DIETARY SUPPLEMENTATION WITH DRIED CHICORY ROOT TRIGGERS CHANGES IN THE BLOOD SERUM PROTEINS ENGAGED IN THE CLOTTING PROCESS AND THE INNATE IMMUNE RESPONSE IN GROWING PIGS
2The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jablonna, Poland
INTRODUCTION
Chicory (Cichorium intybus) root is a natural source of inulin-type fructans such as inulin and oligofructose. Inulin is composed of a set of sucrose molecules of which the fructose moiety is substituted with a linear chain of β (2-1) fructans. The content of inulin in chicory roots ranges from 150 to 200 g·kg–1 of dry matter (1, 2). Chicory roots also contain oligofructose (80–120 g·kg–1 of dry matter) with a chain length shorter than 10 fructose units, organic acids, protein fragments and minerals (3, 4).
Diet is a main factor affecting animal fetal and postnatal development and growth (5, 6). A number of studies have shown that prebiotics exert systemic, health-promoting effect in the gastrointestinal tract, predominantly in the large intestine (7-9). These functional components of the diet show a major influence on the selective growth and activity of microbiota in the colon lumen, such as Bifidobacteria and Lactobacilli. This results in increased production of short-chain fatty acids (SCFA), mainly acetate, propionate and butyrate. SCFA are largely absorbed and metabolized by intestinal epithelium, liver, muscle and adipose tissue. Increased synthesis of SCFA creates an acidic environment in the large intestine that limits the colonization of pathogenic microorganisms and additionally can affect immune cells located along the gut-associated lymphoid tissue (GALT). There is some evidence in the literature suggesting that SCFA in the bloodstream can also interact with the immune cells by binding to G protein-coupled receptors such as GPR41 and GPR43 located in polymorphonuclear cells and peripheral blood mononuclear cells (10-12). Watzl et al. (10) and Seifert and Watzl (11) claim that butyrate can suppress lymphocyte proliferation, inhibit cytokine production of Th1 lymphocytes, induce T-lymphocyte apoptosis and increase production of IL-10. Nevertheless, the exact mechanisms responsible for these observations are still not fully understood, as the influence of inulin-type fructans on local and systemic immune responses in swine is poorly defined.
In young pigs, it has been shown that a diet containing dried chicory root exerts both the antibiotic and parasite-reducing effects (13). In the study of Jensen et al. (13) inclusion of 30% of dried chicory root in the diet of weaned piglets experimentally infected with Ascaris suum and Trichuris suis significantly altered nematodes population. These authors showed that experimental diet reduced the number of A. suum in the small intestine by 64%.
Previous studies have also demonstrated that SCFA in the bloodstream can decrease plasma triglyceride concentrations in humans (14) and rats (15). In the study of Grela et al. (16) feeding diet supplemented with 4% of dried chicory root significantly decreased total cholesterol and LDL-cholesterol concentration in blood plasma of piglets.
These observations may suggest that bioactive compounds present in dried chicory root may affect piglet metabolism by alteration in protein profile of blood serum. Our goal was therefore to determine the effect of feeding diet containing dried chicory root on the systemic immune and metabolic alterations in the blood serum of growing pigs.
MATERIAL AND METHODS
Animals and sample collection
The study was performed on 12 castrated male piglets (PIC × Penarlan P76), randomly divided into two groups (n=6). For experiment piglets were chosen from six different litters (two piglets per litter) offered from the 10th day of life unsupplemented cereal-based diet (control group, C) or a diet supplemented with 4% of dried chicory root (treatment group, T). The remaining components of the diets were as follows: wheat (47.84% in C vs. 43.84% in T), barley (20%), corn starch (2%), full-fat soybean meal (5.9%), whey (9.7%), fish meal (4%), spray-dried blood plasma (4%), soybean oil (3.39%), calcium formate (0.3%), limestone (0.5%), dicalcium phosphate (0.6%), sodium chloride (0.07%), L-lysine (0.614%), DL-methionine (0.232%), L-threonine (0.264%), L-tryptophan (0.089%), mineral-vitamin premix (0.4%) and aroma (0.1%). Both diets contained 20% of crude protein and 14.3 MJ/kg of metabolizable energy. After weaning piglets were kept for 21 days in groups of three animals per pen with free access to feed and water. Body weight of animals was measured twice, at weaning and at the end of the experiment, and feed intake and health status was monitored every day during the experimental period. Piglets were sacrificed at the age of 50 days at final body weight of about 20 kg. The samples of mixed blood were collected during exsanguination and serum for proteome analyses was obtained by blood centrifugation (1500 × g for 15 min at 4°C) after clotting. Blood samples were also collected into heparinized tubes and plasma for biochemical assays was obtained by centrifugation at 3000 rpm for 10 min at 4°C. Serum and plasma samples were stored at –80°C until analyses. The experimental procedures were approved by the Local Commission of Ethics for the Care and Use of Laboratory Animals (No. 13/2012 of 23.05.2012).
