CHANGES IN INTRACELLULAR CALCIUM CONCENTRATION INFLUENCE
BEAT-TO-BEAT VARIABILITY OF ACTION POTENTIAL DURATION
IN CANINE VENTRICULAR MYOCYTES
of Dentistry, University of Debrecen, Debrecen, Hungary; 3Division of Sport Physiology, Department of Physiology, Faculty of Medicine,
University of Debrecen, Debrecen, Hungary
INTRODUCTION
Beat-to-beat variability of action potential duration (also called short term variability, SV) is an intrinsic property of various in vivo and in vitro mammalian cardiac preparations including the human heart (1-3). Although SV is considered one of the best proarrhythmic predictors (4-6), its exact mechanism is still not fully understood. Involvement of several factors, such as stochastic gating of various ion channels (7, 8), intensity of cell-to-cell coupling (9), action potential morphology (10) or stimulation frequency (11), in modulation of SV have already been implicated. Since many cardiac ion currents are influenced by intracellular calcium concentration ([Ca2+]i), we decided to investigate the effects of [Ca2+]i changes on SV in the present study under two experimental conditions: (1) [Ca2+]i was manipulated in an unspecific manner using Ca2+ ionophore and Ca2+ chelator agents, and (2) the role of SR-mediated [Ca2+]i release was tested using ryanodine and cyclopiazonic acid - both are known to diminish [Ca2+]i release from the SR. All the experiments were performed in canine ventricular myocytes, because this preparation is believed to resemble human ventricular cells regarding their action potential morphology and kinetics of the underlying ion currents (12, 13), and also due to the significant amount of experimental data on SV accumulated already in dogs. The results indicate that relative SV is increased by elevation of [Ca2+]i, where contribution of the SR-related [Ca2+]i release seems to be dominant. This finding may help better understanding the mechanisms of [Ca2+]i-related arrhythmogenesis.
MATERIALS AND METHODS
Isolation of single canine ventricular myocytes
Adult mongrel dogs of either sex were anaesthetized with intramuscular injections of 10 mg/kg ketamine hydrochloride (Calypsol, Richter Gedeon, Hungary) + 1 mg/kg xylazine hydrochloride (Sedaxylan, Eurovet Animal Health BV, The Netherlands) according to protocols approved by the local ethical committee (license No: 18/2012/DEMAB) in line with the ethical standards laid down in the Declaration of Helsinki in 1964 and its later amendments. The hearts were quickly removed and placed in Tyrode solution. Single myocytes were obtained by enzymatic dispersion using the segment perfusion technique, as described previously (14). Briefly, a wedge-shaped section of the left ventricular wall supplied by the left anterior descending coronary artery was dissected, cannulated and perfused with oxygenized Tyrode solution. After removal of blood the perfusion was switched to a nominally Ca2+-free Joklik solution (Minimum Essential Medium Eagle, Joklik Modification, Sigma) for 5 min. This was followed by 30 min perfusion with Joklik solution supplemented with 1 mg/ml collagenase (Type II. Worthington, Chemical Co.) and 0.2 % bovine serum albumin (Fraction V., Sigma) containing 50 µM Ca2+. After gradually restoring the normal external Ca2+ concentration, the cells were stored in Minimum Essential Medium Eagle until use. Drugs were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) except ryanodine, which was purchased from Cayman Chemical Company (Michigan, USA).
