INFLAMMATORY RESPONSE IN VISCERAL FAT TISSUE AND LIVER
IS PRENATALLY PROGRAMMED: EXPERIMENTAL RESEARCH
2Department of Pathology, Lviv National Medical University, Ukraine
INTRODUCTION
The worldwide epidemic of obesity has brought considerable attention to research aimed at the understanding of the role of physiological adipocyte's activity and the mechanisms underlying obesity-linked complications (1). Adipose tissue is a key endocrine organ releasing multiple bioactive substances known as adipokines, with pro-inflammatory (leptin, acute-phase serum amyloid A, interleukin-6, tumor necrosis factor-α, macrophage chemoattractant protein-1, and resistin) and anti-inflammatory (adiponectin) properties (2-4). Growing amount of evidence indicates that abnormal secretion of adipokines causes obesity-related chronic low-grade inflammation, which contributes to systemic metabolic dysfunction and several co-morbidities (5, 6). One of them is nonalcoholic fatty liver disease (NAFLD). NAFLD embraces a whole spectrum of liver pathology: from cases of steatosis with virtually no evidence of hepatocellular injury or liver inflammation (often referred to as simple steatosis), to nonalcoholic steatohepatitis (NASH), and to cases with cirrhosis or even hepatocellular carcinoma (7, 8). NAFLD is a disease with a slow and hidden onset. It is recognized as a major health burden in developed countries, with an estimated prevalence from 15% to 45% in general population. NAFLD is presently considered to be hepatic manifestation of metabolic syndrome. In late stages it may lead to a such dangerous complications as hepatic insufficiency (8). Recent data from Mayo Clinic researchers, who studied trends in the prevalence of steatosis and NAFLD among obese and non-obese population showed 3-fold increase in it's prevalence over the last 30 years (9). Similar tendency in prevalence of NAFLD (approximately 10% of all adolescents) was demonstrated in children. Increase in NAFLD prevalence in this population was much greater then the increase in the prevalence of obesity (10).
The basic pathogenesis of NAFLD remains controversial and little is known about possible risk factors leading to dysregulation in signaling mechanisms associated with cross-talk between adipose tissue and liver. Last ones could be early markers of NAFLD. Several recent investigations in both animals and humans have suggested that events that have occurred prenatally may play an important role in the pathogenesis of diseases at adulthood (11, 12) and that the pathogenesis of many lifestyle-linked metabolic disorders and chronic diseases begin not in childhood or adulthood, but during early development - in prenatal period (13, 14). The role of external (environmental) factors, such as maternal nutrition and stress during prenatal period, in NAFLD pathogenesis is still unclear. There are no noninvasive diagnostic tests available as an alternative to liver biopsy to detect early liver changes. Thus, the present study was designed to examine the effects of maternal stress in the first and third trimester of pregnancy and nutritional insults: high dietary content of sugar, fat or their combination on morpho-functional changes and the integrity of liver and adipocytes in the offsprings. Changes in the pro- and anti-inflammatory adipokines production and their possible contribution to liver pathology were assessed as well. The model of liver and adipocyte injury used two combined elements known to contribute to liver injury: maternal nutritional insult (15) and exposure to stress (16, 17). In addition to studying the role of external environmental factors affecting pregnant females and their offspring (males) in liver injury we examined the effects of stress and consumption of HSD, HFD or high sugar and fat diet (HSFD) on adipose tissue and pro-inflammatory (leptin, IL-1β, IL-8) and anti-inflammatory (adiponectin) adipokines production. We hypothesized that the combination of maternal stress and nutritional insults will be valuable in modeling and studying early stages in NAFLD development and selection of a novel laboratory test that can be used as a biomarker to detect early liver damage.
MATERIALS AND METHODS
Animals
All experiments were carried out according to the National Institute of Bioethics Guidelines for the care and use of laboratory animals and the European Council Directive on 24 November 1986 for Care and Use of Laboratory Animals (86/609/EEC), and approved by the Local Ethics Committee of Lviv National Medical University (11.04.2011).
