SUPERACTIVE HUMAN LEPTIN ANTAGONIST REVERSES LEPTIN-INDUCED EXCESSIVE PROGESTERONE AND TESTOSTERONE SECRETION IN PORCINE OVARIAN FOLLICLES BY BLOCKING LEPTIN RECEPTORS
Institute of Zoology, Jagiellonian University, Cracow, Poland
INTRODUCTION
In view of the fact that circulating leptin levels are directly related to body adiposity, elevated leptin concentrations associated with obesity may partly explain the negative impact of obesity on fertility. It has been proposed that hyperleptinemia and leptin resistance may play a role in the pathogenesis of the disease. Iciek et al. (1) found a significant positive correlation between placental leptin, leptin receptor expression and neonatal birth weight in overweight type 1 diabetes mellitus subjects. Obesity is strongly associated with elevated levels of leptin in the circulation, and it has been proposed that obesity directly affects ovarian function, at least in part, by increasing intra-follicular levels of leptin (2). Previously published data showed different patterns of expression for leptin and OB-Rb mRNAs/proteins in the porcine ovarian tissue during estrus cycle (3) and early pregnancy (4).
Over a wide range of doses, leptin hasbeen reported to exert both stimulatory and inhibitory effects on ovarian cell steroid secretion. Ruiz-Cortes et al. (5) demonstrated that leptin at a concentration of 10 ng/ml increased progesterone (P4) accumulation in luteinized porcine granulosa cells in vitro, whereas a 100-fold higher concentration (1000 ng/ml) decreased P4 accumulation. Barb et al. (6) suggested that these results are physiologically significant because 10 ng/ml is on the same order of magnitude as the normal concentration of leptin in the circulation, which ranges from 1.5 to 5 ng/ml. Because circulating leptin levels are directly related to body adiposity, elevated leptin concentrations associated with obesity may partly explain the negative impact of obesity on fertility. Physiological levels of leptin stimulate steroidogenesis and follicle maturation, whereas supraphysiological concentrations of leptin may produce the opposite effect (7). Only at low physiological doses, such as those found in follicular fluid, does leptin act together with insulin-like growth factor I (IGF-I) to stimulate estradiol secretion. High levels of leptin may be perceived by the body as harmful and prevent reproduction through a number of adaptive changes mediated by the hypothalamic-pituitary axis (8). Work performed in our laboratories has shown that relatively high concentrations of leptin (20 and 40 ng/ml) have a stimulatory effect on CYP11A1 and 17β-hydroxysteroid dehydrogenase (17β-HSD) protein expression, resulting in higher amounts of P4 and testosterone (T) secretion (9). These studies suggest that elevated leptin levels could be an independent risk factor for cyst formation in both prepubertal and cycling pigs.
Owing to similarities in size, anatomy, physiology, organ development and disease progression, swine have attracted growing interest as an experimental model for human biomedical research (10). Notably, this animal represents a potentially excellent model for studying leptin. Despite slight differences in the mean levels of plasma leptin reported by different authors (6, 11, 12), all reported values are consistent with the mean plasma leptin concentration (8 ng/ ml) in humans (13). The serum levels of leptin in obese swine are approximately 300% higher than those of contemporary, crossbred swine (14), and leptin concentration in sows is highest in animals exhibiting the greatest amount of back fat (15). Consistent with this, Robert et al. (16) showed that at slaughter, fat swine had a higher level of leptin mRNA than did lean swine.
The recent development of superactive leptin mutants exhibiting antagonistic properties opens new avenues for applications of these tools in research and, eventually, as therapy for cachexia, autoimmune disease, cancer, and other pathologies (17). The development of leptin antagonists in four animal species has been reported by Niv-Spector et al. (18) and Salomon et al. (19). Using a mouse leptin antagonist (MLA), Elinav et al. (20, 21) reported the effects of inhibition of leptin signaling in several mouse models of fibrosis and inflammation. There are also data showing beneficial effects of using leptin antagonists in prostate cancer (22), rheumatoid arthritis (23), and breast cancer (24). Notably, Dupuis et al. (25) showed that administration of pegylated super mouse leptin antagonist (PEG-SMLA) to superovulated mice reduced ovulation rate by 65% compared with control treatment.