Recording of action potentials
All electrophysiological measurements were performed at 37°C. The rod-shaped viable cells showing clear striation were sedimented in a plexiglass chamber of 1 ml volume allowing continuous superfusion (at a rate of 2 ml/min) with modified Krebs solution gassed with a mixture of 95% O2 and 5% CO2 at pH = 7.4. The modified Krebs solution contained (in mM): NaCl, 128.3; NaHCO3, 21.4; KCl, 4.0; CaCl2, 1.8; MgCl2, 0.42; and glucose 10. Transmembrane potentials were recorded using 3 M KCl filled sharp glass microelectrodes having tip resistance between 20 and 40 MΩ. These electrodes were connected to the input of Multiclamp 700A, 700B or Axoclamp-2B amplifiers (Molecular Devices, Sunnyvale, CA, USA). The cells were paced through the recording electrode at steady cycle length of 1 s using 1–2 ms wide rectangular current pulses having amplitudes of twice the diastolic threshold. Since the cytosol was not dialyzed, time dependent changes in action potential morphology were negligible for the period of our experimental protocol lasting typically not longer than 25 min. Action potentials were digitized (at 200 kHz using Digidata 1322A, 1440A and 1200 A/D card, purchased from Axon Instruments Inc., Foster City, CA, USA) and stored for later analysis. Series of 50 consecutive action potentials were analyzed to estimate SV according to the following formula:
SV = Σ (|APDn+1 - APDn|) / [nbeats * √2]
where SV is short term variability, APDn and APDn+1 indicate the durations of the nth and n+1th APs, respectively, at 90% level of repolarization and nbeats denotes the number of consecutive beats analyzed (11, 15). Changes in SV were typically presented as Poincare plots where 50 consecutive APD values are plotted, each against the duration of the previous action potential. Here is to be mentioned that all action potentials analyzed in the present study were regularly paced action potentials, records taken in myocytes displaying early or delayed afterdepolarizations were excluded from evaluation.
Recording of cell shortening
Isolated ventricular cardiomyocytes were placed in a 1 ml volume experimental chamber on the stage of an inverted microscope and superfused with Krebs solution. Cells were field stimulated at a steady-state stimulation rate of 1 Hz using suprathreshold square wave pulses generated by an electronic stimulator (DS-R3, Fonixcomp Ltd, Hungary) through a pair of platinum wires. Cell shortening was measured using a video-edge detector system (VED-105, Crescent Electronics, Sandy, Utah, USA) sampling at 240 Hz. Cell shortening was expressed as a percent of initial diastolic cell length. The analogue signal was amplified (DC amplifier, Fonixcomp Ltd, Hungary), digitized (Digidata 1440A, Molecular Devices) and recorded with pClamp 10 software (Molecular Devices). During subsequent off-line analysis, 10 consecutive curves were averaged and this average was analyzed in every minute before and after drug application (except during the first 5 min of drug superfusion, when this was done twice per minute).
Statistical analysis
Results are expressed as mean ± S.E.M. values. The number of cells / obtained from the number of animals are presented as n. Statistical significance of differences was evaluated using one-way ANOVA followed by Student’s t-test. Differences were considered significant when P was less than 0.05.
RESULTS
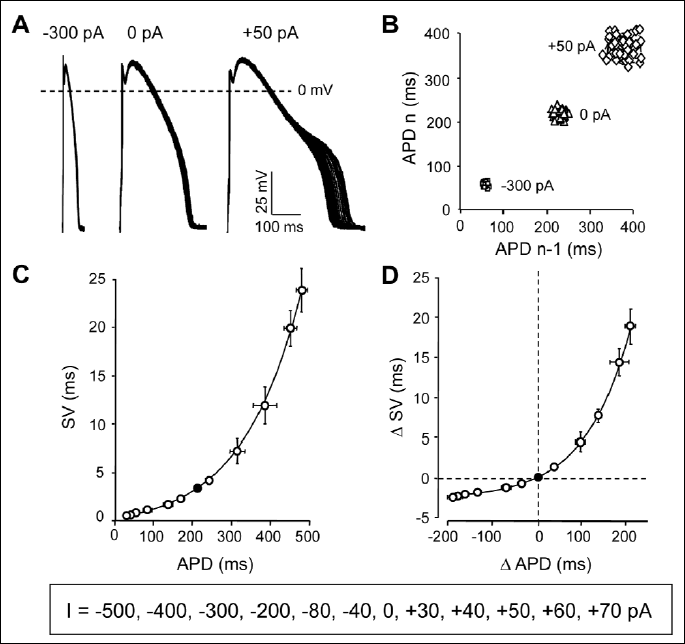
The short term variability-action potential duration (SV-APD) relationship
According to previous results, the beat-to-beat variability of APD is strongly influenced by the baseline value of APD itself (10, 15). As a consequence, the effect of any kind of interventions on SV has to be assessed only as a change in relative SV, i.e. by comparing the observed changes in SV to the concomitant changes in APD. To obtain this baseline SV-APD relationship, outward and inward current pulses were injected in current clamp mode throughout the full repolarization phase of the action potential. Using these current pulses of various amplitudes (ranging from –500 to +70 pA) allowed the modification of APD within a reasonably wide range (between 20 and 500 ms) in a way not related to specific interaction with one or another specific ion current. The results are presented in Fig. 1, where SV obtained with any given current pulse was plotted against the corresponding APD. Similarly, the current-induced changes in SV and APD (ΔSV and ΔAPD, respectively) were also plotted, and the data were fitted to an exponential function yielding the solid curves in Fig. 1C and 1D (the equations and the estimated parameters will be discussed later in Fig. 4). This approach allows to study the effect of any drug or intervention on relative SV directly, since a data point above the curve is a marker of an increased, while below the curve of a reduced relative SV.