Twenty female rats in gestational age and their 3-month old male offspring (n=28, body weight 211 ± 24 g) were used in the experiment. Pregnancy in rats was confirmed by vaginal smear test (18). Gestational rats were divided into 4 groups one control group and three experimental groups (Fig. 1). Control group (n=5) was kept on a standard diet and with free tap water access. All three experimental groups were exposed to a social stress during pregnancy (19). The model of maternal social-emotional stress was produced by daily regrouping of female rats in a cage. Each female rat was misplaced 6 hours per day in the first and third trimesters of their pregnancy. For the night period pregnant females were placed into individual cages. We used different types of nutritional insults. Group 1 (n=5) was kept on high sugar diet (HSD) with chronic access to 30% solution of saccharose ad libitum with a drinking-water (20); group 2 (n=5) was kept on high fat diet (HFD) with 45% calories from fat (21); group 3 (n=5) was kept on combined high sugar and fat diet (HSFD). The experimental diets were started after confirmation of pregnancy in female rats and stopped after delivery of offspring. Study design is presented on Fig. 1.
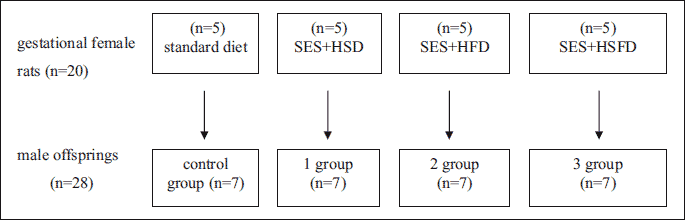
HSD - high sugar diet - standard diet with free access to 30% solution of saccharose with a tap water (by Kozar, et al., 2009); HFD - high-fat diet - high-caloric diet with prevailing of fats (to 45% kcal by A. Lintermans et al., 2009); HSFD - combined high fat diet with free access to 30% solution of saccharose with a tap water.
Experimental animals were housed in plastic cages in a non-barrier-sustained animal room that was maintained at 23 ± 2°C with 50 ± 10% relative humidity and a 12/12-h light/dark cycle. The animals were allowed free access to tap water.
Weight measurement
The total body weight (TBW) in 3-month-old male rats was measured. Then, rats were sacrificed under ketamine anesthesia. Liver, visceral fat and mesenteric fat were removed, washed with ice-cold saline, quickly blotted and weighed. The relative liver weight index (RLW) was calculated as a percent from TBW, relative visceral fat weight (RVFW) was calculated as a percent from TBW, relative mesenteric adipose tissue weight (RMFW) - as percent from visceral fat pad mass.
Histopathological analysis
After killing, the liver and visceral fat were rapidly excised and rinsed in ice-cold saline. All excised organs were then fixed with 10% neutral-buffered formalin and embedded in paraffin for histological analysis. The formalin-fixed, paraffin-embedded tissues were processed and 5-µm-thick serial sections were cut and stained with hematoxylin and eosin (HE). Histopathological imaging was performed using microscope Leica DM 750/4 and digital camera Leica DFC 420 (Germany). Video recording was performed using Leica Application Suit Version 3.8 (Germany), under ×200 and ×400 magnification. Liver histology was scored using semiquantitative scoring system based on complex histology scoring (HS) for human histologic classification of components of NAFLD (22, 23). Visceral fat tissue histology was described based on recommendation proposed by Nov et al. (24).
Liver and fat sections were evaluated in double-blind approach by two individuals and their HS were averaged. HS of liver tissue changes based on the sum of visual analog scales (VAS) points of parenchymal and vascular-stromal changes in each liver section in each group.
Liver cell injury was examined according to the VAS grading: 0 - no changes; 1 - tissue structure is preserved; focal microvacuolar changes and moderate glycogen accumulation in the hepatocytes; 2 - cellular cords are normal, multiple foci of microvacuolar changes, single hepatocytes with large intracytoplasmic vacuoles, irregular accumulation of glycogen in the nuclei and cytoplasm; 3 - severe discomplexation of hepatic cords due to widespread micro/macrovacuolar changes of hepatocytes; single cells with foamy cytoplasm or the signs of necrosis.
Liver vascular-stromal changes analyzed by VAS grading: 0 - no changes; 1 - dilatation and hyperemia of central vein and centrolobular sinusoids; 2 - severe edema and mild infiltration of portal tracts, periportal areas, hyperemia and dilatation of sinusoids associated with the cord discomplexation; 3 - edema and severe mononuclear infiltration of portal/periportal tracts.
