ACUTE ANTI-FIBRILLATING AND DEFIBRILLATING POTENTIAL
OF ATORVASTATIN, MELATONIN, EICOSAPENTAENOIC ACID AND DOCOSAHEXAENOIC ACID DEMONSTRATED IN ISOLATED HEART MODEL
INTRODUCTION
It has been established that rapid spreading of the electrical impulse throughout the heart is essential for synchronized contraction and this is ensured by electrical coupling of the cardiomyocytes at the intercellular gap junction connexin (Cx) channels, predominantly Cx43 (1). In turn, an abnormal myocardial Cx43 distribution, expression and/or phosphorylation contribute to the development of "arrhythmogenic substrate" resulting in myocardial electric disturbances, such as slowing of conduction, dispersion of cell-cell-uncoupling and repolarisation (2-5). Consequently cardiovascular disease related arrhythmogenic substrate promote occurrence of malignant arrhythmias and sudden cardiac death (SCD) due to ventricular fibrillation (VF).
Despite the era of sophisticated devices, such as an implanted cardioverter defibrillator (ICD) to prevent SCD when VF occurs, there is still need to look for novel approaches aimed to prevent development of VF (6). In agreement with this concept the "up-stream" therapy is of interest, i.e. prevention and/or attenuation of "arrhythmogenic substrate" in diseased heart by non-antiarrhythmic drugs and/or cardioprotective compounds (7). Of note, hypolipidemic compounds atorvastatin and omega-3 fatty acids, mainly eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) that are used to treat dyslipidemia exhibit antiarrhythmic effects in clinical practice (8-11) but underlying mechanisms are not sufficiently elucidated. We have previously shown (12, 13) that prolonged treatment of hereditary hypertriglyceridemic (HTG) or spontaneously hypertensive rats (SHR) with these compounds protects from VF and it was attributed to the restoration of normal distribution and expression of myocardial Cx43. Our most recent study (14) revealed for the first time pleiotropic effect of prolonged administration of pineal hormone, melatonin, resulting in up-regulation of myocardial Cx43 associated with decreased incidence of VF in arrhythmia prone SHR. To elucidate further the antiarrhythmic potential of atorvastatin, EPA, DHA and melatonin we aimed to explore their acute antiarrhythmic effects as well as their possible efficacy to defibrillate the heart and/or to facilitate sinus rhythm restoration.
MATERIAL AND METHODS
All animal experiments were performed in accordance with the rules issued by the State Veterinary Administration of Slovak Republic, legislation No 289/2003, and with the regulations of the Animal Research and Care Committee of Institute for Heart Research. Animals were housed with food and water ad libitum and exposed to a cycle of 12 h of light and 12 h of darkness. The experiments were performed each day between 10 am and 3 pm.
This study was conducted on 5-month old male (n=40) and female (n=44) HTG rats since the long-term effect of atorvastatin and omega-3 fatty acids was realized using this strain (12). In addition, we also used aged 24-month old male (n=12) and female (n=12) guinea pigs (GP) to test acute effect of melatonin. Ageing is known to be associated with reduced levels of melatonin and we have previously shown (5) that old GP are much prone to malignant arrhythmias comparing to young ones. The rats were anesthetized by thiopental (50 mg/kg, i.p.) and GP were euthanized by cervical dislocation. Thereafter the chest was opened and the heart was rapidly excised followed by the cannulation of the aorta. The spontaneously beating heart was perfused in Langendorff mode with oxygenated Krebs-Henseleit (KH) solution (containing in mmol/l: 120 NaCl; 4.2 KCl; 1.75 CaCl2; 1.25 MgSO4. 4H2O; 12.5 glucose; 25.0 NaHCO3) at constant flow (10 – 14ml/min for rats and 16 – 18ml/min for GP), temperature 37°C and 7.4 of pH. Left ventricular pressure (LVP) was measured isovolumetrically by a water-filled latex balloon inserted into the ventricle and connected to the pressure transducer. Bipolar ECG, LVP and maximum rates of pressure development and fall (+dP/dt and –dP/dt, as the indexes of contraction and relaxation) were continuously monitored.