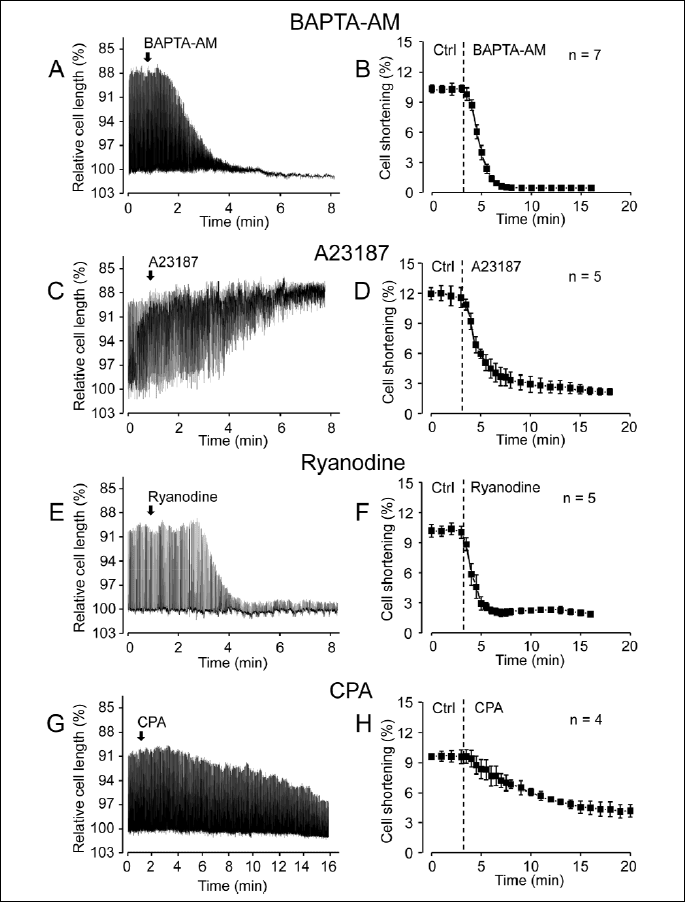
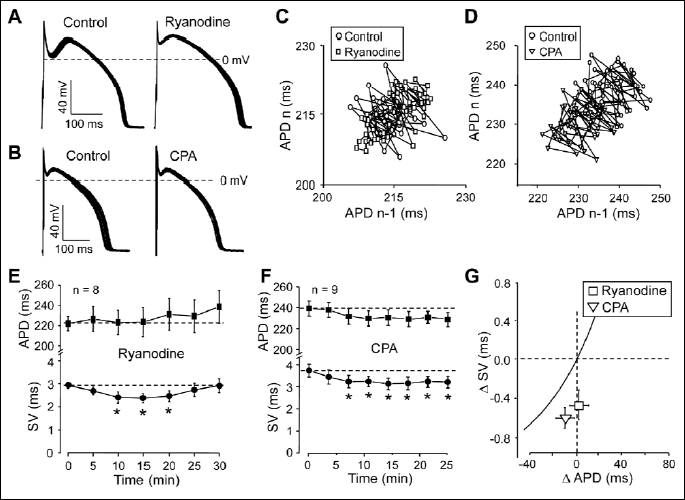
Effects of BAPTA-AM, A23187, ryanodine and cyclopiazonic acid on cell shortening
Since in the following sections the effects of BAPTA-AM, A23187, ryanodine and cyclopiazonic acid on relative SV are studied, the effects of these agents on unloaded cell shortening, considered as an indicator of [Ca2+]i changes, had to be first monitored. Drugs were superfused after reaching steady-state amplitudes of shortening. According to Fig. 2, cells shortening was suppressed by all of the drugs studied. While in the case of BAPTA-AM, ryanodine and cyclopiazonic acid the decreased cell length approximated the diastolic level, indicating a suppressed calcium release, the enveloping curve of the cell shortening was close to the systolic level in the presence of A23187. This is in line with the calcium accumulation expected in the presence of a calcium ionophore.