Visceral fat tissue changes including inflammation was scored based on following VAS grading: 0 - normal histological structure; 1 - mild polymorphism of adipocytes; 2 - severe polymorphism of adipocytes and focal mononuclear infiltration with admixtures of leucocytes; 3 - severe polymorphism of adipocytes and multifocal mononuclear infiltration with admixtures of leucocytes.
Serum cytokines levels
Immediately after the termination of experiment, venous blood samples were drawn from the abdominal vein and placed into EDTA-containing vials and used for the measurement of levels of leptin, adiponectin, interleukin-1β (IL-1β) and growth-regulated oncogene/cytokine-induced neutrophil chemoattractant-1 (GRO/CINC-1), which is a rat chemokine with structural and functional homology to human interleukin-8 (IL-8). The blood samples were centrifuged at 3500 rpm for 15 min at 15°C. Serum was collected with a micropipette and stored in –60°C until the ELISA assay was performed according to the manufacturer's protocols. Following ELISA kits were used: leptin (rat) (Enzo Life Sciences, United Kingdom), adiponectin (rat) (Adipogen, Korea), IL-8 - a GRO/CINC-1 kit (Enzo Life Sciences, United Kingdom), and IL-1β (rat) kit (Enzo Life Sciences, United Kingdom).
All cytokines levels were measured in duplicate series. Leptin/adiponectin (L/A) ratio was calculated.
Statistical analysis
Statistical analysis is carried out in two stages using the module of a one-way analysis of variance (ANOVA package) of programs STATISTICA for Windows 5.0 (StatSoft, USA, 1999). The first stage was a two-factor analysis of variance, the second - post-hoc comparisons of pairs of groups using the Newman-Keuls test (25).
RESULTS
Body and organ weight
The analysis of rat body weight results (Table 1) has shown increased in 1 group on 4% (P<0.05), in 2 and 3 groups - 7% and 16% (P<0.05), respectively, versus control (P<0.05). The liver weight was significantly elevated evidently in rats of 2 group on 54%, 3–71%, 4–90% versus control (P<0.05). The approximately 2 fold increased RLW was in rats in 3 and 2 groups with fatty diets vs control. The visceral fat pad weight was highest in rats of 1 and 2 groups, increasing on 217 and 237%, respectively, versus control (P<0.05). The greatest RMFW was observed in the rats with HSD, in 1,4 times higher than control (P<0.05).
Thus, the data of relative weight of fat tissue in TBW demonstrated that both sugar and fat overload in diet had an independent effect on weight gain in general, liver and visceral fat, as well as RLW and RVFW. In addition, it demonstrates significant interaction between those factors in relation to the estimated parameters. The relative amount of mesenteric fat to visceral fat pad had independent influence only in group with HSD.
Pairwise comparison of groups showed that body weight and liver weight in rats with HFD increased more significantly comparatively not only to control, but also in comparison with rats with HSD. HSFD was accompanied by further increasing of RLW, RMFW, RVFW.
Histology
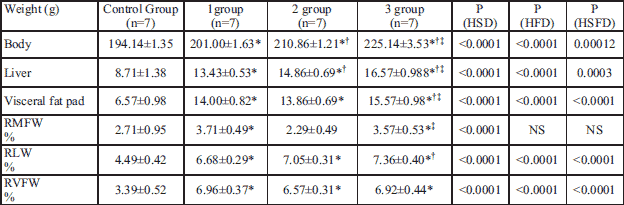
Offspings of female rats treated with standard diet and free access to tap water (control group) exhibited normal liver and visceral fat appearance, with HS of zero and normal histological structure (Fig. 2).
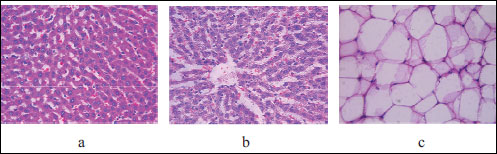 |
Fig. 2. Light microscopic histological appearance of liver: (a) - parenchyma, (b) - microvasculature and (c) fat tissue obtained from control rats. Magnification ×200, ×400. |
Histopathological analysis of liver damage in rats whose mothers were kept on HSD has shown moderate changes (Fig. 3). We demonstrated that the lobular structure was broken due to irregular cellular accumulations and abnormalities in the tissue; hepatocytes were swollen, with focal microvacuolar changes, irregular glycogen accumulations in the nuclei and cytoplasm. Discomplexation of hepatic cords developed due to accumulation of extravascular fluid and focal diapedesis of red blood cells in the Disse space. Severe dilatation and hyperemia of periportal sinusoids were visualized and HS of the liver changes was 2.5 point which is similar histological findings of steatohepatitis in humans. In the fat tissue obtained from the rat of the same group we also observed moderate changes: moderate polymorphism of adipocytes and single focuses of inflammatory infiltration. HS of the fat tissue damage was 2 points and it has correlated with minor accumulation of leukocytes in human visceral adipose tissue, which participates in the cellular mechanisms assisting hepatic fibroinflammatory lesions in obese patients (26).
