GLUCOCORTICOID RECEPTOR MEDIATES THE EXPANSION OF SPLENIC LATE ERYTHROID PROGENITORS DURING CHRONIC PSYCHOLOGICAL STRESS
INTRODUCTION
Stress has become an important aspect of daily life. Exposure to a stressor initiates an integrated response, known as a stress response, which is composed of multiple interactions between neuroendocrine and other physiological systems (1). Each stressor evokes distinct repertoire of adaptive changes, thus contributing to the complexity of the stress response and its multifaceted manifestations (2). To address different aspects of stress-induced adaptive response, a wide variety of experimenta lmodels have been developed (3). In particular, restraint stress has been commonly used as an experimental model of psychological stress that robustly induces the entire spectrum of known allostatic responses (3, 4).
Glucocorticoids are the main effector end point of neuroendocrine system and key mediators of the stress. Their increased secretion in response to adverse stimuli normally results in a cascade of physiological mechanisms that allow survival and adaptation (5, 6). Exerting the effects by activating the glucocorticoid receptor (GR) that is expressed in virtually all mammalian cell types (7), glucocorticoids affect different physiological processes including erythropoiesis (8).
Erythropoiesis is a complex multistep process whereby multipotent hematopoietic stem cells differentiate toward a progressively committed erythroid lineage through intermediate progenitors, specifically, burst forming units-erythroid (BFU-E), followed sequentially by colony forming units-erythroid (CFU-E). CFU-E progenitors further differentiate through the proerythroblast, basophilic, polychromatic, and orthochromatic erythroblast stages and reticulocytes to red blood cells. The production of red blood cells is regulated at multiple levels through cytokines, transcription factors and cofactors, post-translation modifications of histones and microRNAs (9).
Stress erythropoiesis is defined as a process of increased production of red blood cells in physiological and clinical settings that threaten tissue oxygen tension (10). In addition to erythropoietin, stem cell factor and bone morphogenetic protein 4, glucocorticoids stimulate stress erythropoiesis. Thus, Bauer et al. demonstrated an essential role for GR in stress erythropoiesis induced by hemolytic anemia and hypoxia (11). Although the regulation of stress erythroid response has been extensively studied, the target cell and the physiological effects of glucocorticoids remain elusive (12).
It has been recognized that stress erythropoiesis, critical for survival, is more than simply an expansion of steady-state erythropoiesis (10, 13). Occurring predominantly in the adult spleen, stress erythropoiesis is highly dependent on integrating signals derived from spleen microenvironment (14). Recent evidence suggests that spleen microenvironment acts as a specialized niche which promotes the rapid expansion of erythroid progenitors during stress conditions (15, 16). Stress erythropoiesis has been studied mainly in murine models of acute anemia (17, 18), but the erythropoietic response to chronic psychological stress is largely unknown. Therefore, the purpose of the present study was to investigate the influence of chronic restraint stress on committed erythroid progenitors in the mouse spleen as well as to examine the role of GR in observed effects.
MATERIALS AND METHODS
Animals
Male, 6–8 week-old CBA mice were obtained from the Breeding Facilities of the Institute for Medical Research, Military Medical Academy, Belgrade. The animals were housed six to eight per cage under conventional conditions (lights on at 06.00 h, lights off at 18.00 h, 21°C) with standard laboratory diet and water provided ad libitum. The experimental protocol was approved by the Ethic Committee of the Institute for Medical Research, University of Belgrade, Serbia (No O112-1/12), according to the National Law on Animal Welfare that is consistent with guidelines for animal research and principles of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes (Official Daily N. L 358/1-358/6, 18, December 1986) and Directive on the Protection of Animals Used for Scientific Purposes (Directive 2010/63/EU of the European Parliament and of the Council, 22 September 2010).
Experimental procedure
Experiment 1
Chronic psychological stress was applied in the form of repeated restraint, which consisted of placing the mice in 50 ml conical centrifuge tubes with multiple ventilation holes, for 2 hours per day. To avoid possible changes associated with new environment, restrained mice were maintained horizontally in their home cages during the restraint sessions and released into the same cage thereafter. Mice were subjected to randomly timed (between 07.00 and 11.00 h) restraint for 7 or 14 consecutive days. All experiments were performed during light phase considering that rodents subjected to repeated restraint during light phase did not habituate to this stressor as seen in rodents during transient and dark phases (19). Furthermore, as stressor predictability could influence habituation to repeated stimuli, we changed the start time of restraint exposure each morning. Control mice were handled once daily in the housing room. The mice were sacrificed by cervical dislocation immediately after last restraint session. The controls were sacrificed at an equivalent time-point. Blood was collected in heparinized tubes and subsequently centrifuged (2700 rpm, 4°C, 10 min) to obtain plasma samples for corticosterone determination. Spleens were harvested under sterile conditions and used for colony assays. Since different technical procedure is required to prepare spleen tissue for histological examination, we had to repeat this experiment using additional, age-matched mice for these analyses.