Two-dimensional electrophoresis (2-DE)
Serum samples were processed in duplicate using a ProteoMiner™ Protein enrichment Large-Capacity kit (Bio-Rad) to decrease the concentration of high-abundance proteins and increase the low-abundance protein concentration. Samples were then precipitated with four volumes of cold acetone (–20°C) for two hours and resulting protein pellets were dissolved in the lysis buffer containing 7 M urea, 2 M thiourea, 4% w/v CHAPS, 1% w/v DTT, 0.2% w/v 3–10 ampholytes and 2 mM TBP. Protein samples (800 µg) were applied to 3–10, 24 cm NL (nonlinear) ReadyStrip™ IPG Strips (Bio-Rad). The isoelectrofocusing (IEF) was run in total of 90 000 Vh using a Protean® IEF Cell (Bio-Rad). After IEF, the IPG strips were reduced with DTT in equilibration buffer (6 M urea, 0.5 M Tris/HCl, pH 6.8, 2% w/v SDS, 30% w/v glycerol and 1% w/v DTT) for 15 minutes and then alkylated with iodoacetamide (2.5% w/v) for 20 minutes at ambient temperature. The second dimension was performed in 12% SDS polyacrylamide gels (20 × 25 cm) at 40 V for 2.5 hours and then at 100 V for 16 hours at 10°C using a Protean Plus™ Dodeca Cell™ electrophoretic chamber (Bio-Rad). After 2-DE separation, the gels were stained for 72 hours with colloidal Coomassie Brilliant Blue G-250 according to Westermeier (17).
Image analysis
The gels were scanned using a GS-800™ Calibrated Densitometer (Bio-Rad). Analysis of 2-D images was performed using a PDQuest Analysis software version 8.0.1 Advanced (Bio-Rad). To measure the variability within the group, the coefficient of variation (CV) was calculated for each replicate group. Qualitative and quantitative comparisons between the groups were performed to show the differences in the pattern of protein spots between groups and to examine changes in the protein expression level. Experiment normalization was performed using a local regression model (LOESS). Molecular mass (kDa) was computed for each identified protein spot based on the molecular range standard.
Mass spectrometry and functional protein clustering
The protein spots showing statistically significant differences were manually excised from the gels and decolorized by washing in buffer containing 25 mM NH4HCO3 in 5% v/v acetonitrile (ACN), followed by two washes with a solution of 25 mM NH4HCO3 in 50% v/v ACN. The gel pieces were dehydrated (100% ACN), vacuum dried and incubated overnight with trypsin (20 µl/spot of 12.5 µg trypsin/ml in 25 mM NH4HCO3; Promega, Madison, USA) at 37°C. The resulting peptides were extracted with 100% ACN, combined with an equal volume of matrix solution (5 mg/ml CHCA, 0.1% v/v TFA, 50% v/v ACN) and loaded onto a MALDI-MSP AnchorChip™ 600/96 plate (Bruker Daltonics, Germany). Peptide Mass Standard II was used (mass range 700–3200 Da, Bruker Daltonics) for calibration of the mass scale. Mass spectra were acquired in positive-ion reflector mode using a Microflex™ MALDI-TOF (matrix-assisted laser desorption/ionization time of flight) mass spectrometer (Bruker Daltonics, Germany). Peptide mass fingerprinting (PMF) data were compared to mammalian databases (SWISS-PROT; http://us.expasy.org/uniprot/ and NCBI; http://www.ncbi.nlm.nih.gov/) with the aid of MASCOT search engine (http://www.matrixscience.com/). Search criteria included: trypsin as an enzyme, carbamidomethylation of cysteine as a fixed modification, methionine oxidation as a variable modification, mass tolerance to 150 ppm and a maximum of one missed cleavage site. The results were further validated by the MASCOT score (only statistically significant hits were applied) and sequence coverage.
In order to integrate the data, the identified proteins were uploaded to DAVID (v 6.7) Functional Annotation Clustering tool (http://david.abcc.ncifcrf.gov/). The analysis comprised functional classification of biological processes in the context of Gene Ontology (GO) terms and cellular signalling pathways based on the KEGG database. Cluster names were extracted from the most biologically relevant GO and KEGG terms. Clusters with a minimum of three GO terms and enrichment score ≥4 were used.