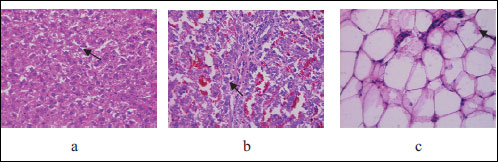 |
Fig. 3. Light microscopic histological appearance of liver: arrows: (a) - microvacuolar changes of cytoplasm and irregular accumulation of glycogen, (b) - dilation and hyperemia of periportal sinusoids and fat tissue: arrow: (c) - single focuses of inflammatory infiltration) obtained from experimental rats with high sugar diet. Magnification ×200, ×400. |
Mild histological liver changes in the offspring of rats fed with HFD were consistent of preserved parenchymal cells with rare hepatocytes with clearing of cytoplasm, hyperemia of central vein and insignificant dilatation of centrolobular sinusoids, and their HS was on average 1 point (Fig. 4). These findings are similar to what is observed in human low-power parenchymal involvement and would be assigned a grading possible/borderline steatohepatitis, in accordance with the NASH clinical research network scoring system (22). The structure of the visceral fat tissue presented with solitary polymorphic and hypertrophic adipocytes. Those changes were scored 1 point.
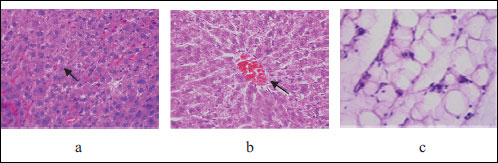 |
Fig. 4. Light microscopic histological appearance of liver: arrows: (a) - single hepatocytes with small intracytoplasmic vacuoles, (b) - hyperemia of central vein and mild dilation of centrolobular sinusoids and fat tissue (c) - mild polymorphism of adipocytes obtained from experimental rats with high fat diet, HE staining. Magnification ×200, ×400. |
The most severe liver changes demonstrated offspring of rats kept on HSFD. Histopathological studies in this group showed preserved lobular structure, discomplexation of hepatic cords; hepatocytes with the signs of the moderate damage - micro- and macrovacuolar changes in 25% of hepatocytes, irregular accumulation of glycogen in 75% of cells (Fig. 5). Signs of interstitial edema were observed mainly in the centrolobular compartments, leucocyte infiltration was determined in 25% of portal tracts and liver damage by HS scoring was 3.5 points. The histological changes of visceral fat tissue in this group were also the severe and were characterized by hyperplastic proliferative changes and intensive leukocyte infiltrations, with HS grading reaching 2.5 points. Those findings correspond histologically to the extent of ectopic lipid accumulation in human liver (27).
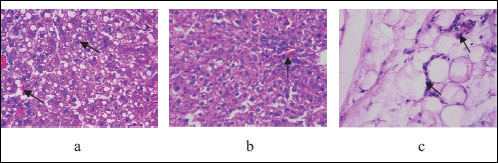 |
Fig. 5. Light microscopic histological appearance of liver: arrows: (a) - micro- and macrovacuolar changes and discomplexation of hepatic cords, (b) - leucocyte infiltration and fat tissue: arrow: (c) - leukocyte infiltrations, obtained from experimental rats with high sugar and fat diet, HE staining. Magnificationi ×200, ×400. |
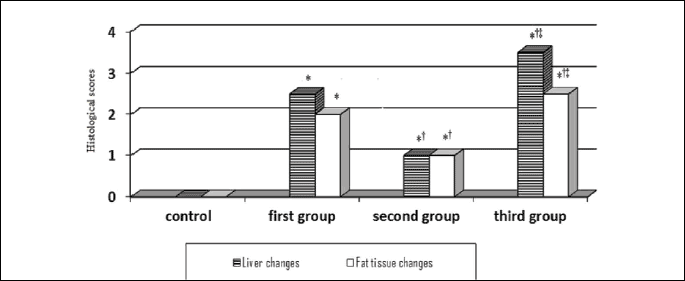
Fig. 6 illustrates the pattern of changes in histological scores of liver and fat tissues in all experimental groups that we had studied.