Experiment 2
The role of GR was assessed by pretreatment of mice with GR antagonist mifepristone (RU486, Sigma-Aldrich, St Louis, MO, USA) dissolved in sterile filtered dimethylsulfoxide (DMSO, ampoules A7248, 0005, AppliChem) just prior to use, and injected subcutaneously 60 min before each restraint stress. Thus, the mice were randomly assigned to following weight-matched groups: 1) R - restraint group exposed to 2 h daily restraint stress during 7 consecutive days; 2) RU486+R group, treated with mifepristone (50 mg/kg) 60 min prior to daily restraint; 3) RU486 group, received only the daily dose of mifepristone; and 4) C - control group, simply handled daily. As no effect of DMSO was shown on BFU-E, CFU-GM and CFU-GEMM (20), to evaluate whether this solvent affects CFU-E, we used additional group consisting of the mice injected with DMSO 60 minutes prior to each restraint exposure for 7 days. All animals were sacrificed by cervical dislocation. The stressed mice were sacrificed immediately after last restraint session, and the controls were sacrificed at an equivalent time-point. Blood was collected in heparinized tubes and subsequently centrifuged (2700 rpm, 4°C, 10 min) to obtain plasma samples for corticosterone determination. Spleens were harvested under sterile conditions and used for colony assays.
Corticosterone determination
Plasma corticosterone levels were determined using a 125I-coupled double antibody radioimmunoassay (MP Biomedicals, LLC, USA). The low limit of detection of corticosterone kit was 7.7 ng/ml. The inter- and intra-assay coefficients of variation (CV) were 4.4 and 6.5%, respectively. Corticosterone concentration was expressed as ng/ml.
Colony forming assays
Spleens were harvested under sterile conditions, passed through a 70 µm wire mash and monodispersed in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, CA, USA) supplemented with 5% fetal calf serum (FCS, Invitrogen, CA,USA). Then, 4×105/ml nucleated splenocytes were plated in methylcellulose media (StemCell Technologies, Vancouver, BC, Canada) containing either 3 U/ml EPO (MethoCult M3334) or 3 U/ml EPO supplemented with 50 ng/ml SCF, 10 ng/ml IL-3, and 10 ng/ml IL-6 (MethoCult GF M3434). Splenocytes were plated in duplicate in 35 mm tissue culture plates (Sarstedt, Germany) and incubated at 37°C in a humidified atmosphere containing 5% CO2. Following an incubation period of 7 days in MethoCult GF M3434 medium, BFU-Es were enumerated whereas CFU-E-derived colonies were scored after 2 days of culture in MethoCult M3334. After above-mentioned incubation periods, the culture plates were removed and colonies counted using an inverted microscope and a scoring grid. The number of colonies was obtained per each culture plate.
Histology and immunohistochemistry
The central part of the spleen was dissected under sterile conditions, fixed in formalin and embedded in paraffin wax. Embedded tissue was sectioned at 3 µm thickness and used for immunohistochemical and hematoxylin and eosin staining.
The sections were dewaxed, rehydrated and treated with a 0.3% hydrogen peroxide solution to quench endogenous peroxidase activity. Antigen retrieval was aided by placing the slides in a preheated 10 mM citrate buffer (pH 6.0) in a microwave oven for 20 min. Slides were then rinsed in phosphate buffered saline (PBS) and incubated for 1 h with rabbit polyclonal anti-GR (1:400 dilution, Santa Cruz Biotechnology, Cat No. sc-1004, CA, USA), recommended for GR detection of mouse (21) and rat origin by immunohistochemistry and Western blot. After a brief wash in PBS, immunostaining was performed using the streptavidin-biotin technique and DAB Substrate/Chromogen System for visualization (Novocastra Peroxidase Detection System kit, Leica Biosystems, Wetzlar, Germany). Control sections in which the primary antibody was omitted were processed in parallel. The nuclei were counterstained with Mayer’s hematoxylin.
Immunoreactive cells were analyzed and scored using a computer-supported imaging system (analysis Pro 3.1) connected to the light microscope (Olympus AX70) with an objective magnification of ×40. Quantitative analysis was performed using 5 high power fields of the red pulp in each tissue section. The number of GR-positive cells was determined by scoring all GR-immunoreactive cells, regardless if they exhibit cytoplasmic, nuclear, or both cytoplasmic and nuclear staining. Data were presented as the number of GR-immunoreactive cells per mm2 of red pulp area.
Statistical analysis
Data are expressed as mean ± S.E.M. of 4–6 mice per each group. Statistical significance was assessed by either one-way or two-way analysis of variance (ANOVA). Following the one-way ANOVA, post hoc comparisons using Bonferroni-corrected t-test or Fisher’s least significant difference (LSD) test were performed as appropriate. In the case of significant results with two-way ANOVA, pairwise comparisons with Bonferroni adjustment were performed. Significance was determined at the P<0.05 level.