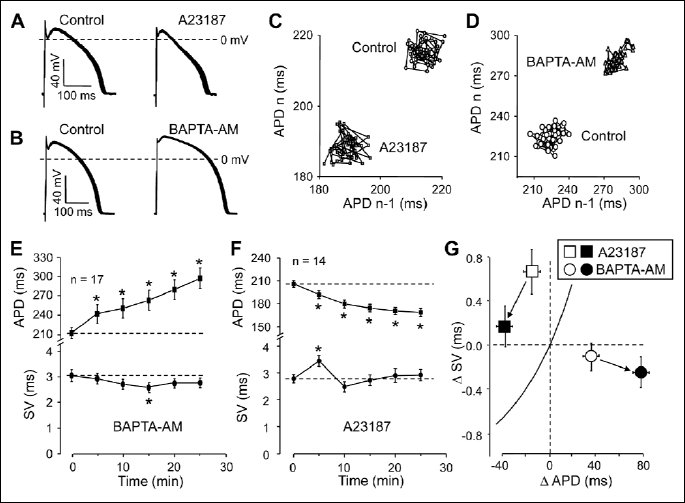
Effects of unspecific changes in [Ca2+]i on relative short term variability
Unspecific elevation and reduction of [Ca2+]i was based on the simple assumption that if the cell is loaded directly with Ca2+, it must increase [Ca2+]i, and conversely, [Ca2+]i must be reduced by an intracellularly applied Ca2+ chelator. Therefore, the cells were exposed to the Ca2+ ionophore A23187 (1 µM for 25 min) or they were loaded with the cell-permeant acetoxy-methylesther form of the Ca2+ chelator BAPTA (5 µM BAPTA-AM was applied for 25 min). As demonstrated in Fig. 3A, 3C, 3F and 3G, 1 µM A23187 significantly increased relative SV (shortening of APD with unchanged SV). Interestingly, an absolute increase in SV was also observed at the beginning of superfusion with A23187. In contrast, loading the cells with BAPTA-AM caused a significant reduction in relative SV primarily due to the progressive lengthening of APD as more and more intracellular BAPTA was released by the esterases (Fig. 3B, 3D, 3E and 3G). Reduction of SV was not significant statistically - except for the 15th min superfusion with BAPTA-AM, where the decrease in SV was significant. In summary, relative SVwas increased by elevation of cytosolic Ca2+ concentration.
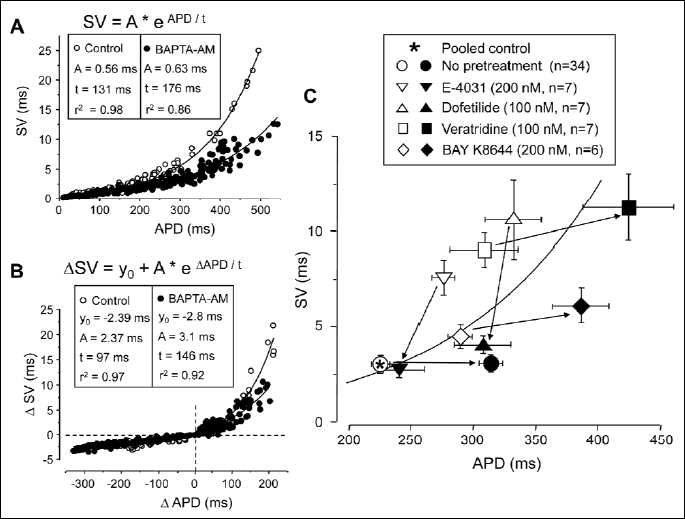
The effect of BAPTA-AM was further investigated by combining current injections with BAPTA-AM pretreatment. In these experiments action potentials were prolonged or shortened with the application of appropriately tailored depolarizing or hyperpolarizing pulses, respectively. Thus the SV-APD relationship could be determined in the presence of BAPTA-AM and compared to that obtained under control conditions. According to the results presented in Fig. 4A and 4B, the time constant (t values) obtained by fitting the individual data points to a monoexponential function were much longer in BAPTA-AM than in control, indicating that relative SV is reduced by BAPTA-AM at any APD value, however, this reduction was greater and more prominent at longer APDs.