Analysis of biochemical parameters of blood plasma samples
The levels of blood plasma immunoglobulin A (IgA), immunoglobulin G (IgG) and immunoglobulin M (IgM) were determined by the immuno-turbidimetry method using ready-to-use reagents according to the manufacturer’s instruction (APTEC Diagnostics nv, Sint-Niklaas, Belgium). Turbidity was measured at 340 nm and Igs concentrations were calculated using a four-parameter logistic curves.
Fibrinogen concentration was analyzed chronometrically, according to the method of Clauss (18), using reagents of DIAGON Ltd. (Budapest, Hungary). Plasma samples were diluted in imidazole buffer, mixed with thrombin and the clotting time was measured using photometers supplied with 470 nm LEDs. The concentration of fibrinogen was determined based on a bilogarithmic standard curve.
The levels of total cholesterol, high-density lipoprotein (HDL) and triglycerides were determined spectrophotometrically using ELITech ready-to-use reagents (ELITech Group, Puteaux, France).
All biochemical analyses were performed on a multidisciplinary diagnostic platform MAXMAT PL (Erba Diagnostics France SARL, Montpellier, France). The level of low-density lipoprotein (LDL) was calculated on the basis of the Friedewald’s formula (LDL=TC – HDL – [TG/2.2]).
Statistics
The mean values and standard error of the mean (S.E.M.) were calculated. Significance of the differences in protein expression pattern was confirmed during image analysis by Student’s t-test using PDQuest Analysis software. Body weight gain and biochemical blood parameters were also analyzed by Student’s t-test using STATGRAPHICS Centurion XVI version 16.1.03.
RESULTS
Production traits
All animals remained healthy during the experimental period. Feeding experimental diets did not affect significantly body weight gain and final body weight (data not shown). Feed intake was similar in both groups, however it could not be analyzed statistically since it was registered collectively per pen.
Differentially regulated serum proteins
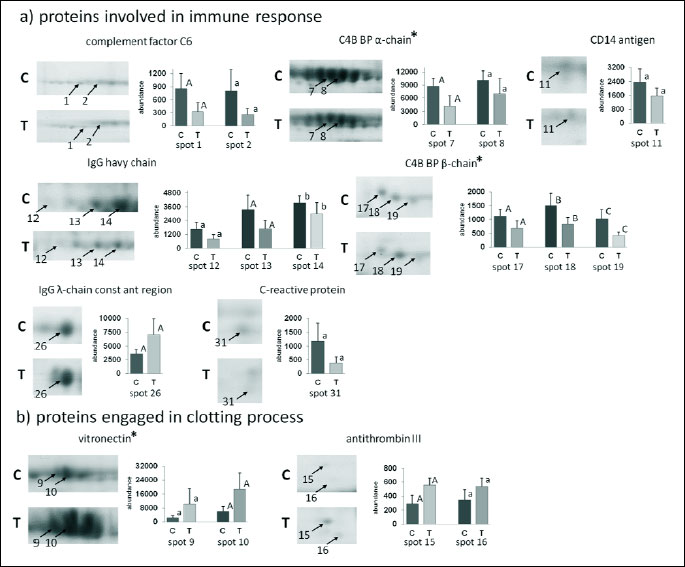
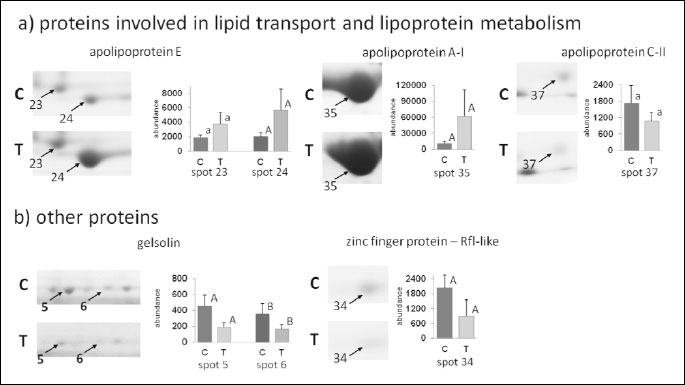
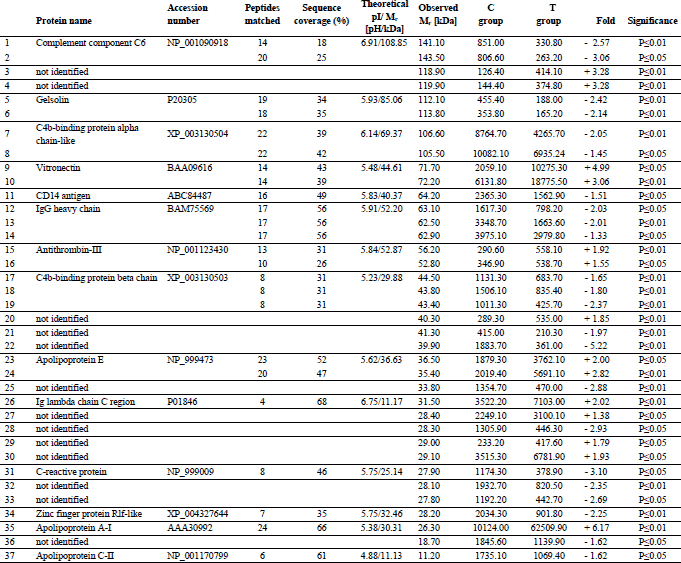