On the basis of these observations, we here sought to determine whether blocking leptin receptors using super human leptin antagonist (SHLA) could reverse the actions of elevated leptin in ovarian follicles. Taking into consideration data of Gonzalez-Anover et al. (26) who compared leptin levels in prepubertal gilt pigs of obese and lean genotypes identified a profound increase in plasma leptin levels in the obese genotypes in the current study, we used both prepubertal and mature animals. First, we determined the effects of SHLA on ObR and leptin protein expression and leptin secretion into the culture medium. We then investigated the effects of SHLA on P4 and T secretion, and expression of the enzymes involved in their synthesis. Finally, we examined whether SHLA was capable ofpreventing the adverse effects of high concentrations of leptin on steroid secretion.
MATERIAL AND METHODS
Reagents
M199, fetal bovine serum (FBS; heat-inactivated), antibiotic/antimycotic solution (100X), Tris, Na-deoxycholate, Nonidet NP-40, sodium dodecyl sulfate (SDS), protease inhibitor (EDTA-free), DTT, Tween 20, bromophenol blue, and leptin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). SHLA (Cat.no.SLAN-2) was obtained from Protein Laboratories Rehovot (PLR) Ltd., Israel.
Animals
Porcine ovaries were obtained from a local slaughterhouse. Ovaries were collected in a bottle filled with sterile saline and transported to the laboratory. Medium (4–5 mm) follicles from prepubertal animals and 7–9 mm from mature animals, correspond respectively to the estrus cycle at days 10–12 as described previously were collected for study (27). For each experiment particular size follicles were selected from three prepubertal and three mature animals. Each experiment was repeated 3 times (n=3).
Experimental procedure
To evaluate the action of SHLA, follicles were individually placed in 24-well plates in M199 medium without phenol red supplemented with antibiotic/antimycotic solution and 5% FBS and exposed to 100, 250 and 500 ng/ml SHLAor combination of SHLA with 40 ng/ml leptin. Dose of leptin, as an elevated dose, have been chosen based on physiological serum leptin levels in the pig to be <10 ng/ml reported by Barb et al. (6), Ruiz-Cortes et al. (5), Estienneet et al. (11), Amills et al. (12). Studies Ramsay et al. (14) and Robert et al. (16) showed that obese swine have approximately 300% higher levels of porcine leptin in sera. Doses of SHLA was based on the earlier dose response experiments (unpublished data). After 24 h of incubation, medium was frozen for measuring leptin, P4 and T levels. Ovarian follicular wallswere frozen at 70°C for ObR, leptin, CYP11A1 and 17βHSD protein expression analysis.
Analysis of leptin levels
Leptin concentrations in incubation medium of medium size follicles collected from prepubertal and mature pigs were quantified using the enzyme immunoassay porcine leptin ELISA (Cat. no. E0084p, Wuhan EIAab Science Co.,Ltd., Wuhan, China) according to the manufacturer’s instructions. The sensitivity of the assay was 0.104 ng/ml, and the detection range was 0.312–20 ng/ml.
Analysis of steroid levels
The concentrations of P4 and T in culture media were determined by enzyme immunoassay (EIA) using commercial ELISA kits (DRG Diagnostic, Germany). All samples were run in duplicate in the same assay. The sensitivity limits for P4 and T were 0.045 ng/ml and 0.083 ng/ml, respectively. The coefficients of variation for inter- and intra-run precision were 4.34% and 6.99%, respectively, for P4 and 6.71% and 3.28% for T. The range of each assay was 0–40 ng/ml for P4; 0–16 ng/ml for T. The absorbance values were measured at 450 nm using an ELISA reader (ELx808 BIO-TEK Instruments, USA).