Serum adipokines and cytokines levels
Despite only minor and moderate liver and fat tissue inflammation following maternal stress and nutritional insulting, there were marked changes in serum levels of anti-inflammatory and a pro-inflammatory cytokines. The levels of IL-1β (28.29 ± 2.06 pg/ml), IL-8 (321 ± 2.85 pg/ml), leptin (2.08 ± 0.05 ng/ml) adiponectin (1.71 ± 0.13 mg/ml), and L/A index (1.22 ± 0.11) in control group were considered as a baseline levels. Changes in serum cytokine levels under the stress and different nutritional insults during the prenatal development are showed in Table 2.
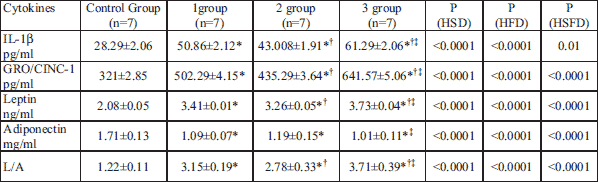
There were significant changes in serum adipokines and IL levels in all experimental groups. The most significant GRO/CINC-1 elevation was detected in the offspring of rat treated with HSFD (99%, group 3), 56% GRO/CINC-1 elevation was detected in offsprings from rat kept on HSD, and 46% - in the HFD group. Similar trend was noticed in term of IL-1β which reached 217% comparatively to control in a HSFD group, 100% elevation in HSD group and 57% in HFD group respectively. Leptin level was more or less equally elevated in all groups - on 64% in HSD group, 60% in HFD group and 79% in HSFD group. Serum adiponectin level went down to 36% in 1 (HSD) group, to 32% in 2 (HFD) group, and to 41% in 3 (HSFD) group. The L/A index was elevated in all experimental groups but most dramatic elevation was observed in HSFD group - 204% comparatively to control.
Further analysis of the cytokines level changes showed that especially diet with increased content of both sugar and fat have an independent effect on their synthesis (with the exception of adiponectin, which is shown to decrease), as evidenced by the significant interaction between these factors on all evaluated parameters. Pairwise comparison groups showed that HSD leads to a stronger increase in the level IL-1β, GRO/CINC-1, leptin, L/A index, than HFD (not only significant difference compared with the control, but also compared with a group of fat). At the same time simultaneous HSFD caused a synergistic effect - significant excess over the two other researched groups.
Changes in adiponectin level were opposite: in all experimental groups it was decreased comparatively to control, without meaningful differences between groups with fat and sugar consumption. In the group with binary influence of these nutritional insults there was recorded important significant decrease of adiponectin level vs control group.
DISCUSSION
NAFLD is among the most common diseases in the world, and largely because of this high incidence and comorbidity to various lifestyle metabolic associated disorders (e.g., type-2 diabetes, metabolic syndrome, obesity). NAFLD has doubled during last 20 years, whereas the prevalence of other chronic liver diseases has remained stable or even decreased (28). These concerns highlight the importance of better understanding the pathogenesis of NAFLD and identifying risk factors, ethiopathogenesis and effective diagnostic tools of this disorder. Among several well-known risk factors of NAFLD recent studies have reveled significant impact of maternal stress, lifestyle factors (e.g., unbalanced diet, micronutrient intake disorders, lack of physical activity), endocrine status, pernicious habits, which play key role in the concept of "fetal origins of adult disease". In the present study, the long-term social-emotional stress and different kinds of nutritional insults during pregnancy were shown to play important role in functioning of adipose tissue, as a highly dynamic endocrine organ, secreting a number of bioactive adipokines, mediators that regulate insulin sensitivity, energy metabolism and vascular homeostasis (29-31). Adipokines and other soluble factors released by inflammated adipose tissue have a direct impact on other insulin-sensitive tissues, especially on the liver (32). In fact, both adipose and hepatic tissues have immediate access to a vast network of blood vessels that implicate a direct connection between these two tissues, and the circulating fatty acid pool derived from fat is the primary contributor to hepatic steatosis, the initial stage in NASH (7, 33).