In HTG rat hearts VF inducibility was tested using stimulations by 1 s burst of electrical rectangular pulses (100 pulses/s), 1 ms in duration at current strength 35 mA (electrostimulator ST-30; Medicor, Budapest, Hungary) delivered via stimulating electrodes attached to the epicardium of the right ventricle (15). Maximum 10 stimuli were delivered to the each heart unless sustained VF occured earlier. The incidence of sustained VF (i.e. 2 min lasting according to Lambeth convention, 1998) was registered using continuous recording of bipolar ECG. After 2 min lasting VF (in cases when it was induced), the perfusion of the heart was stopped for brief global ischemia, which terminated sustained VF followed by sinus rhythm restoration (sSR). The time from the onset of stop perfusion until sSR was registered and evaluated as the ability of the heart to recover normal rhythm (Fig. 3, panel B and for details reference (15). In GP hearts was examined VF threshold by stepwise increase of current strength from 10 mA until 50 mA (i.e. when sustained VF was not induced after the first stimulus, the intensity of current was increased in 5 mA steps).
Experimental protocol of Langendorff-perfused rat hearts in short
Twenty min stabilization-10 min test compound - intermittent burst stimulation to induce sustained VF - 2 min sustained VF - stop perfusion until the restoration of sinus rhythm.
In addition to this protocol, the bolus of atorvastatin, EPA or DHA was injected to the origin of the aorta of fibrillating hearts (n=6 for each compound) to evaluate defibrillation efficacy.
Experimental protocol of Langendorff-perfused GP hearts in short
Twenty min of KH perfusion, ten min of KH perfusion with melatonin or without melatonin (control condition), electrical stimulation by stepwise increase of current strength to induce sustained VF.
All experiments were continuously recorded using BioLab-1 software into the PC for the evaluations. The rat hearts were perfused with atorvastatin (Zentiva, Bratislava, SK), EPA or DHA (Sigma Saint Louis, MO, USA) at 15 µmol concentration. Bolus 150 µM of these compounds was injected to test defibrillation of HTG rat hearts. GP hearts were perfused with melatonin (Sigma-Aldrich, Saint Louis, MO, USA) at 50 µmol concentration.
All tested compounds were solved in absolute ethanol, whereby 100 L of stock solution was added to 1 liter of KH solution. To prevent light exposition when testing melatonin Langendorff set up was covered by alluminium.
Statistical analysis
The data are expressed as means ± standard deviations (S.D.). Normalization of the data was analyzed by Shapiro-Wilk test. Comparison between two groups was performed using the unpaired Student's t-test. To evaluate percentage of fibrillating heart was used Chi-Quadrat-Test. Values were considered to differ significantly at P<0.05.
RESULTS
Experiments performed on the HTG rat hearts showed that in the control (test compound free) group, sustained VF was electrically-induced in all male (n=10) as well as female (n=12) hearts as shown in Fig. 1A and 1B. However, the propensity to induce sustained VF was higher in males comparing to females since 3 – 4 burst stimuli were needed in males versus 9 – 10 burst stimuli in females. Administration of atorvastatin significantly reduced the incidence of sustained VF in both male and female groups to 30% and 60%, respectively (Fig. 1A and 1B), i.e. VF was not induced in seven of ten male and in four of ten female rat hearts. Pre-treatment with EPA prevented VF induction in three of ten male and three of 12 female rat hearts (Fig. 1A and 1B). However, the difference did not reach statistical significance. Pre-treatment of the hearts with DHA resulted in significant decrease of sustained VF incidence to 60% in both male and female HTG rat hearts (Fig. 1A and 1B), i.e. sustained VF was not induced in four of ten either male or female rat hearts.
