study suggests that the imbalance between antioxidants and prooxidants may result in cellular damage (54), predisposing OSAS patients to, for example, cardiovascular and cerebrovascular diseases (54). In OSAS there is also a relationship between oxidative stress and oral/dental diseases, particularly periodontitis (31, 63). On the other hand, the question of whether or not OSAS patients exhibit amplified oxidative stress is still controversial (64, 64). Indeed, some studies do not support the association between oxidative stress and OSAS (54, 59). Recent works by Tothova et al. (65) have shown that the levels of salivary markers of oxidative stress (thiobarbituric acid-reacting substances, advanced glycation end-products and advanced oxidation protein products) did not change after one-night treatment with continuous positive airway treatment (CPAP), whereas a decrease was observed after one month of CPAP therapy. Tothova et al. (65) postulate that further studies should focus on finding the optimum sampling frequency to clarify the potential saliva monitoring during OSAS treatment. Moreover, different studies assessing oxidative stress levels may produce conflicting results due to the difference in applied methods or in the size of patient group under investigation, to difficulties in the determining of a kinetic profile or the measuring of ROS instability, or to confounding factors such as malnutrition. At the present moment, the measurement and evaluation of oxidative-antioxidative balance in OSAS performed in the samples of saliva may be used to assist clinicians in making therapeutic choices and to monitor the efficacy of such therapies (66).
RENAL FAILURE
In uremia, several endogenous metabolites accumulate in blood. The accumulation of these substances is thought to contribute to certain uremic symptoms, such as hypertension (10, 21), lipid metabolism disorders (20) or uremic stomatitis (67). In 2007 Ben-Zivi et al. (68) reported an increase in the oxidative stress burden in both serum and saliva of diabetic and uremic patients. Recently Pawlak et al. (69) have shown a correlation between the levels of Cu/Zn superoxide dismutase and some elevated levels kynurenine, 3-hydroxykynurenine and anthranilic acid in plasma and saliva of uremic patients compared with the values observed in healthy volunteers. The concentration of kynurenine assessed in saliva of uremic patients may be a sensitive marker in the evaluation of the activity and severity of uremia (70). Thus we recommend that the metabolites in kynurenine pathway present in saliva should be regarded as new biomarkers of oxidative stress.
A summary of the presented data is included in the Table 1.
CONCLUSION
The oral cavity is a very complex and unique environment characterized by numerous interactions between different surfaces: soft and hard tissues, food, air and microorganisms. Saliva is in the middle of this environment and to some extent it reflects the state of health of the human body. Since saliva is rich in antioxidants it can constitute the first line of defense against oxidative stress. Over the recent years it has been postulated that no single marker can validate the presence or prognosis of a disease, a panel of biomarkers would be more helpful. As the effects of antioxidants can be additive and the measuring of individual antioxidants separately is time-consuming and labor-intensive, measurement of the combined activities of all antioxidants or the total antioxidant status is often used to estimate the overall antioxidant capacity (71). Similarly, total oxidant status is measured to determine the overall oxidation state (72). Moreover, it is suggested that the TOS (total oxidant status) to TAS ratio should be calculated as a more accurate indicator of oxidative stress in the body (73-75) (Fig. 1). Nevertheless, we may recommend saliva as a diagnostic biological material containing easily measurable indicators of oxidative processes that may provide a tool for the development and monitoring of certain diseases such as rheumatoid arthritis, chronic renal failure or diabetes, as well as local pathologic conditions including periodontal disease or cancer of oral cavity. The present advances and emerging nanotechnology coupled with the use of proteomic and genomic markers of oxidative stress contribute to the fact that salivary diagnostics is becoming a very sensitive and important tool.
Acknowledgements: Supported by the Medical University in Bialystok, Poland.
Conflict of interests: None declared.
REFERENCES
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 1993; 90: 7915-7922.
- Bagchi K, Puri S. Free radicals and antioxidants in health and disease. East Mediterr Health 1998; 14: 350-360.
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 2002; 30: 620-650.
- Forrest CM, Mackay GM, Stoy N, et al. Tryptophan loading induces oxidative stress. Free Radic Res 2004; 38: 1167-1171.
- Gonzalez D, Marquina R, Rondon N, Rodriguez-Malaver AJ, Reyes R. Effects of aerobic exercise on uric acid, total antioxidant activity, oxidative stress, and nitric oxide in human saliva. Res Sports Med 2008; 16: 128-137.
- Cofta S, Wysocka E, Piorunek T, Rzymkowska M, Batura-Gabryel H, Torlinski L. Oxidative stress markers in the blood of persons with different stages of obstructive sleep apnea syndrome. J Physiol Pharmacol 2008; 59 (Suppl. 6): 183-190.
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol 2013; 64: 409-421.
- Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gyneacol 2011; 25: 287-299.
- Mastalerz-Migas A, Steciwko A, Pokorski M, et al. What influences the level of oxidative stress as measured by 8-hydroxy-2-deoxyguanosine in patients on hemodialysis. J Physiol Pharmacol 2006; 57 (Suppl. 4): 199-205.