Western blotting
Whole different size follicles collected from prepubertal and mature pigs were homogenized twice in ice-cold lysis buffer (50 mMTris-HCl, pH 7.5, 100 mM NaCl, 0.5% Na-desoxycholate, 0.5% NP-40, 0.5% SDS, and EDTA-free protease inhibitor). For each experiment, three follicles per treatment group were used. The homogenate was centrifuged at 15.000×g at 4°C for 30 min, and the protein concentration in the lysates determined using the Bradford reagent (Bio-Rad Protein Assay; Bio-Rad Laboratories, Munchen, Germany) with bovine serum albumin (BSA) as a standard. Samples containing 50 µg of total protein were reconstituted in the appropriate amount of sample buffer (125 mMTris, pH 6.8, 4% SDS, 25% glycerol, 4 mM EDTA, 20 mM DTT, and 0.01% bromophenol blue). The samples were separated by 7% (for ObR), 10% (CYP11A, 17βHSD) or 15% (for leptin) for SDS-polyacrylamide gel electrophoresis in a Bio-Rad Mini-Protean II Electrophoresis Cell and then electrotransferred to a PVDF membrane. The membrane was washed and the non-specific binding sites were blocked with 5% milk/0.2% Tween 20 in 0.02 M TBS for 2 hours. The membranes were incubated overnight at 4°C with anti-CYP11A, anti-17βHSD, anti-ObR antibodies, diluted 1:200 (sc-18040, sc-26963 and sc-8391, respectively; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-leptin antibodies (L3160, Sigma Chemical Co.) diluted 1:1000. Anti-β-actin antibody (A5316, Sigma Chemical Co.), diluted 1:3000, was used as a loading control. After incubation with the primary antibody, the membrane was washed with TBS/0.02% Tween 20 and then incubated for 1 hour with horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG (sc-2020, Santa Cruz Biotechnology), diluted 1:2000 for CYP11A, and 17β-HSD, or an HRP-conjugated secondary antibody (P0447, DakoCytomation, Denmark), diluted 1:5000 for β-actin or 1:2000 for ObR and leptin. Signals were detected by enhanced chemiluminescence (ECL) using Western blotting luminol reagent (sc-2048; Santa Cruz Biotechnology) and visualized using the ChemiDoc-It Imaging System (UVP, LLC). All bands visualized by chemiluminescence were quantified using ImageJ analysis software (US National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each experiment was repeated three times (n=3). For ELISA each sample was run in quadruplicate. Statistical analysis was performed using Graph Pad Prism 5. The data were analyzed by one-way ANOVA with Tukey’s honestly significant difference (HSD) test. The data are presented as mean ±S.E.M. (or S.D.)
RESULTS
Effect of superactive human leptin antagonist on ObR and leptin protein expression and leptin secretion to the medium
In prepubertal animals, SHLA at a dose of 100 ng/ml had no effect on ObR protein expression, while 30% and 50% inhibition was noted under the influence of 250 and 500 ng/ml of SHLA, respectively (P<0.05). Independent of the SHLA doses, 50% inhibition of leptin expression was noted in ovarian follicles from prepubertal animals (Fig. 1A). In ovarian follicles collected from mature pigs SHLA in a dose of 100 ng/ml had no effect on both ObR and leptin protein expression. 30% and 50% of inhibition of ObR protein expression and 40% and 70% of inhibition of leptin protein expression was noted under the influence of 250 and 500 ng/ml of SHLA, respectively, (P<0.05) (Fig. 1B). SHLA at a dose of 100, 250 or 500 ng/ml decreased significantly leptin secretion from 0.5 ng/follicle to 0.09, 0.15 and 0.07, respectively, in incubated ovarian follicles from prepubertal animals and from 0.7 ng/follicle to 0.2, 0.18 and 0.08 ng/follicle in mature animals (P<0.05) (Fig. 1C).