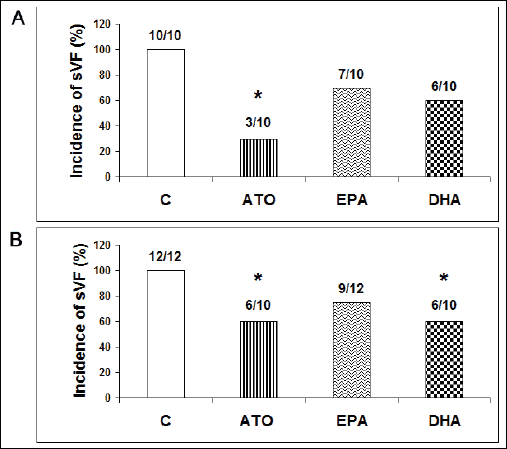 |
Fig. 1. Incidence of sustained VF (sVF) in male (A) and female (B) hypertriglyceridemic rat hearts pretreated with atorvastatin (ATO), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and without pretreatment (C). |
The ability of the fibrillating heart to restore sinus rhythm by the stop-perfusion manoeuvre was determined by the time period from the onset of stop-perfusion until the occurrence of sinus rhythm. Restoration of sinus rhythm was significantly faster in both male and female groups exposed to either atorvastatin, EPA or DHA compared to control groups without the treatment as shown in Fig. 2A and 2B.
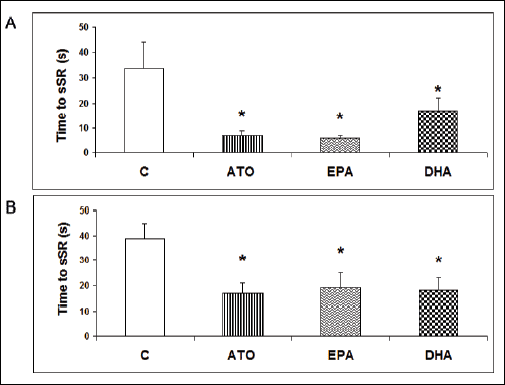 |
Fig. 2. Time to sinus rhythm restoration (sSR) in male (A) and female (B) hypertriglyceridemic rat hearts pretreated with atorvastatin (ATO), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and without pretreatment (C). |
Administration of bolus of either EPA or DHA did defibrillate six out of six hearts and bolus of atorvastatin defibrilated four out of six HTG rat hearts. Fig. 3 demonstrates representative recordings from the experiments showing burst stimulation induced sustained VF (Fig. 3A), stop perfusion-induced termination of sustained VF followed by sinus rhythm restoration (Fig. 3B) and the termination of sustained VF by bolus of DHA (Fig. 3C).
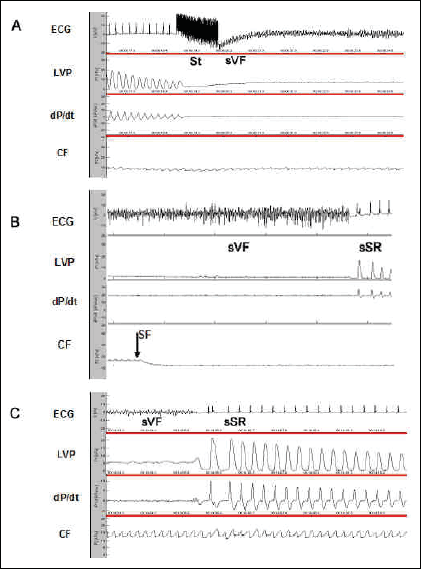 |
Fig. 3. Representative recordings showing electrical stimulation-induced sustained VF (A), stop perfusion-induced termination of sustained VF (B), restoration of sinus rhythm after bolus of DHA infused to the fibrillating heart (C). Abbreviations: ECG - bipolar electrocardiogram, LVP - left ventricular pressure; dP/dt - derivation of LVP development; CF - coronary flow; St - burst stimuli; sVF - sustained ventricular fibrillation; sSR - sinus rhythm restoration; SF - stop perfusion. |
Perfusion of the HTG rat heart for 10 min with either tested agents did not change significantly functional parameters, such as heart rate, LVP or indexes of contraction and relaxation (not shown).