The effect of BAPTA-AM on relative SV was studied also when APD was pharmacologically lengthened (Fig. 4C). The rapid delayed rectifier K+ current (IKr) was suppressed by 100 nM dofetilide or 200 nM E-4031 (16), while 100 nM veratridine and 200 nM BAY K8644 were used to increase late Na+ current (INa-late) and L-type Ca2+ current (ICa), respectively (17, 18). Although the effects of BAPTA-AM superfusion were different when applying after IKr-blockade and following enhancement of inward currents, the reduction of relative SV following the chelation of [Ca2+]i by BAPTA-AM was a common observation in these studies - independently of the actual experimental conditions.
Contribution of sarcoplasmic reticular Ca2+ release
Contribution of transient changes of [Ca2+]i due to Ca2+ released from the sarcoplasmic reticulum was studied using ryanodine and cyclopiazonic acid (Fig. 5). The former blocks Ca2+ release from the SR at 10 µM concentration by decreasing the open probability of the Ca2+ release channel in the SR membrane (19). Cyclopiazonic acid is a selective inhibitor of the SR Ca2+ pump resulting in a depletion of the SR Ca2+ release pool (20, 21). As shown in Fig. 5G, application of these agents resulted in a comparable reduction of relative SV. Ryanodine caused a transient decrease in SV with no significant change in APD (Fig. 5A, 5C and 5E), while 1 µM cyclopiazonic acid decreased SV significantly, accompanied with a small, statistically not significant reduction of APD (Fig. 5B, 5D and 5F). These results clearly indicate that systolic Ca2+ release, experienced by the surface membrane, is a relevant signal for SV modulation.
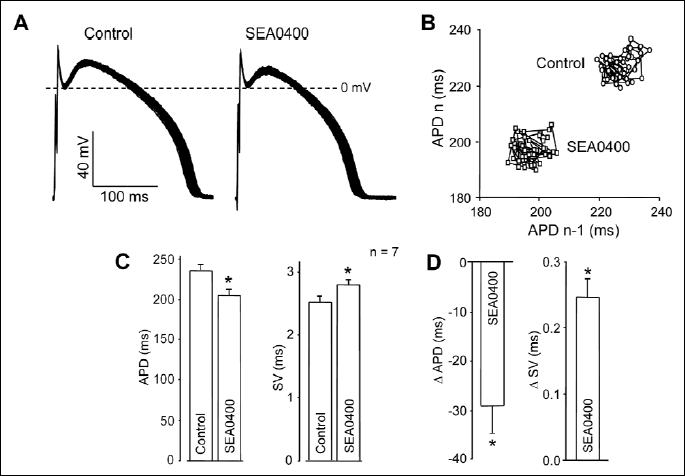
Role of the Na+-Ca2+ exchange
The influence of the Na+-Ca2+ exchanger (NCX) on SV was studied after inhibition of the exchanger by SEA0400. This agent is thought to be a selective blocker of NCX, when applied at a sufficiently low concentration of 300 nM (22). Exposure of the cells to 300 nM SEA0400 for 25 min increased SV and decreased APD significantly, clearly resulting in a marked enhancement of relative SV (Fig. 6).
DISCUSSION
In the present study we have shown that relative SV was increased by elevation of [Ca2+]i and decreased by its reduction. This was true practically independently of the actual experimental conditions. Best demonstration of this finding is in Fig. 4C, where chelation of [Ca2+]i by BAPTA-AM decreased relative SV in all cases. This reduction in relative SV was mainly due to an actual decrease in SV when BAPTA-AM was applied following IKr blockade by dofetilide or E-4031. Under these conditions APD was shortened by BAPTA-AM. On the other hand, when APD was lengthened by veratridine or BAY K8644 (due to enhancement of INa-late and ICa, respectively), as well as under control conditions, relative SV was decreased by BAPTA-AM due to the marked prolongation of APD. The BAPTA-AM induced prolongation was likely the consequence of a direct blocking effect of BAPTA-AM on IKr (23). Although the underlying ionic mechanism was basically different under these conditions (i.e. the set of operating Ca2+-sensitive ion channels were likely different), reduction of [Ca2+]i decreased relative SV uniformly.