Analysis of 2D images allowed to detect approximately 240 protein spots in each gel in the molecular mass range from 10 to 250 kDa and a pH ranging from 3 to 10. CV for replicate groups was 58.86% and 54.79% for C and T group, respectively. Thirty-seven protein spots were found to be significantly altered in the group T compared to the group C (Fig. 1). Among these spots, 14 were up-regulated, while 23 showed down-regulation. Twenty four protein spots, representing 14 distinct gene products, were successfully identified. The expression of C4b-binding protein alpha chain-like (C4b-BPα), C4b-binding protein beta chain (C4b-BPβ), IgG heavy chain, complement component 6 (C6), C-reactive protein (CRP), CD14 antigen, zinc finger protein Rlf-like, gelsolin and apolipoprotein C-II (apo C-II) was significantly lower in T than in C group. The major changes in spot intensities induced by the experimental diet was demonstrated in case of complement C6 and CRP (3.06 and 3.02-fold greater reduction in expression, respectively). The expression of vitronectin (VTN), antithrombin III (AT III), Ig lambda chain C region (IGLC), apolipoprotein A-I (apo A-I) and apolipoprotein E (apo E) was significantly greater in T than in C group. It should be emphasised that apo A-I displayed a six-fold higher expression in the T than in the C group. Graphs showing differences in abundance and changes in the intensity of spots of the identified proteins are presented in Figs. 2 and 3. Additionally, detailed information concerning the average abundance, fold change and significance of differences of the spots analyzed are summarized in Table 1.
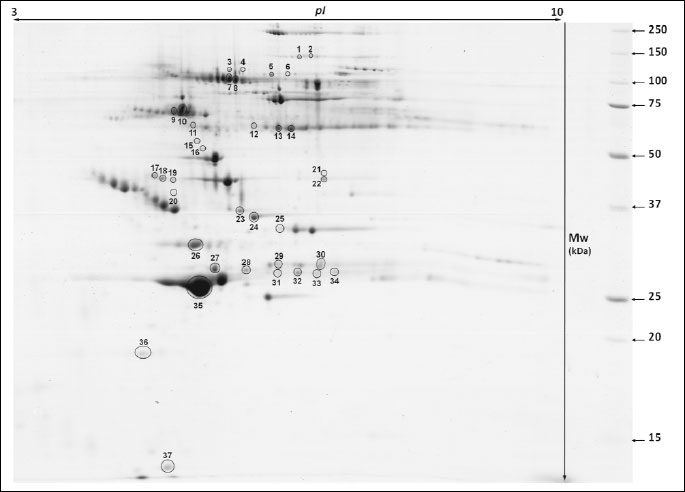
Functional annotation clustering of identified proteins
The most significant (P - 1.3E-4) cellular pathway, according to KEGG database, found for our proteins was assigned to “complement and coagulation cascades”, which included 4 of the 14 identified proteins: C4b-BPα, C4b-BPβ, C6 and AT III.
The two most significant functional annotation clusters were selected. The first one (P - 1.2E-5) was associated with “immune response” and included 7 of the 14 proteins identified, i.e.: C4b-BPα, C4b-BPβ, C6, CRP, CD14 antigen, IGLC and VTN. The second one (P - 1.1E-5) was associated with “high-density lipoprotein particle clearance” and comprised: apolipoprotein A-I, apolipoprotein E and apolipoprotein C-II.
Biochemical blood parameters
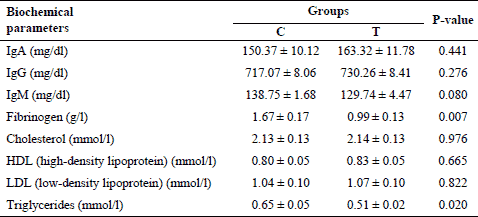
Mean levels of plasma biochemical parameters are shown in Table 2. Piglets fed diet supplemented with dried chicory root had similar levels of IgA, IgG and IgM compared to the C group. There was only a decreasing tendency observed for the concentration of IgM in the T group. The level of fibrinogen and triglycerides was significantly lower in the T than in C group, whereas total cholesterol, HDL and LDL cholesterol were not affected by the diet.