Experiments conducted on GP hearts showed that the threshold to induce sustained VF was 21.7 ± 3.8 mA in males and 38.3 ± 2.9 mA in females. While sustained VF was not induced even by the strongest 50 mA stimulus in GP heart pre-treated with melatonin regardless the sex (Fig. 4). Perfusion of the heart for 10 min with melatonin did not change registered functional parameters, such as coronary flow, heart rate, left ventricular pressure, indexes of contraction and relaxation (not shown).
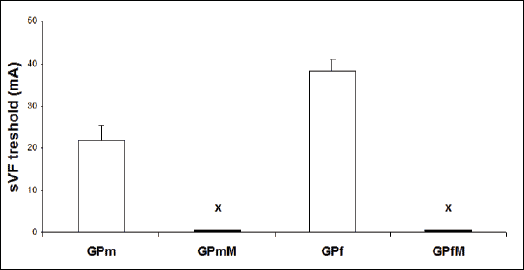 |
Fig. 4. Thresholds to induce sustained VF (sVF) in male and female guinea pig hearts. Abbreviations: GPm - males GP heart without pretreatment; GPmM - males GP heart pretreated with melatonin; GPf - females GP heart without pretreatment; GPfM - females GP heart pretreated with melatonin. Note that sustained VF was not induced in male and female GP hearts (x) even after the highest stimulus strength 50 mA. |
DISCUSSION
In this study we have demonstrated for the first time the acute aniarrhythmic effects of melatonin in male and female guinea pig's heart and atorvastatin in male and female HTG rat's heart. Accordingly, pretreatment of the heart with melatonin resulted in increase of threshold to induce sustained VF and pretreatment with atorvastatin resulted in suppression of electrically-inducible sustained VF. Furthermore, we demonstrated the protection from VF by acute administration of EPA and DHA that is consistent with previously reported direct anti-arrhythmic effects in the rabbit heart (16), dog model of ischemia-induced SCD (17) and in patients during electrophysiological testing for inducible ventricular tachycardia (18). Noteworthy, we revealed that EPA, DHA and atorvastatin facilitate sinus rhythm restoration and exhibit defibrillating efficacy.
Nonetheless, underlying mechanisms of malignant arrhythmias prevention are poorly understood. Acute administration of omega-3 FA has been reported (19) to suppress dose-dependently fast sodium current (INa) that is responsible for the upstroke of the action potential (AP) and affects electrical impulse conduction, L-type calcium current that is responsible for plateau of AP and potassium (Ik) currents that are responsible for repolarisation. Accordingly, omega-3 FA alter cardiac electrophysiology by wide spectrum of actions and thereby may be anti-arrhythmic. These effects were taken into consideration when explaining acute antiarrhythmic effects in the rabbit heart (16). While pro-arrhythmic effect of omega-3 FA was rarely reported (19). Circulating omega-3 FA, in addition, modulate intracellular Ca2+ homeostasis, whereby the prevention of Ca2+ overload is important for protection from the arrhythmias (19, 5). Of note, increased circulating levels of omega-3 FA due to infusion leads to fast incorporation to the cardiac phospholipid membranes, where they can exert their anti-arrhythmic potential. This may play a role in the prevention of ischemia-induced VF by acute infusion of omega-3 FA in dog hearts (17). The relative contribution of circulating and incorporated omega-3 FA to electrophysiology has not been discriminated. Taken into consideration the electrophysiological data, it is most likely that DHA and EPA may efficiently modulate Cx43 channel's conductivity and hence myocardial conduction velocity. However, further studies should prove it.