More importantly, the contribution of the SR-related [Ca2+]i release seems to be a dominant factor in Ca2+-sensitive modulation of SV. Ryanodine, the blocker of the SR Ca2+ release channel (19) decreased SV directly. Cyclopiazonic acid, the selective inhibitor of the SERCA Ca2+ pump resulting in a depletion of the SR Ca2+ release pool (20, 21) caused similar effect. Both agents are known to decrease the amplitude of the intracellular calcium transient in cardiac cells (24, 25), and in line with these results, they decreased unloaded cell shortening in our experiments. Although the time-dependent pattern of changes in SV and APD were different, the resultant reduction in relative SV was similar with both drugs. The biphasic effect of A23187 on SV suggests that probably more than one mechanism is involved in the effect of [Ca2+]i on SV (26). These observations are in accordance with the results of Johnson et al. (27) demonstrating the increment of SV during diastolic spontaneous [Ca2+]i release induced by β-adrenergic stimulation. The important role of [Ca2+]i transients in modulation of SV supports the view that it is manifested in fact by the Ca2+-sensitive ion channels in the surface membrane, which are known to directly experience [Ca2+]i changes in the narrow submembrane compartment, also known as fuzzy space.
In line with this approach, SEA0400, the potent inhibitor of NCX strongly increased relative SV.NCX is believed to be the main mechanism of Ca2+ extrusion from the intracellular space in cardiac cells (28). Suppression of this component of Ca2+ elimination is expected to increase [Ca2+]i beneath the sarcolemma, which effect was well documented in rat myocytes (29). Although no significant increase in [Ca2+]i could be detected in canine cells on exposure to SEA0400 when measuring [Ca2+]i in the bulk phase of the cytosol (30), it is likely that suppression of NCX results in an elevated [Ca2+]i in the submembrane compartment of canine myocytes as well. Indeed, selective suppression of NCX by SEA0400 increased SV while APD was reduced. Both changes were likely consequences of the elevated submembrane Ca2+ concentration. It is worthy of note that under markedly different experimental conditions (i.e. after prolongation of APD and development of early afterdepolarizations by dofetilide in hypertrophied remodeled cells) 1 µM SEA0400 decreased the short term variability of APD and suppressed early afterdepolarizations, while the elevated amplitude of [Ca2+]i transient was also decreased by SEA0400 (31). Although the effects of NCX inhibitors on [Ca2+]i are strongly dependent on experimental conditions (32), reduction of [Ca2+]i released from the SR was accompanied by a decreased SV, supporting further our conclusions.
Regarding the underlying mechanisms (i.e. which Ca2+ sensitive ion current may be responsible for the Ca2+ sensitivity of SV), it has been shown that there are 3 ion currents in canine myocytes which may have a larger impact on beat-to-beat variability, these are: late INa, IKr and ICa (10, 15). Although activation of INa or suppression of IKr were reported to increase SV drastically (10, 15), these alterations cannot be easily deduced from elevation of [Ca2+]i.The third current is ICa, which is suppressed in amplitude by high [Ca2+]i due to its Ca2+ dependent inactivation (33). Indeed, relative SV was reduced by BAY K8644 and increased by nisoldipine, indicating that ICa is an important regulator of SV under normal conditions (15) as well as in Ca2+ overloaded cells (27). In conclusion, the major effect of [Ca2+]i on SV is likely to occur via reduction of ICa by enhancing its Ca2+ dependent inactivation, however, minor contribution of other Ca2+ sensitive ion currents, such as INCX, ICl and a variety of Ca2+ sensitive K+ currents, cannot fully be ruled out.