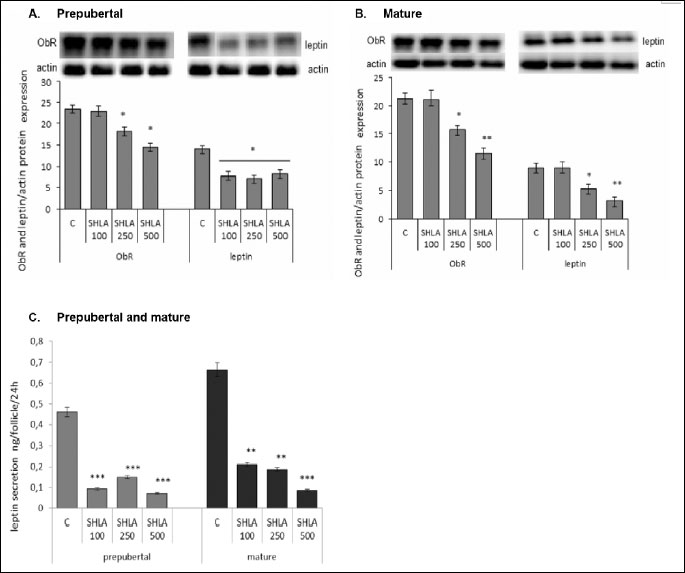
Effect of superactive human leptin antagonist on progesterone secretion and CYP11A1 protein expression
SHLA at all doses used decreased progesterone secretion (Fig. 2A) and CYP11A1 protein expression in follicles collected from prepubertal (P<0.01) and in a doses 250 ng/ml and 500 ng/ml in mature animals (P<0.05; Fig. 2B).
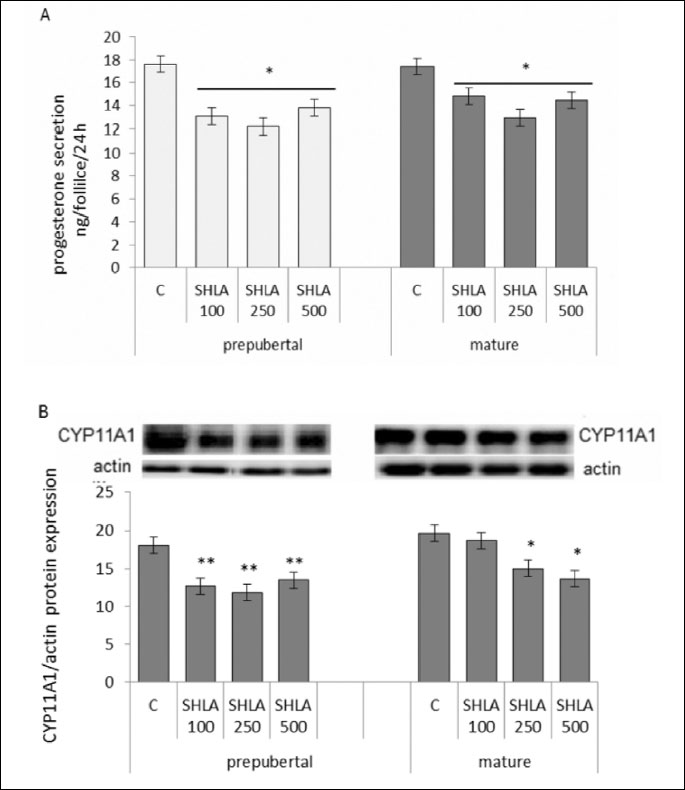
Effect of superactive human leptin antagonist on testosterone secretion and 17βHSD protein expression
SHLA at all doses used decreased statistical significantly testosterone secretion by follicles collected from prepubertal and mature pigs (Fig. 3A). Inhibitory action on 17βHSD protein expression was noted under the influence of 100 ng/ml, 250 ng/ml and 500 ng/ml in both prepubertal and mature pigs (P<0.05, P<0.01) (Fig. 3B).