Unlike EPA and DHA there is no data whether melatonin can affect functioning of specific ion channels. Nevertheless, it is likely since melatonin has been shown to lengthen APD (20). Moreover, melatonin can modulate redox state and cell signalling, including activation of PKC (21). These effects were suggested to be involved in up-regulation of myocardial Cx43 after long-term treatment of SHR (14). Of note, melatonin prevented reoxygenation-induced cellular uncoupling and redistribution of Cx43 in cultured rat astrocytes (22). Thus, we can suppose that melatonin may acutely affect myocardial Cx43 channels mediated electrical coupling via Cx43 phosphorylation. In this context it would be interesting to know whether melatonin-related acute antiarrhythmic effects are mediated through its membrane receptors or regardless of them. Perhaps interesting to note that melatonin may affect cell-to-cell coupling also via modulation of fibroblast activity and turnover of extracellular matrix proteins (23).
As for acute antiarrhythmic effects of atorvastatin we can speculate that likewise omega-3 FA, perhaps atorvastatin may modulate cell membrane composition and properties (such as fluidity) due to inhibition of cholesterol synthesis and its availability. Acute administration of simvastatin was shown to improve post-ischemic reperfusion-induced contractile dysfunction (24) that might be, in part, attributed to improvement of myocardial synchronization via cell-to-cell coupling. We assume that antiarrhythmic actions of circulating atorvastatin, EPA and DHA most likely take place via modulation of Cx43 channel's function by the alterations of membrane properties close to the channels and/or by the phosphorylation of channels. This assumption is supported by our previous study (12) as well as by recent findings that statins prevent electrical remodelling of diabetic rat heart (25) and modulate PKC signalling in SHR (26). Moreover, atorvastatin has been shown to inhibit the secretion of pro-inflammatory mediators, such as cytokines (IL-6, IL-8, MCP-1) as well as adhesion molecules (sICAM-1) and extracellular matrix proteins (27). These factors may affect Cx43 channels mediated intercellular communication as well.
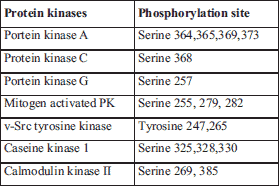
In the context of Cx43 channel function it should be noted that phosphorylation of Cx43 affects its trafficking to the membrane, assembly into gap junctions, cellular distribution, degradation and Cx43 channel function (28). Consequently it may affect myocardial conduction (2, 29). Of note, long-term administration of atorvastatin, melatonin and omega-3 FA was accompanied with modulation of Cx43 phosphorylated status (i.e. enhancement or normalization) that was linked with attenuation of abnormal Cx43 distribution and associated with the protection from VF (12-14). The changes in Cx43 phosphorylated forms were attributed mainly to increased PKCe expression. However, there are several protein kinases, which directly phosphorylate Cx43 (Table 1) and their involvement in acute effects of atorvastatin, melatonin, EPA and DHA is needed to be explored in further studies. It is of great interest because some of these protein kinases enhance and some inhibit Cx43 channel function (30).
Taken into consideration available data including ours, it appears that protection from malignant arrhythmias can be achieved either by improving (31) or by partial inhibition of Cx43 mediated intercellular communication (32) depending on actual heart condition. Accordingly, we suppose that termination of fibrillating activity and recovery of sinus rhythm may be attributed to partial cell-to-cell uncoupling at the Cx43 channels due to brief ischemia (induced by stop perfusion) as previously reported (15). Similarly this mechanism could explain termination of VF followed by sinus rhythm restoration induced by bolus of high concentration of atorvastatin, EPA and DHA.
In addition, it is important to note that EPA, DHA, melatonin and atorvastatin exert anti-inflammatory or antioxidant and free radical's scavenging effects that can contribute to their antiarrythmic potential during both acute and chronic treatment. Protection of Cx43 from increased level of reactive oxygen species during VF can support ability of the heart to restore sinus rhythm. Indeed, effective antioxidant therapy was suggested for the management of arrhythmia (33). These facts point out that healthy life style (34) avoiding diet rich in saturated fatty acids, "light pollution" and exercise deficiency may improve heart function and decrease a risk for arrhythmias.