There are also evidences that in utero programming seems to create lesions in neural networks that regulate energy metabolism. Moreover, the external environmental factors, as stress and nutrition, alter differentiation of hypothalamic neurons in offspring and it may be related with hyperphagia, overweight, adiposity and insulin resistance at adulthood (34-36). Despite the role of excess stress hormones and nutritional insult during fetal period on adipocytes functions in association with liver morpho-functional condition in offspring had previously reported (37, 38), however, among risk factors of NAFLD the role of external environmental risk factors during prenatal period and prognostic non-invasive biomarkers of early changes in liver and fat tissues have not introduced. The developing novel non-invasive biomarkers of NAFLD remain an urgent priority, since laboratory investigation will improve early detection changes in liver injury and avoid the need of liver biopsy (e.g., screening for prognosis of NAFH arising in NAFLD).
Indeed, experimental unbalanced maternal diet and stress contribute in insulin resistance, dyslipidemia, hypertension in offspring, since physiological anomalies of gestational period are responsible for the onset of diseases in offspring at adulthood, such as type 2 diabetes, atherosclerosis, obesity, that are key criteria for metabolic syndrome and the most wide common comorbidities (39-41). This impairment includes ischemia and hypoxia secondary to endothelial dysfunction and neuron damage. The overload of stress hormones and excessive intake of glucose and fatty acids trigger infiltration of immune cells during fetal life via a number of physiological pathways that can be linked to obesogenic effects via glucocorticoid programming (42), which was previously described, as "thrifty phenotype hypothesis of fetal programming" (43), and in nowadays, as a concept of "metabolic memory" (14, 44).
In the present study, we demonstrated that this clinical scenario could be mimicked in the laboratory by inducing stress and imbalanced diet in prenatal period in rats through supplementation of drinking water with sugar, fat rich diet or combination of both factors. Our results have shown that the relative liver weight was elevated in offspring of rats with combined HSFD in 1.7 times and in group with HFD in 1.6 versus control, while relative mesenteric and visceral fat pad weight were in twice elevated in groups with high-carbohydrate and combined high-carbohydrate and high-fat diet versus control that demonstrated that excessive intake of fat is primary driver of increased weight of liver, but excessive intake of carbohydrate has been implicated in increased weight of fat pad weight. Also we had observed different elevation of liver damage histological scores and worsen vascular changes in liver structural functional reorganization dominated in rats, who were exposed in prenatal period to excessive overload of carbohydrates (Fig. 4b and 6), while combined parenchymal and vascular changes were presented in offspring of mothers from group fed with combined HSFD (Fig. 5a and 6). It has been proposed that excessive uptake of glucose and fatty acids initiate a shift in pro- and anti-inflammatory and redox systems, characterized by cellular membrane injury by free radicals, as well as rised circulating proteolytic enzymes that induced increased expression of protease-activated receptors 2 (PAR2) (45). These changes are attributed to transformation of the normal liver organization into steatosis, hepatocellular ballooning, and lobular inflammation, key histologic components of NASH, the disease, which can remain asymptomatic for years, or progress to cirrhosis and hepatocellular carcinoma (46).
Recent studies have suggested that acute inflammation is a primary driver of impaired liver structure, with subsequent loss of "barrier function" resulting in abnormal metabolic and defense reactions with dramatically increased expression of pro-inflammatory mediators (7, 47). Similar findings from Duval et al., pointed on significant role of adipokines and demonstrated elevation of leptin and adipose tissue-derived IL-1β in C57Bl/6 mice fed with HFD that links adipose tissue dysfunction and NASH (48). Our results also demonstrated 3-fold rise of leptin and 1.5 fold fall of adiponectin with sharply increased levels of IL-1β and IL-8 in offspring from rats fed with HSFD.
Similar results were obtained in the offspring from rat kept on HSD. This suggests that the alteration of cytokine levels in rats that were exposed to stress and nutritional insults during prenatal period was likely a systemic effect contributing to the observed changes in liver and fat inflammation, and injury related to excessive glucose uptake. However, the relative index of leptin/adiponectin ratio was more elevated (3 versus 2.5 times) in offspring with HSFD diet, comparatively to HSD, and correlated with histological data of liver damage with alteration and combined hyperplastic and hypertrophic type of transformation fat tissue.