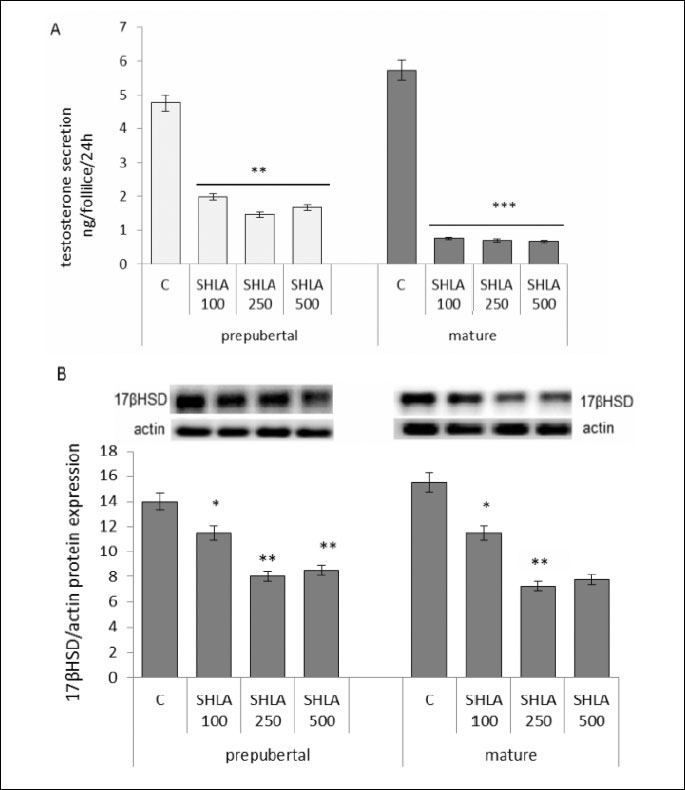
Effect of superactive human leptin antagonist on leptin stimulated progesterone and testosterone secretion
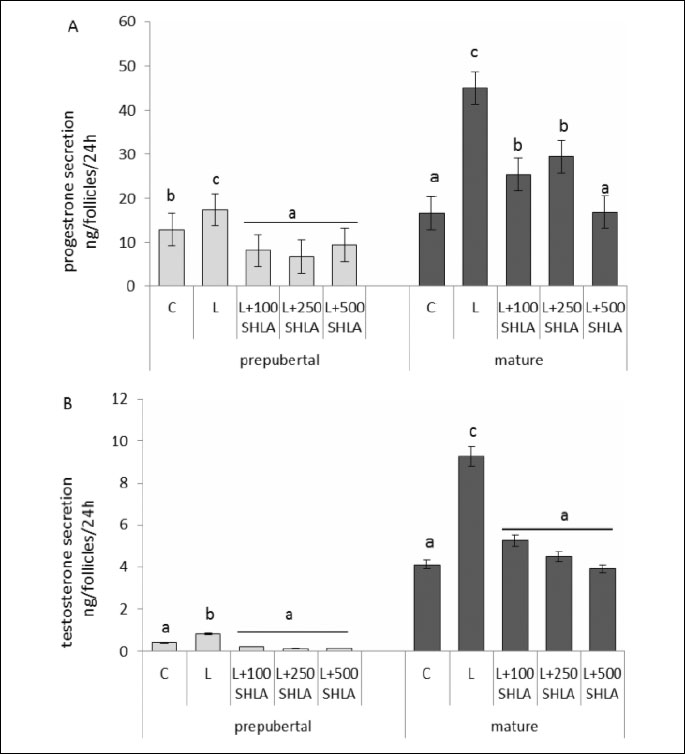
SHLA at all doses used decreased statistical significantly leptin stimulated progesterone (Fig. 4A) and testosterone (Fig. 4B) secretion by follicles collected from prepubertal and mature pigs.