Finally, it should be noted that this study showed the sex-related differences in susceptibility of the rat and guinea pig hearts to malignant arrhythmias. This fact can be in part explained by differences in expression of myocardial Cx43, i.e. higher levels of Cx43 in females comparing to males as indicated in our previous studies (35, 36). Interestingly, acute antiarrhythmic effects of atorvastatin were more pronounced in males comparing to females. This feature should be elucidated more in details in further studies.
In conclusions, findings of this study indicate clear cut acute protection of the heart from lethal arrhythmia by atorvastatin, melatonin, EPA and DHA as well as their efficacy to facilitate sinus rhythm restoration. It challenges to investigate the implication of specific protein kinases in modulation of Cx43 channels function by acute administration of these compounds. It may contribute to the progress in novel antiarrhythmic strategy in clinical praxis.
Acknowledgements: This study was supported by VEGA 2/0046/12, 1/0032/14, 2/0167/15 and APVV-0241-11, APVV-0348-12 grants.
Conflict of interests: None declared.
REFERENCES
- Severs NJ. Pathophysiology of gap junctions in heart disease. J Cardiovasc Electrophysiol 1994; 5: 462-475.
- Severs NJ. Gap junction remodeling and cardiac arrhythmogenesis: cause or coincidence? J Cell Mol Med 2001; 5: 355-366.
- Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 2004; 84: 431-488.
- Tribulova N, Manoach M, Varon D, Okruhlicova L, Zinman T, Shainberg A. Dispersion of cell-to-cell uncoupling precedes low K+-induced ventricular fibrillation. Physiol Res 2001; 50: 247-259.
- Tribulova N, Seki S, Radosinska J, et al. Myocardial Ca2+ handling and cell-to-cell coupling, key factors in prevention of sudden cardiac death. Can J Physiol Pharmacol 2009; 87: 1120-1129.
- Lopshire JC, Zipes DP. Sudden cardiac death: better understanding of risks, mechanisms, and treatment. Circulation 2006; 114: 1134-1136.
- Singh BN. Augmenting maintenance of sinus rhythm in the control of atrial fibrillation by antiarrhythmic drug combinations. J Cardiovasc Pharmacol Ther 2010; 15: 31-35.
- Anderson KP. Lipid-lowering therapy for prevention of ventricular tachyarrhythmias. J Am Coll Cardiol 2003; 42: 88-92.
- Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation 2003; 107: 2646-2652.
- Mitchell LB, Powell JL, Gillis AM, Kehl V, Hallstrom AP, AVID Investigators. Are lipid lowering drugs also antiarrhythmic drugs? An analysis of the antiarrhythmics versus implantable defibrillators (AVID) trial. J Am Coll Cardiol 2003; 42: 81-87.
- London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 2007; 116: e320-e335.
- Bacova B, Radosinska J, Knezl V, et al. Omega-3 fatty acids and atorvastatin suppress ventricular fibrillation inducibility in hypertriglyceridemic rat hearts: implication of intracellular coupling protein, connexin-43. J Physiol Pharmacol 2010; 61: 717-723.
- Radosinska J, Bacova B, Knezl V, et al. Dietary omega-3 fatty acids attenuate myocardial arrhythmogenic factors and propensity of the heart to lethal arrhythmias in a rodent model of human essential hypertension. J Hypertens 2013; 31: 1876-1885.
- Benova T, Viczenczova C, Radosinska J, et al. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can J Physiol Pharmacol 2013; 91: 633-639.
- Radosinska J, Knezl V, Benova T, Urban L, Tribulova N, Slezak J. Alterations of the intercellular coupling protein, connexin-43, during ventricular fibrillation and sinus rhythm restoration demonstrated in male and female rat hearts. A pilot study. Exp Clin Cardiol 2011; 16: 116-120.