DISCUSSION
The addition of 4% of dried chicory root to the diet of piglets triggered significant changes in the serum proteome. It is rather unlikely that observed serum protein changes could be related to differences in feed intake or overall energy and protein content in the two types of diets as these parameters were similar and body weight gains of pigs did not differ between groups. Thus, it can be postulated that it may be attributed to differences in the chemical composition of the diet as chicory root is characterised by a high content of inulin, oligofructose, organic acids, protein fragments and minerals. Unexpectedly, the results of this proteomic study reveals that of 14 identified different gene products, 7 were classified as being involved in not only the innate immune response but also in the clotting process. However, there is a large body of evidence demonstrating that the interaction exists between the coagulation, complement cascades and lipid metabolism (19-22). It is noteworthy, that this interaction has a bidirectional character manifested in mutual activation: complement effectors can induce coagulation process and components of the coagulation cascade can trigger complement activation.
The complement component system plays a pivotal role as part of the innate immune system of mammals. It is not only engaged in the protection against exogenous antigens, due to its opsonic, inflammatory and lytic abilities, but it also participates in the regulation of adaptive immune system (23, 24). The complement cascade can be activated either by the classical pathway (CP), lectin pathway (LP) or the so-called alternative pathway (AP). The inflammatory response, e.g. to infections or tissue damage results in the activation of both the complement and haemostatic systems, resulting in a rapid increase in plasma levels of acute phase proteins (APP), which may directly affect the process of coagulation. Among the APP we could distinguish proteins that were also identified in our experiment. These included: C-reactive protein (CRP), C4b-binding protein (C4b-BP) and fibrinogen (Fb) (25). However, in the present study, the above-mentioned proteins as well as complement component C6, were down-regulated in response to the experimental diet, which may result from anti-inflammatory properties of chicory inulin-type fructans. Unfortunately, there is no information available in the literature regarding the effect of dietary supplementation with dried chicory roots on serum protein changes in animals or human subjects. Nevertheless, chicory is characterised by a high content of inulin and oligofructose, for that reason comparison of our data with the results obtained with the supplementation of a diet with those fructans seems to be adequate.
CRP is a liver-derived APP that is released as one of the main mediators of the inflammatory cascade in pigs (26). It has been shown, that CRP not only activates the complement system but it can also enhance expression of tissue factor and plasminogen activator inhibitor-1 (PAI-1), thereby promoting procoagulant state (25, 27). Nova et al. (28) also observed a decrease in serum CRP after synbiotic supplementation in healthy human subjects. These authors incorporated into the diet a synbiotic product containing certain species of Lactobacillus and Bifidobacterium with fructooligosaccharides (FOS) for a total period of six weeks (28). Similar results were obtained by Malaguarnera et al. (29) after synbiotic treatment of humans with non-alcoholic steatohepatitis. The results of this study demonstrated that a 24-week supply of Bifidobacterium longum and FOS reduced plasma CRP level.
C4b-BP is a regulatory protein of the complement system that is composed of α- and β-chains linked together at their terminal parts. Alpha-chains have binding sites for C4b molecules and heparin, whereas the β-chain is mainly engaged in binding vitamin K-dependent protein S. The latter promotes clot lysis and it is estimated that approximately 60% of circulating protein S is bound to C4b-BP. When the protein complex S-C4b-BP is formed, the anticoagulant activity of protein S is inhibited (20, 30). C4b-BP is an APP and its level rises significantly in response to inflammatory stimuli. However, Garcia de Frutos et al. (31) found that a significant increase in the plasma level of human C4b-BP is mainly caused by the elevation of the α-chain form, as the β-chain form remains virtually unaltered. According to these authors, such a mechanism prevents a reduction in the level of free protein S even if the concentration of C4b-BP is considerably higher (31). Unfortunately, there is no information in the available literature concerning changes in the level of C4b-BP in animals or human subjects after prebiotic or probiotic supplementation.
We have also demonstrated that supplementation of the diet with dried chicory root leads to a significant decrease in the levels of fibrinogen in blood plasma of pigs. It should be emphasised that the increased level of plasma fibrinogen - the final substrate in the clot formation - has been also associated with inflammatory reactions in pigs (32) and humans (33). Studies in humans have also shown that the addition of Lactobacillus plantarum to the diet can reduce plasma fibrinogen both in smokers (34) and mildly hypercholesterolemic patients (35). Naruszewicz et al. (34) showed a marked 21% decrease in the concentration of plasma fibrinogen in a group of smokers supplemented with L. plantarum. These authors also measured plasma levels of interleukin-6 (Il-6), an inflammatory mediator that is tightly linked to the activation of APP, including fibrinogen. They demonstrated a significant (41%) decrease in the level of circulating Il-6 in the treatment group (34).