DISCUSSION
Depending on the dose, leptin has been reported to have both stimulatory and inhibitory effects on ovarian cell steroid secretion (5). Moreover, circulating leptin levels are strongly correlated with obesity, which is frequently associated with PCOS. This association of elevated leptin concentrations with obesity may partly explain the negative impact of obesity on fertility, and suggests that leptin antagonists would be beneficial in infertility syndromes.
As reviewed recently by Zabeau et al. (28), four strategies are currently being used to antagonize leptin signaling: (1) leptin antagonistic mutants that bind to, but do not activate, the receptor; (2) leptin peptide antagonists that consist of parts of the leptin sequence; (3) leptin and leptin receptor (LR)-specific (monoclonal) antibodies or nanobodies that prevent productive binding of leptin; and (4) soluble LR variants that trap free leptin in the circulation. Only a few studies on the blockade of leptin or ObR with specific antibodies that provide information concerning reproductive organs have been published. Kawamura et al. (29) showed that an anti-ObR antibody that targets the extracellular domain inhibits the rates of expanded mouse blastocyst formation and hatching in vitro model of embryo development. Gonzalez et al. (30) found that blockade of ObR with a specific antibody inhibits the stimulatory effect of leptin on the expression of β3 integrin and the interleukin (IL)-1 system in human endometrial cell cultures. In a report published the same year (31), these authors showed that LPA-2, a peptide comprising helix III (residues 70–95), inhibited the effects of leptin on secretion of leukemia-inhibiting factor (LIF) and expression of the LIF receptor by rabbit endometrial cells-critical steps in the development of endometrial receptivity and/or implantation. They suggested that leptin receptor blockers might be useful as novel tools for studying leptin functions in the endometrium and as agents for controlling fertility. LPA-2 may also be useful for studying leptin regulation in disease states associated with abnormalities in leptin function, such as preeclampsia, endometriosis, diabetes mellitus, and obesity.
The results of the current study showed that the human leptin antagonist SHLA significantly decreased ObR and leptin protein expression and leptin secretion by follicles. Moreover, bysuppression of CYP11A1 and 17β-HSD expression, SHLA decreased basal P4 and T secretion and, importantly, reversed the excessive T secretion induced by leptin. The findings confirm the usefulness of studying disease associated with abnormalities in leptin function, as suggested by Gonzalez and Leavis (30). Brzechffa et al. (32) reported a positive correlation between leptin levels and body mass index (BMI). They further showed that 29% of women with PCOS had leptin levels above the 99% prediction interval for their BMI, and none had low leptin levels. These results suggest that abnormalities in leptin signaling in the reproductive system may be involved in certain cases of PCOS. Our most recent study reported that leptin at elevated doses (20 and 40 ng/m) exerted a stimulatory effect on CYP11A1 and 17β-HSD protein expression, resulting in higher amounts of P4 and T secretion in both prepubertal and cycling pigs (9). A number of studies have described hyperandrogenism and menstrual disturbancesas symptoms of PCOS (33-35). Leptin acts mainly to increase T secretion, resulting in follicular atresia of antral follicles and a failure to produce ovulatory follicles.
Considerable evidence from both clinical and animal studies suggests that PCOS is a developmental syndrome that manifests in adolescence and adulthood in which exposure to excess androgens during the fetal or prepubertal phases of growth essentially ‘reprograms’ multiple tissues (36, 37). Moreover, Gonzalez-Anover et al. (26), demonstrated that blocking leptin receptor signaling in obese prepubertal individuals might prevent the development of PCOS in adults. In addition, Shpilman et al. (38) showed that mouse leptin antagonists, used in pegylated form, resulted in a remarkably rapid increase in body weight owing to an increase in food consumption. The authors of this study suggested that these antagonists are useful for studying the role of leptin in metabolic and immune processes in vivo, and hold promise for future therapeutic use in disease pathologies involving leptin. In this context, SHLA could be novel, potent research tool for elucidating the role of leptin in the pathology of reproduction.