It is generally accepted that elevation of [Ca2+]i is highly proarrhythmic (34). High [Ca2+]i -induced arrhythmias are generated mainly by two mechanisms: elevation of [Ca2+]i causes uncoupling of myocytes due to closure of gap junctions, resulting in increased longitudinal resistance, slower conduction, and ultimately re-entry. The second - not less important - mechanism is development of delayed afterdepolarizations, mediated by Ca2+-overloaded SR. Based on the present results a third potential Ca2+ dependent proarrhythmic mechanism has to be considered: the increased beat-to-beat variability of APD. Further studies are required to assess the actual significance of the increased beat-to-beat variability, induced by high [Ca2+]i, in arrhythmogenesis.
Acknowledgements: Financial support was provided by grants from the Hungarian Scientific Research Fund (OTKA-K100151, OTKA-K109736, OTKA-K101196, OTKA-PD101171 and OTKA-NK104331). Further support was obtained from the Hungarian Government and the European Community (TAMOP-4.2.2.A-11/1/KONV-2012-0045). Research of KK and BH was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TAMOP-4.2.4.A/2-11/1-2012-0001 ‘National Excellence Program’. The authors thank Miss Eva Sagi for excellent technical assistance.
Conflict of interests: None declared.
REFERENCES
- Hinterseer M, Beckmann BM, Thomsen MB, et al. Relation of increased short-term variability of QT interval to congenital long-QT syndrome. Am J Cardiol 2009; 103: 1244-1248.
- Hinterseer M, Beckmann BM, Thomsen MB, et al. Usefulness of short-term variability of QT intervals as a predictor for electrical remodeling and proarrhythmia in patients with nonischemic heart failure. Am J Cardiol 2010; 106: 216-220.
- Tereshchenko LG, Han L, Cheng A, et al. Beat-to-beat three-dimensional ECG variability predicts ventricular arrhythmia in ICD recipients. Heart Rhythm 2010; 7: 1606-1613.
- Abi-Gerges N, Valentin JP, Pollard CE. Dog left ventricular midmyocardial myocytes for assessment of drug-induced delayed repolarization: short-term variability and proarrhythmic potential. Br J Pharmacol 2010; 159: 77-92.
- Jacobson I, Carlsson L, Duker G. Beat-by-beat QT interval variability, but not QT prolongation per se, predicts drug-induced torsades de pointes in the anaesthetised methoxamine-sensitized rabbit. J Pharmacol Toxicol Methods 2011; 63: 40-46.
- Thomsen MB, Verduyn SC, Stengl M, et al.Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation 2004; 110: 2453-2459.
- Lemay M, de Lange E, Kucera JP. Effects of stochastic channel gating and distribution on the cardiac action potential. J Theor Biol 2011; 281: 84-96.
- Pueyo E, Corrias A, Virag L, et al. A multiscale investigation of repolarization variability and its role in cardiac arrhythmogenesis. Biophys J 2011; 101: 2892-2902.
- Zaniboni M, Pollard AE, Yang L, Spitzer KW. Beat-to-beat repolarization variability in ventricular myocytes and its suppression by electrical coupling. Am J Physiol Heart Circ Physiol 2000; 278: H677-H687.
- Heijman J, Zaza A, Johnson DM, et al. Determinants of beat-to-beat variability of repolarization duration in the canine ventricular myocyte: a computational analysis. PLOS Comput Biol 2013; 9: e1003202.
- Johnson DM, Heijman J, Pollard CE, et al. IKs restricts excessive beat-to-beat variability of repolarization during beta-adrenergic receptor stimulation. J Mol Cell Cardiol 2010; 48: 122-130.
- Szabo G, Szentandrassy N, Biro T, et al. Asymmetrical distribution of ion channels in canine and human left ventricular wall: epicardium versus midmyocardium. Pflugers Arch 2005; 450: 307-316.
- Szentandrassy N, Banyasz T, Biro T, et al. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res 2005; 65: 851-860.
- Banyasz T, Magyar J, Szentandrassy N, et al. Action potential clamp fingerprints of K+ currents in canine cardiomyocytes: their role in ventricular repolarization. Acta Physiol Scand 2007; 190: 189-198.
- Szentandrassy N, Kistamas K, Hegyi B, et al. Contribution of ion currents to beat-to-beat variability of action potential duration in canine ventricular myocytes. Pflugers Arch 2014; Aug 2. Epub ahead to print. doi: 10.1007/s00424-014-1581-4.