The present study also showed that the ingestion of experimental diet resulted in increased expression of vitronectin (VT) and antithrombin III (AT III) in the porcine serum. AT III is not only one of the physiological anticoagulants that inhibit thrombin activation through the formation of thrombin-antithrombin complexes (TAT) but it also exerts anti-inflammatory effect (36). Moreover, the antithrombin III is also known as negative acute phase protein (37), thus the up-regulation of this protein observed in the present study might be another evidence for the anti-inflammatory properties of chicory inulin-type fructans. Vitronectin is a multifunctional glycoprotein that binds a variety of ligands including TAT complexes, thereby forming a ternary complex - VN-TAT (38). Vitronectin displays procoagulant properties, as it forms VN-TAT complexes that protect thrombin against antithrombin activity (39). Increased expression of serum VT and AT III after the supplementation of dried chicory root remains unclear. De Boer et al. (39) reported an increase in the VT level when the coagulation cascade was activated. In our study, we have not measured the parameters related to this process, thus it is difficult to assess whether it was the main cause of the observed phenomenon. Nevertheless, we postulate that the increased AT III expression was caused by the up-regulation of VT as a compensatory mechanism in response to increased coagulation.
CD14 antigen was another multifunctional molecule found to be statistically down-regulated in the serum of pigs due to feeding diet containing dried chicory root. This protein is present both as a membrane-bound (mCD14) and a circulating soluble (sCD14) form (40). The sCD14 form is recognized as an APP and its elevated concentrations are associated with various diseases in humans (40). Gori et al. (41) showed that dietary supplementation with a mixture of short chain galactooligosaccharides (scGOS), long-chain fructooligosaccharides (lcFOS) and pectin hydrolyzates-derived acidic oligosaccharides (pAOS), resulted in decreased sCD14 plasma levels at week 16 in the virus type 1 (HIV-1) infected patients, treated with 15 g daily dose of prebiotic oligosaccharides. Significantly reduced levels of sCD14 were also observed by Prescott et al. (42) in the cord blood of human neonates, whose mothers were supplemented Bifidobacterium lactis two weeks prior to delivery.
Dietary supplementation with dried chicory root only slightly affected the levels of systemic IgG and IgM. Surprisingly, three protein spots identified as IgG heavy chains were down-regulated, whereas the total plasma IgG did not differ significantly between. This phenomenon is hard to explain and remains unclear. Similar effects were observed by Grela et al. (16). Authors showed that supplementation of a diet with dried chicory root did not affect the IgG concentration in piglets fed the diet with this additive for 74 days. Nevertheless, authors observed increased IgG concentration at weaning in animals receiving diet with addition of chicory root for two weeks. Taranu et al. (43) showed moderately elevated plasma IgG in pigs fed diet supplemented with 1.5% inulin for 11 days after weaning. The current study demonstrated a tendency to a lower level of plasma IgM in piglets fed diet with dried chicory roots, what is in accordance to results obtained by Grela et al. (16). Studies conducted by Taranu et al. (43) in pigs showed that the level of plasma IgM was not affected by inulin.
We have also demonstrated that the diet supplemented with 4% of dried chicory root affected serum proteins involved in the transport of lipids and lipoprotein metabolism. Two of these proteins, i.e., apo E and apo A-I, showed increased expression, whereas apo C-II was found to be down-regulated in response to the experimental diet. Apo A-I and apo E are the main protein components of high-density lipoproteins (HDL) and they both act as cofactors for the lecithin-cholesterol acyltransferase (LCAT). LCAT is an enzyme responsible for the production of cholesteryl esters, which are transported into the core of HDL particles (44). The concentration of plasma total cholesterol, HDL cholesterol and LDL cholesterol remained practically unchanged in the experimental group. A study by Kim and Shin (45) showed that a diet supplemented with 1 or 5% chicory extract or 5% inulin for 4 weeks increased the serum HDL levels in Sprague-Dawley rats. Similar findings were reported in the study where oat bran fiber was added to the rats’ diet (80% of basal mix and 20% of oat bran) for 4 weeks (46). On the other hand, no changes in both human serum apo A-I levels and HDL cholesterol were observed after 4-week supplementation with short chain FOS (47). Apolipoprotein C-II is one of the protein components of the triglyceride-rich chylomicrons and very low density lipoproteins (VLDLs). Moreover, apo C-II is known to activate lipoprotein lipase (LPL), thus it is a key molecule engaged in the metabolism of plasma triglycerides (48). In the present study, we have demonstrated that experimental diet induced down-regulation of apo C-II as well as reduction of plasma triglyceride concentrations. This is consistent with previous studies where inulin-type fructans have been shown to decrease plasma triglyceride concentrations both in human and rats (14, 15).