In summary, the increasing scale of the problems of obesity and infertility highlights the need for additional studies. It also argues for greater participation in such research. As it stands,the paucity of research along the lines reported here limits the opportunities for the type of interactive discussion with other researchers that is critically important in propelling research forward. Although numerous reports have investigated the potential of leptin antagonists as a novel strategy in oncology, our study is the first to present preliminary data suggesting that this approach may also be relevant in infertility.
Acknowledgements: We thank Dr. Anna Karpeta for assistance with Western blotting. These studies were supported by a grant (K/ZDS/00 4194) from Jagiellonian University, Cracow, Poland.
Conflict of interests: None declared.
REFERENCES
- Iciek R, Wender-Ozegowska E, Zawiejska A, et al. Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol 2013; 64: 579-585.
- Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health SciRes 2013; 3: 191-196.
- Gregoraszczuk EL, Ptak A, Wojciechowicz T, Nowak K. Action of IGF-I on expression 378 of the long form of the leptin receptor (ObRb) in the prepubertal period and throughout 379 the estrous cycle in the mature pig ovary. J Reprod Dev 2007; 53: 289-295.
- Siawrys G, Smolinska N. in vitro effects of luteinizing hormone, progesterone and oestradiol-17β on leptin gene expression and leptin secretion by porcine luteal cells obtained in early pregnancy. J Physiol Pharmacol 2013; 64: 513-520.
- Ruiz-Cortes ZT, Martel-Kennes Y, Gevry NY, Downey BR, Palin MF, Murphy BD. Biphasic effects of leptin in porcine granulosa cells. Biol Reprod 2003; 68: 789-796.
- Barb CR, Hausman GJ, Houseknecht KL. Biology of leptin in the pig. Domest Anim Endocrinol 2001; 21: 297-317.
- Cervero A, Dominguez F, Horcajadas J.A, Quinonero A, Pellicer A, Simon C. The role of the leptin in reproduction. Curr Opin Obstet Gynecol 2006; 18: 297-303.
- Svensson J, Herlitz H, Lundberg PA, Johannsson G. Adiponectin, leptin, and erythrocyte sodium/lithium countertransport activity, but not resistin, are related to glucose metabolism in growth hormone-deficient adults. J Clin Endocrinol Metab 2005; 90: 2290-2296.
- Gregoraszczuk EL, Rak-Mardyla A. Supraphysiological leptin levels shift the profile of steroidogenesis in porcine ovarian follicles toward progesterone and testosterone secretion through increased expressions of CYP11A1 and 17β-HSD: a tissue culture approach. Reproduction 2013; 145: 311-317.
- Lunney JK. Advances in swine biomedical genomics. Int J Biol Sci 2007; 3: 179-184.
- Estienne MJ, Harper AF, Kozink DM, Knight JW. Serum and milk concentrations of leptin in gilts fed a high- or low-energy diet during gestation. Anim Reprod Sci 2003; 75: 95-105.
- Amills M, Villalba D, Tor M, et al. Plasma leptin levels in pigs with different leptin and leptin receptor genotypes. J Anim Breed Genet 2008; 125: 228-233.
- Sone M, Osamura RY. Leptin and the pituitary. Pituitary 2001; 4: 15-23.
- Ramsay TG, Yan X, Morrison C. The obesity gene in swine: sequence and expression of porcine leptin. J Anim Sci 1998; 76: 484-490.
- Estienne MJ, Harper AF, Barb CR, Azain MJ. Concentrations of leptin in serum and milk collected from lactating sows differing in body condition. Domest Anim Endocrinol 2000; 19: 275-280.
- Robert C, Palin MF, Coulombe N, et al. Backfat thickness in pigs is positively correlated with leptin mRNA levels. Can J Anim Sci 1998; 8: 473-482.