- Dhein S, Michaelis B, Mohr FW. Antiarrhythmic and electrophysiological effects of long-chain omega-3 polyunsaturated fatty acids. Naunyn Schmiedebergs Arch Pharmacol 2005; 371: 202-211.
- Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation 1999; 99: 2452-2457.
- Schrepf R, Limmert T, Claus Weber P, Theisen K, Sellmayer A. Immediate effects of n-3 fatty acid infusion on the induction of sustained ventricular tachycardia. Lancet 2004; 363(9419): 1441-1442.
- Den Ruijter HM, Berecki G, Opthof T, Verkerk AO, Zock PL, Coronel R. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res 2007; 73: 316-325.
- Diez ER, Prados LV, Carrion A, Ponce AZ, Miatello RM. A novel electrophysiologic effect of melatonin on ishcemia/reperfusion-induced arrhythmias in isolated rat hearts. J Pineal Res 2009; 46: 155-160.
- Strosberg AD, Nahmias C. G-protein-coupled receptor signalling through protein networks. Biochem Soc Trans 2007; 35: 23-27.
- Martinez AD, Saez JC. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res Brain Res Rev 2000; 32: 250-258.
- Drobnik J, Tosik D, Piera L, et al. Melatonin-induced glycosaminoglycans augmentation in myocardium remote to infarction. J Physiol Pharmacol 2013; 64: 737-744.
- Szarszoi O, Maly J, Osadal P, et al. Effect of acute and chronic simvastatin treatment on post-ischemic contractile dysfunction in isolated rat heart. Physiol Res 2008; 57: 793-796.
- Ozturk N, Yaras N, Ozmen A, Ozdemir S. Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart. J Bioenerg Biomembr 2013; 45: 343-352.
- Qiu Z, Zhang W, Fan F, et al. Rosuvastatin-attenuated heart failure in aged spontaneously hypertensive rats via PKCa/b2 signal pathway. J Cell Mol Med 2012; 16: 3052-3061.
- Korybalska K, Kawka E, Breborowicz A, Witowski J. Atorvastatin does not impair endothelial cell wound healing in an in vitro model of vascular injury. J Physiol Pharmacol 2012; 63: 389-395.
- Herve JC. The communicating junctions, composition, structure and charasteristic. Biochim Biophys Acta 2012; 1818: 1803-1806.
- Meyer R, Schreckenberg R, Kraus D, Kretschmer F, Schulz R, Schlüter KD. Cardiac effects of osteostatin in mice. J Physiol Pharmacol 2012; 63: 17-22.
- Chen VC, Gouw JW, Naus CC, Foster LJ. Connexin multi-site phosphorylation: Mass spectrometry-based proteomics fills the gap. Biochim Biophys Acta 2013; 1828: 23-24.
- Dhein S, Hagen A, Jozwiak J, et al. Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. Naunyn Schmiedebergs Arch Pharmacol 2010; 381: 221-234.
- Rohr S, Kucera JP, Fast VG, Kleber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science 1997; 275: 841-844.
- Sovari AA, Bonini MG, Dudley SC. Effective antioxidant therapy for the managment of arrhythmia. Expert Rev Cardiovasc Ther 2011; 9: 797-800.
- Sakr HF. Modulation of metabolic and cardiac dysfunctions by swimming in overweight rats on a high cholesterol and fructose diet: possible role of adiponectin. J Physiol Pharmacol 2013; 64: 231-240.
- Tribulova N, Dupont E, Soukup T, Okruhlicova L, Severs NJ. Sex differences in connexin-43 expression in left ventricles of aging rats. Physiol Res 2005; 54: 705-708.
- Mitasikova M, Smidova S, Macsaliova A, et al. Aged male and female spontaneously hypertensive rats benefit from n-3 polyunsaturated fatty acids supplementation. Physiol Res 2008; 57: 39-48.
A c c e p t e d : December 10, 2014