In conclusion, the implementation of the 4% of dried chicory root into the diet of growing pigs caused a significant down-regulation of not only proteins directly or indirectly engaged in the innate immune response but also those related to clotting process, including complement component C6, C-reactive protein, CD14 antigen, C4b binding protein α and β chains, and fibrinogen. This may indicate that bioactive components of chicory root, mainly inulin-type fructans, may exert anti-inflammatory properties. Moreover, it is concluded that diet with dried chicory root has a lowering effect on lipid metabolism. However, further work is required to assess the health-promoting effect of this functional food ingredient at the global level and determine possible mechanisms involved in these processes.
Acknowledgements: This work was supported by grant from National Centre of Science, Poland (Project No. 2012/05/D/NZ9/01604).
Conflict of interests: None declared.
REFERENCES
- Robefroid MB. Inulin-type fructans: functional food ingredients. J Nutr 2007; 137: 2493-2502.
- Robefroid MB. Introducing inulin-type fructans. Br J Nutr 2005; 93 (Suppl. 1): 13-25.
- Molbak L, Thomsen LE, Jensen TK, Bach Knudsen KE, Boye M. Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J Appl Microbiol 2010; 103: 1853-1867.
- Van Loo J. How chicory fructans contribute to zootechnical performance and well-being in livestock and companion animals. J Nutr 2007; 137: 2594S-2597S.
- Mickiewicz M, Zabielski R, Grenier B, et al. Structural and functional development of small intestine in intrauterine growth retarded porcine offspring born to gilts fed diets with differing protein ratios throughout pregnancy. J Physiol Pharmacol 2012; 63: 225-239.
- Tomaszewska E, Dobrowolski P, Wydrych J. Postnatal administration of 2-oxoglutaric acid improves articular and growth plate cartilages and bone tissue morphology in pigs prenatally treated with dexamethasone. J Physiol Pharmacol 2012; 63: 547-554.
- Mikkelsen LL, Bendixen C, Jakobsen M, Jensen BB. Enumeration of bifidobacteria in gastrointestinal samples from piglets. Appl Environ Microbiol 2003; 69: 654-658.
- Yasuda K, Maiorano R, Welch RM, Miller DD, Lei XG. Cecum is the major degradation site of ingested inulin in young pigs. J Nutr 2007; 137: 2399-2404.
- Mair C, Plitzner C, Domig KJ, Schedle K, Windisch W. Impact of inulin and a multispecies probiotic formulation on performance, microbial ecology and concomitant fermentation patterns in newly weaned piglets. J Anim Physiol Anim Nutr (Berl) 2010; 94: e164-e177.
- Watzl B, Girrbach S, Roller M. Inulin, oligofructose and immunomodulation. Br J Nutr 2005; 93 (Suppl. 1): 49-55.
- Seifert S, Watzl B. Inulin and oligofructose: review of experimental data on immune modulation. J Nutr 2007; 137 (Suppl. 11): S2563-S2567.
- Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol 2008; 59 (Suppl. 2): 251-262.
- Jensen AN, Mejer H, Molbak L, et al. The effect of a diet with fructan-rich chicory roots on intestinal helminths and microbiota with special focus on Bifidobacteria and Campylobacter in piglets around weaning. Animal 2011; 5: 851-860.
- Williams CM. Effects of inulin on lipid parameters in humans. J Nutr 1999; 129: 1471S-1473S.
- Cieslik E, Kopec A, Pisulewski PM. Effects of fructooligosaccharides and long-chain inulin on serum lipids in rats. Pol J Food Nutr Sci 2005, 14: 437-441.
- Grela ER, Sobolewska S, Kowalczuk-Vasilev E, Krasucki W. Effect of dietary inulin source on piglet performance, immunoglobulin concentration, and plasma lipid profile. Bull Vet Inst Pulawy 2014; 58: 453-458.
- Westermeier R. Sensitive, quantitative and fast modifications for coomassie blue staining of polyacrylamide gels. Proteomics 2006, 6 (Suppl. 2): 61-64.
- Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol 1975; 17: 237-246.
- Williams JL. Cross talk between the inflammation and coagulation systems. Clin Lab Sci 2007; 20: 224-229.
- Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 2005; 131: 417-430.
- Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement – their role in inflammation. Semin Immunopathol 2012; 34: 151-165.
- Siennicka A, Drozdzynska M, Chelstowski K, Cnotliwy M, Jastrzebska M. Haemostatic factors and intraluminal thrombus thickness in abdominal aortic aneurysm. Is secondary fibrinolysis relevant? J Physiol Pharmacol 2013; 64: 321-330.
- Salvesen B, Mollnes TE. Pathway-specific complement activity in pigs evaluated with a human functional complement assay. Mol Immunol 2009; 46: 1620-1625.
- Georgiou AS, Gil MA, Alminana C, et al. Effects of complement component 3 derivatives on pig oocyte maturation, fertilization and early embryo development in vitro. Reprod Dom Anim 2011; 46: 1017-1021.
- Davidson SJ. Inflammation and acute phase proteins in haemostasis. In: Acute Phase Proteins, S. Janciauskiene (ed.). InTech, 2013, pp. 31-54.
- Chen HH, Lin JH, Fung HP, et al. Serum acute phase proteins and swine health status. Can J Vet Res 2003; 67: 283-290.
- Deveraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation 2003; 107: 398-404.
- Nova E, viadel B, Warnberg J, Carreres JE, Marcos A. Beneficial effects of a synbiotic supplement on self-perceived gastrointestinal well-being and immunoinflammatory status of healthy adults. J Med Food 2011; 14: 79-85.
- Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non-alcoholic steatohepatitis. Dig Dis Sci 2012; 57: 545-553.
- Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol 2004; 40: 1333-1346.
- Garcia de Frutos P, Alim RI, Hardig Y, Zoller B, Dahlback B. Differential regulation of alpha and beta chains of C4b-binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant protein S. Blood 1994; 84: 815-822.
- Skovgaard K, Mortensen S, Boye M, et al. Rapid and widely disseminated acute phase protein response after experimental bacterial infection of pigs. Vet Res 2009; 40: 23.
- Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM 2003; 96: 711-729.
- Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr 2002; 76: 1249-1255.
- Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis 1998; 137: 437-438.
- Oelschlager C, Romisch J, Staubitz A, et al. Antithrombin III inhibits nuclear factor kB activation in human monocytes and vascular endothelial cells. Blood 2002; 99: 4015-4020.
- Niessen RW, Lamping RJ, Jansen PM, et al. Antithrombin acts as a negative acute phase protein as established with studies on HepG2 cells and in baboons. Thromb Haemost 1997; 78: 1088-1092.
- Goswami S, Thompson LC, Wickman L, Peterson CB. The cellular microenvironment modulates the role of PAI-1 and vitronectin in mediating cell-matrix interactions. Adv Biol Chem 2013; 3: 114-132.
- de Boer HC, Preissner KT, Bouma BN, de Groot PG. Binding of vitronectin-thrombin-antithrombin III complex to human endothelial cells is mediated by the heparin binding site of vitronectin. J Biol Chem 1992; 267: 2264-2268.
- Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol 2004; 172: 4470-4479.
- Gori A, Rizzardini G, Van’t Land B, et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol 2011; 4: 554-563.
- Prescott SL, Wickens K, Westcott L, et al. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin A detection. Clin Exp Allergy 2008; 38: 1606-1614.
- Taranu I, Marin DE, Untea A, et al. Effect of dietary natural supplements on immune response and mineral bioavailability in piglets after weaning. Czech J Anim Sci 2012; 57: 332-343.
- Birchbauer A, Knipping G, Juritsch B, Aschauer H, Zechner R. Characterization of the apolipoprotein AI and CIII genes in the domestic pig. Genomics 1993; 15: 643-652.
- Kim M, Shin HK. The water-soluble extract of chicory influences serum and liver lipid concentrations, cecal short-chain fatty acid concentrations and fecal lipid excretion in rats. J Nutr 1998; 128: 1731-1736.
- Schneeman BO, Cimmarusti J, Cohen W, Downes L, Lefevre M. Composition of high density lipoproteins in rats fed various dietary fibers. J Nutr 1984; 114: 1320-1326.
- Luo J, Rizkalla SW, Alamowitch C, et al. Chronic consumption of short-chain fructooligosaccharides by healthy subjects decreased basal hepatic glucose production but had no effect on insulin-stimulated glucose. Am J Clin Nutr 1996; 63: 939-945.
- Andersson Y, Majd Z, Lefebvre AM, et al. Developmental and pharmacological regulation of apolipoprotein C-II gene expression. Comparison with apo C-I and apo C-III gene regulation. Arterioscler Thromb Vasc Biol 1999; 19: 115-121.
A c c e p t e d : November 10, 2014