We conclude that maternal stress and unbalanced nutrition contribute significantly to regulation of liver defense and inflammation. Changes in fat tissue in offsprings (validated by histological scores grading), marked increase in the leptin/adiponectin index, increased production of pro-inflammatory mediators (IL-1β and IL-8) lead to exacerbation of liver damage. This may play a key role in the impairment of liver functions and increasing of relative liver and visceral fat weight noted in rats exposed to intra-uterine stress and unbalanced diet. The marked induction of fat tissue damage and inflammation by prenatal stress and nutritional insult by HFD or HSD alone or in their combination suggest that these external environmental factors could be implicated as critical risk factors in development of NAFLD in adulthood. Leptin/adiponectin index may be a novel, non-invasive and more sensitive biomarker for liver alteration and inflammation comparatively to separate values of those adipokines levels.
Perspectives and study limitations
These and other results (49-51) suggest that fetal exposure to excess of maternal glucocorticoids and maternal nutritional status may reprogram expression of the pro- and anti-inflammatory adipokines. There is substantial potential for nutritional interventions during prenatal period. Nutritional interventions during prenatal period potentially could be added to preventive programs for NAFLD. This study has several limitations: we did not conduct antropometric measurements of female rats during pregnancy and we did not assess glucocorticoids and catecholamines plasma levels. Also, we did not study offsprings from female rats exposed to stress but having standart diet. Future investigations should consider these issues to provide further insight into the studied mechanisms and variables.
Conflict of interests: None declared.
REFERENCES
- Lappalainen T. Obesity, low-grade inflammation and cardiovascular diseases - special emphasis on fat mass and obesity associated (FTO) and serum amyloid A (SAA) Genes. Publications of the University of Eastern Finland. Dissertations in Health Sciences, Kuopio. 2010.
- Duvnjak L., Duvnjak M. The metabolic syndrome - an ongoing story. J Physiol Pharmacol 2009; 60 (Suppl. 7): 19-24.
- Konturek PC, Konturek SJ, Brzozowski T, Hahn EG. Gastroprotection and control of food intake by leptin. Comparison with cholecystokinin and prostaglandins. J Physiol Pharmacol 1999; 50: 39-48.
- Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne) 2012; 3: 181.
- Krawczynska A, Olczak E, Rembiszewska A, Herman AP, Gromadzka-Ostrowska J. Time-dependent supplementation of vitamin E influences leptin expression in the aortic layers of rats fed atherogenic diet. J Physiol Pharmacol 2014; 65: 33-39.
- Zelber-Sagi S, Ratziu V, Zvibel I, et al. The association between adipocytokines and biomarkers for nonalcoholic fatty liver disease-induced liver injury: a study in the general population. Eur J Gastroenterol Hepatol 2012; 24: 262-269.
- Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 2010; 298: G634-G642.
- Farell GC, van Rooyen D, Gan L, Chitturi S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 2012; 6: 149-171.
- Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol 2010; 105: 1567-1573.
- Vos MB, Welsh J. Prevalence of suspected NAFLD is increasing among U.S. adolescents. Gastroenterology 2012; 5 (Suppl. 1) abstract 705.
- Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of prepregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr 2011; 158: 221-226.
- Nicoletto SF, Rinaldi A. In the womb's shadow: the theory of prenatal programming as the fetal origin of various adult diseases is increasingly supported by a wealth of evidence. EMBO Rep 2011; 12: 30-34.
- Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of "metabolic memory". Exp Diabetes Res 2011; 2011: 218598.
- Tarry-Adkins LJ, Ozanne ES. Mechanisms of early life programming: current knowledge and future directions. Am J Clin Nutr 2011; 94 (Suppl. 6): 1765S-1771S.
- Nivoit P, Morens C, Van Assche FA, et al. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009; 52: 1133-1142.
- Method of induction the hepatocellular reorganization in offspring of experimental animals at the term of prenatal programming. Patent No 81947. UA, G09B 23/28, G01N 33/48. No request u 2013 02294 (25.02.2013). Published 10.07.2013, Bul. No 13, 2013. / Co-authors: L. Bezpalko, O. Zayachkivska, M. Gzhegotsky.
- Li YY. Genetic and epigenetic variants influencing the development of nonalcoholic fatty liver disease. World J Gastroenterol 2012; 18: 6546-6551.
- Kotsumbas IY. Preclinical Researches of Medications in Veterinary Science. Lviv, Triada Plus, 2006.