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 1990; 96: 195-215.
- Ulbricht W. Effects of veratridine on sodium currents and fluxes. Rev Physiol Biochem Pharmacol 1998; 133: 1-54.
- Sanguinetti MC, Krafte DS, Kass RS. Voltage-dependent modulation of Ca++ channel current in heart cells by BAY K8644. J Gen Physiol 1986; 88: 369-392.
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem 1986; 261: 6300-6306.
- Yard NJ, Chiesi M, Ball HA. Effect of cyclopiazonic acid, an inhibitor of sarcoplasmic reticulum Ca2+-ATPase, on the frequency-dependence of the contraction-relaxation cycle of the guinea-pig isolated atrium. Br J Pharmacol 1994; 113: 1001-1007.
- Takahashi S, Kato Y, Adachi M, Agata N, Tanaka H, Shigenobu K. Effects of cyclopiazonic acid on rat myocardium: inhibition of calcium uptake into sarcoplasmic reticulum. J Pharmacol Exp Ther 1995; 272: 1095-1100.
- Birinyi P, Acsai K, Banyasz T, et al. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedeberg’s Arch Pharmacol 2005; 372: 63-70.
- Tang Q, Jin MW, Xiang JZ, et al. The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go-related gene potassium channels stably expressed in HEK 293 cells. Biochem Pharmacol 2007; 74: 1596-1607.
- Weisser-Thomas J, Piacentino V, Gaughan JP, Margulies K, Houser SR. Calcium entry via Na/Ca exchange during the action potential directly contributes to contraction of failing human ventricular myocytes. Cardiovasc Res 2003; 57: 974-985.
- Zahanich I, Sirenko SG, Maltseva LA, et al. Rhythmic beating of stem cell-derived cardiac cells requires dynamic coupling of electrophysiology and Ca cycling. J Mol Cell Cardiol 2011; 50: 66-76.
- Bebarova M, Matejovic P, Pasek M, Simurdova M, Simurda J. Dual effect of ethanol on inward rectifier potassium current IK1 in rat ventricular myocytes. J Physiol Pharmacol 2014; 65: 497-509.
- Johnson DM, Heijman J, Bode EF, et al. Diastolic spontaneous calcium release from the sarcoplasmic reticulum increases beat-to-beat variability of repolarization in canine ventricular myocytes after β-adrenergic stimulation. Circ Res 2013; 112: 246-256.
- Ginsburg KS, Weber CR, Bers DM. Cardiac Na+Ca2+ exchanger: dynamics of Ca2+-dependent activation and deactivation in intact myocytes. J Physiol (Lond) 2013; 591: 2067-2086.
- Acsai K, Kun A, Farkas AS, et al. Effect of partial blockade of the Na+/Ca2+ exchanger by SEA0400 on Ca2+ handling in isolated rat ventricular myocytes. Eur J Pharmacol 2007; 576: 1-6.
- Birinyi P, Toth A, Jona I, et al. The Na+/Ca2+ exchange blocker SEA0400 fails to enhance cytosolic Ca2+ transient and contractility in canine ventricular cardiomyocytes. Cardiovasc Res 2008; 78: 476-484.
- Bourgonje VJ, Vos MA, Ozdemir S, et al. Combined Na+/Ca2+ exchanger and L-type calcium channel block as a potential strategy to suppress arrhythmias and maintain ventricular function. Circ Arrhythm Electrophysiol 2013; 6: 371-379.
- Mackiewicz U, Lewartowski B. The effect of sarcoplasmic reticulum Ca2+ leak on contractile activity of guinea pig heart myocytes depends in activity of sarcoplasmic reticulum Ca2+-ATPase and Na+/Ca2+ exchanger. J Physiol Pharmacol 2008; 59: 287-300.
- Szentandrassy N, Papp F, Hegyi B, Bartok A, Krasznai Z, Nanasi PP. Tetrodotoxin blocks native cardiac L-type calcium channels but not CaV1.2 channels expressed in HEK cells. J Physiol Pharmacol 2013; 64: 807-810.
- Clusin WT. Calcium and cardiac arrhythmias: DADs, EADs, and alternans.Crit Rev Clin Lab Sci 2003; 40: 337-375.
A c c e p t e d : January 16, 2015