- Gertler A, Solomon G. Leptin-activity blockers: development and potential use in experimental biology and medicine. Can J Physiol Pharmacol 2013; 91: 873-882.
- Niv-Spector L, Gonen-Berger D, Gourdou I, et al. Identification of the hydrophobic strand in the A-B loop of leptin as major binding site III: implications for large-scale preparation of potent recombinant human and ovine leptin antagonists. Biochem J 2005; 391: 221-230.
- Salomon G, Niv-Spector L, Gussakovsky EE, Gertler A. Large-scale preparation of biologically active mouse and rat leptins and their L39A/D40A/F41A muteins which act as potent antagonists. Protein Expr Purif 2006; 47: 128-136.
- Elinav E, Ali M, Bruck R, et al. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology 2009; 49: 278-286.
- Elinav E, Niv-Spector L, Katz M, et al. Pegylated leptin antagonist is a potent orexigenic agent. Preparation and mechanism of activity. Endocrinology 2009; 150: 3083-3091.
- Samuel-Mendelsohn S, Inbar M, Weiss-Messer E, Niv-Spector L, Gertler A, Barkey RJ. Leptin signaling and apoptotic effects in human prostate cancer cell lines. Prostate 2011; 71: 929-945.
- Otvos L, Shao WH, Vanniasinghe AS, et al. Toward understanding the role of leptin and leptin receptor antagonism in preclinical models of rheumatoid arthritis. Peptides 2011; 32: 1567-1574.
- Otvos L, Kovalszky I, Riolfi M, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer 2011; 47: 1578-1584.
- Dupuis L, Schuermann Y, Cohen T, et al. Role of leptin receptors in granulosa cells during ovulation. Reproduction 2013; 147: 221-229.
- Gonzalez-Anover P, Vigo E, Encinas T, et al. Prepubertal evolution of plasma leptin levels in gilts of thrifty genotype (Iberian pig) and lean commercial crosses (Large White X Landrace). Res in Vet Sci 2012; 93: 100-102.
- Rak-Mardyla A, Gregoraszczuk EL. Expression of ghrelin and its receptor in porcine ovarian follicles collected from prepubertal and estrous cycle animals. J Physiol Pharmacol 2012; 63: 195-199.
- Zabeau L, Peelman F, Tavernier J. Antagonizing leptin: current status and future directions. Biol Chem 2014; 395: 499-499.
- Kawamura K, Sato N, Fukuda J, et al. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology 2002; 143: 1922-1931.
- Gonzalez RR, Leavis PC. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine 2003; 21: 185-195.
- Gonzalez RR, Leary K, Petrozza JC, Leavis PC. Leptin regulation of the interleukin-1 system in human endometrial cells. Mol Hum Reprod 2003; 9: 151-158.
- Brzechffa PR, Jakimiuk AJ, Agarwal SK, Weitsman SR, Buyalos RP, Magoffin DA. Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1996; 81: 4166-4169.
- Norman RJ. Hyperandrogenaemia and the ovary. Mol Cell Endocrinol 2002; 191: 113-119.
- Spritzer PM. Polycystic ovary syndrome: reviewing diagnosis and management of metabolic disturbances. Arq Bras Endocrinol Metabol 2014; 58: 182-187.
- Melo AS, Vieira CS, Romano LG, Ferriani RA, Navarro PA. The frequency of metabolic syndrome is higher among PCOS Brazilian women with menstrual irregularity plus hyperandrogenism. Reprod Sci 2011; 18: 1230-1236.
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 2005; 11: 357-374.
- Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab 2006; 91: 1660-1666.
- Shpilman M, Niv-Spector L, Katz M, et al. Development and characterization of high affinity leptins and leptin antagonists. Biol Chem 2011; 286: 4429-4442.
A c c e p t e d : December 4, 2015