- Pratt NC, Lisk RD. Effects of social stress during early pregnancy on litter size and sex ratio in the golden hamster (Mesocricetusauratus). J Reprod Fertil 1989; 87: 763-769.
- Kozar VV, Kudria MY, Ustenko NV, Pavlenko TO, Zhurakovs'ka MV. The state of the humoral component of immunity under condition of metabolic syndrome with underlying hypoestrogenia and farmacological correction. Buk Med Herald 2009; 13: 141-144.
- Verhulst PJ, Lintermans A, Janssen S, et al. GPR39, a receptor of the ghrelin receptor family, plays a role in the regulation of glucose homeostasis in a mouse model of early onset diet-induced obesity. J Neuroendocrinol 2011; 23: 490-500.
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313-1321.
- Bezpalko L, Gavrylyuk O, Zayachkivska O, Gzhegotskiy M. Role of metabolic stress in process of cellular reorganization of liver (experimental research). Tavricheskiy Medicko-Biologicheskiy Vestnik 2012; 1: 301-302.
- Nov O, Shapiro H, Ovadia H, et al. Interleukin-1 regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS One 2013; 8: e53626.
- Sawyer FS. Analysis of variance: the fundamental concepts. J Manual Manipulative Ther 2009; 17: E27-E38.
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012; 56: 952-964.
- Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J 2012; 59: 849-857.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274-285.
- Gu JJ, Gao FY, Zhao TY. A preliminary investigation of the mechanisms underlying the effect of berberine in preventing high-fat diet-induced insulin resistance in rats. J Physiol Pharmacol 2012; 63: 505-513.
- Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012; 61: 346-354.
- Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008; 29: 274-281.
- Sarr O, Yang K, Regnault TR. In utero programming of later adiposity: the role of fetal growth restriction. J Pregnancy 2012; 2012: 134758.
- Rius B, Lopez-Vicario C, Gonzalez-Periz G, et al. Resolution of inflammation in obesity-induced liver disease. Front Immunol 2012; 3: 257.
- Freeman DJ. Effects of maternal obesity on fetal growth and body composition: implications for programming and future health. Semin Fetal Neonatal Med 2010; 15: 113-118.
- Vuguin PM, Hartil K, Kruse M, et al. Shared effects of genetic and intrauterine and perinatal environment on the development of metabolic syndrome. PLoS One 2013; 8: e06302.
- Fall CH. Evidence for the intra-uterine programming of adiposity in later life. Ann Hum Biol 2011; 38: 410-428.
- Hyatt MA, Gardner S, Sebert S, et al. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 2011; 141: 119-126.
- Stanton MC, Chen S-C, Jackson JV, et al. Inflammatory signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J Inflamm (Lond) 2011; 8: 8. doi: 10.1186/1476-9255-8-8.
- Touwslager RN, Gielen M, Mulder AL, et al. Changes in genetic and environmental effects on growth during infancy. Am J Clin Nutr 2011; 94: 1568-1574.
- Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the etiology of obesity. Mol Cell Endocrinol 2009; 304: 90-96.
- Benklaft NB, Merzouk H, Bouanane S, et al. Altered adipose tissue metabolism in offspring of dietary obese rat dams. Clin Sci 2011; 121: 19-28.
- Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes 2008; 32 (Suppl. 7): S62-S71.
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001; 60: 5-20.
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Eng J Med 2008; 359: 61-73.
- Gotoh K, Inoue M, Masaki T. A novel anti-inflammatory role for spleen-derived interleukin-10 in obesity induced inflammation in white adipose tissue and liver. Diabetes 2012; 61: 1994-2003.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274-285.
- Kondro M, Gzhegotsky M, Zayachkivska O, Gavryluk O. Analyses activity system NO/NOS by result of specifics in liver structural and functional changes under the conditions of individual and complex persistent effect of lead acetate in various dosages and stress. Pract Med 2008; 1 (Suppl. 14): 82-92.
- Duval C, Thissen U, Keshtkar S, et al. Adipose tissue disfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57Bl/6 Mice. Diabetes 2010; 59: 3181-3191.
- Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 2006; 88: 234-243.
- Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Reg Integr Comp Physiol 2010; 299: R711-R722.
- McCurdy CE, Bishop JM, William SM, et al. A Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009; 119: 323-335.
A c c e p t e d : November 15, 2014