- Popolo A, Autore G, Pinto A, Marzocco S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic Res 2013; 47: 346-356.
- Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal 2014; 20: 164-82.
- Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012; 2012: 428010. doi.: 10.1155/2012/428010.
- Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci 2013; 14: 24438-24475.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010; 49: 1603-1616.
- Gupta RK, Patel AK, Shah N, et al. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac J Cancer Prev 2014; 15: 4405-4409.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408: 239-247.
- Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol 2010; 23 (Suppl. 15): S29-S36.
- Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY) 2013; 5: 144-150.
- Benkhai H, Kohler F, Lademann J, et al. Comparison of the antioxidant potential in urine, saliva and skin. GMS Krankenhaushyg Interdiszip 2011; 6: Doc02. doi: 10.3205/dgkh000159.
- Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol 2002; 29: 189-194.
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 1994; 344: 721-724.
- Buczko W, Cylwik D, Stokowska W. Metabolism of tryptophan via the kynurenine pathway in saliva. Post Hig Med Dosw 2005; 59: 283-289.
- Tomas SR, Stocker R. Redox reactions related to IDO and tryptophan metabolism along the kinurenine pathway. Redox Rep 1999; 4: 199-220.
- Buczko P, Stokowska W, Gorska M, Kucharewicz I, Pawlak D, Buczko W. Tryptophan metabolites via kynurenine pathway in saliva of diabetic patients. Dent Med Probl 2006; 43: 208-214.
- Nagler RM. Saliva as a tool for oral cancer diagnosis and prognosis. Oral Oncol 2009; 45: 1006-1010.
- Greabu M, Battina M, Mohora M, et al. Could saliva constitute the first line of defense against oxidative stress? Rom J Intern Med 2007; 45: 209-213.
- Leszczynska A, Buczko P, Buczko W, Pietruska M. Periodontal pharmacotherapy - an update review. Adv Med Sci 2011; 56: 123-131.
- Racz GZ, Kadar, K, Foldes A, et al. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol 2014; 65: 327-329.
- Tothova L, Celecova V, Celec V. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis Markers 2013; 34: 9-15.
- Chapple IL, Mason GI, Garner I, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem 1997; 34: 412-421.
- Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci 2003; 105: 167-172.
- Nagler R, Dayan D. The dual role of saliva in oral carcinogenesis. Oncology 2006; 71: 10-17.
- Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis. J Clin Periodontol 2000; 27: 453-465.
- Bostanci V, Toker H, Senel S, Ozdemir H, Aydin H. Effect of chronic periodontitis on serum and gingival cereviscular fluid oxidant and antioxidant status in patients with familial mediterranean fever before and after periodontal treatment. J Periodontol 2014; 85: 706-712.
- Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in chromic periodontitis patients with diabetes. J Periodontol 2014; 85: 713-714.
- Takane M, Sugano N, Ezawa T, Uchiyama T, Ito K. A marker of oxidative stress in saliva: association with periodontally-involved teeth of a hopeless prognosis. J Oral Sci 2005; 47: 53-57.
- Pendyala G, Thomas B, Joshi SR. Evaluation of total antioxidant capacity of saliva in type 2 diabetic patients with and without periodontal disease: a case-control study. North Am J Med Sci 2013; 5: 51-57.
- Akalin FA, Baltacioglu E, Alver A, Karabulut E. Total antioxidant capacity and superoxide dismutase activity levels in serum and gingival celevis cular fluid in pregnant women with chronic periodontitis. J Periodontol 2009; 80: 457-467.
- Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesion based on serum and salivary markers of oxidative stress. J Oral Sci 2010; 52: 251-256.
- Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM. Salivary analysis in cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer 2007; 109: 54-59.
- Prasad RB, Sharma A, Babu HM. An insight into salivary markers in oral cancer. Dent Reo J (Isfahan) 2013; 10: 287-295.
- Ravindran R. Salivary tumor markers in oral cancer: brief reviev. Oral Maxillofac Pathol J 2012; 2: 238-244.
- Gokul S, Patil VS, Jailkhani R, Hallikeri K, Kattappagari KK. Oxidant-antioxidant status in blood and tumor tissue of oral squamous cell carcinoma patients. Oral Dis 2010; 16: 29-33.
- Giebultowicz J, Wroczynski P, Samolczyk-Wanyura D. Comparison of antioxidant enzymes activity and the concentration of uric acid in the saliva of patients with oral cavity cancer, odontogenic cysts and healthy subjects. J Oral Pathol Med 2011; 40: 726-730.
- Hershkovich O, Shafat I, Nagler RM. Age-related changes in salivary antioxidant profile: possible implications for oral cancer. J Gerontol A Biol Sci Med Sci 2007; 62: 361-366.
- Tankiewicz-Kwedlo A, Buczko P, Dziemianczyk-Pakiela D, et al. Tryptophan metabolism in patients with oral squamous cell carcinoma. Pol J Environ Stud 2007; 16: 120-124.
- Olteanu C, Muresan A, Daicoviciu D, Tarmure V, Olteanu I, Keularts IM. Variations of some saliva markers of the oxidative stress in patients with orthodontic appliances. Physiology 2009; 19: 26.
- Ozcan SS, Ceylan I, Ozcan E, Kurt N, Dagsuyu IM, Canakci CF. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. Dis Markers 2014; 2014: 597892. doi: 10.1155/2014/597892.
- Szarmach IJ, Buczko P, Tankiewicz-Kwedlo A, Kasacka I, Tankiewicz J, Pawlak D. The concentration of kynurenine and anthranilic acid in saliva of patients with fixed orthodontic appliances. Pol J Environ Stud 2007; 16 (Suppl. 2C): 125-127.
- Arana C, Cutando A, Ferrera MJ, et al. Parameters of oxidative stress in saliva from diabetic and parenteral drug addict patients. J Oral Pathol Med 2006; 35: 554-559.
- Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res 2011; 8: 22-28.
- Reznick ZA, Shehadeh N, Shafir Y, Nagler RM. Free radicals related effects and antioxidants in saliva and serum of adolescents with type 1 diabetes mellitus. Arch Oral Biol 2006; 51: 640-648.
- Nagler RM, Salameh F, Reznick AZ, Livshits V, Nahir AM. Salivary gland involvement in rheumatoid arthritis and its relationship to induced oxidative stress. Rheumatology (Oxford) 2003; 42: 1234-1241.
- Brik R, Livnat G, Pollak S, Catz R, Nagler R. Salivary gland involvement and oxidative stress in juvenile idiopathic arthritis novel observations in oligoarticular-type patients. J Rheumatol 2006; 33: 2532-2537.
- Brik R, Rosen I, Savulescu D, Borovoi I, Gavish M, Nagler R. Salivary antioxidants and metalloproteinases in juvenile idiopathic arthritis. Mol Med 2010; 16: 122-128.
- Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA 2005; 294: 1671-1684.
- Antczak J, Horn B, Richter A, et al. The differences in sleep profile changes under continuous positive airway pressure (cpap) therapy between non-obese, obese and severely obese sleep apnea patients. J Physiol Pharmacol 2012; 63: 263-269.
- Kang IG, Jung JH, Kim ST. The effect of obstructive sleep apnea on DNA damage and oxidative stress. Clin Exp Otorhinoloryngol 2013; 6: 68-72.
- Sofic E. Rustembegovic A, Kroyer G, Cao G. Serum antioxidant capacity in neurological, psychiatric, renal diseases and cardiomyopathy. J Neural Transm 2002; 109: 711-719.
- Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Grammiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003; 124: 1386-1392.
- Celec P, Hodosy J, Behuliak M, et al. Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 2012; 16: 393-398.
- Sales LV, Sales de Bruin UM, D'Ameida V, et al. Cognition and biomarkers of oxidative stress in obstructive sleep apnea. Clinics 2013; 68: 449-455.
- Kamodynova N, Tothova L, Celec P. Salivary markers of oxidative stress and antioxidant status: influence of external factors. Dis Markers 2013; 34: 313-321.
- Lavie L, Visknevsky A, Levie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004; 27: 123-128.
- Tothova L, Hodosy J, Mucska I, Celec P. Salivary markers of oxidative stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 2014; 18: 563-570.
- Zhang J, Veasey S. Making sense of oxidative stress in obstructive sleep apnea: mediator or distracter? Neurology 2012; 3: 1-8.
- Antoniades DZ, Markopoulos AK, Andreadis D, Balaskas I, Patrikalou E, Grekas D. Ulcerative uremic stomatitis associated with untreated chronic renal failure report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101: 608-613.
- Ben-Zivi I, Green Y, Nakhoul F, Kanter Y, Nagler RM. Effects of diabetes mellitus, chronic renal failure and hemodialysis on serum and salivary antioxidant status. Nephron Clin Pract 2007; 105: c114-c120.
- Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009; 204: 309-314.
- Buczko P, Tankiewicz-Kwedlo A, Buraczewska A, Mysliwiec M, Pawlak D. Accumulation of kynurenine pathway metabolites in saliva and plasma of uremic patients. Pharmacol Rep 2007; 59 (Suppl. 1): 199-204.
- Erel O. A novel automated method to measure total antioxidant response against potent free radicals reactions. Clin Biochem 2004; 37: 112-119.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005; 38: 1103-1111.
- Horoz M, Bolukbas C, Bolukbas FF, et al. Measurement of the total antioxidant response using a novel automated method in subjects with nonalcoholic steatohepatitis. BMC Gastroenterol 2005; 5: 35-42.
- Wang D, Feng JF, Zeng P, Yang YH, Luo J, Yang YW. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr Relat Cancer 2011; 18: 773-782.
- Baltacioglu E, Kehribar MA, Yuva P, et al. Total oxidant status and bone resorption biomarkers in serum and gingival creviscular fluid of patients with periodontitis. J Periodontol 2014; 85: 817-826.
A c c e p t e d : October 13, 2014