RESULTS
Enhanced spleen weights with increased number of erythroid progenitor cells after exposure to chronic restraint stress
Chronic restraint stress caused an increase in absolute and relative weights of the spleen (Fig. 1A). Exposure to 2 hours daily restraint stress for 7 or 14 days did not affect body mass [one-way ANOVA, F(2,12)=3.27; P>0.05], while significantly increased the absolute weight of the spleen in stressed mice [one-way ANOVA, F(2,12)=6.497; P<0.05]. In addition, the spleen index, defined as spleen/body weight ratio multiplied by 100, was significantly increased [one-way ANOVA, F(2,12)=7.014; P<0.05] in mice subjected to either 7 or 14 days of restraint stress. Furthermore, the splenic red pulp exhibited increased cellularity in chronically stressed mice (Fig. 1C, 1D), as compared to controls (Fig. 1B).
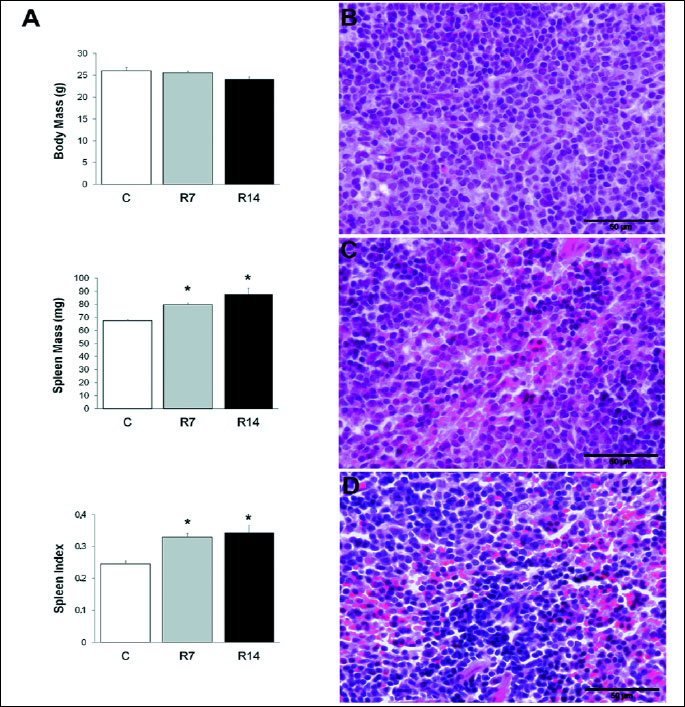
To further evaluate whether the increased cellularity of the red pulp reflects enhanced erythropoiesis in the spleen of stressed animals, we examined the number of splenic BFU-E and CFU-E progenitors in control and restrained mice. Since BFU-E and CFU-E can only be detected by their ability to form colonies in functional assays in vitro, we quantified BFU-E and CFU-E using this methodology. In parallel, spleen cells of restrained and control mice are cultivated in the appropriate medium under the same conditions and the obtained number of BFU-E and CFU-E colonies reflects the number of these cells at the time of plating. Statistical analysis showed that 7 and 14 days of restraint stress resulted in markedly increased number of both BFU-E [one-way ANOVA, F(2,15)=35.592;P<0.001] and CFU-E [one-way ANOVA, F(2,15) =16.081;P<0.001] progenitors in the spleen (Fig. 2).
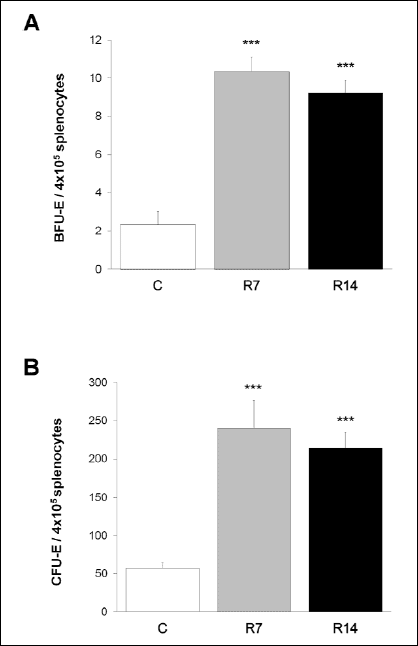 |
Fig. 2. The effects of repeated restraint stress on BFU-E and CFU-E in the spleen. Exposure to chronic restraint stress resulted in markedly increased number of BFU-E (A) and CFU-E (B) progenitors in the spleen. Data are expressed as mean ± S.E.M., n=6 animals per group; ***P<0.001, ANOVA followed by Bonferroni post hoc test. BFU-E, burst forming units-erythroid; CFU-E, colony-forming unit-erythroid; C, control animals; R7, animals stressed for 7 consecutive days; R14, animals stressed for 14 consecutive days. |
Increased corticosterone concentration and decreased number of GR-immunoreactive cells in the spleen during chronic restraint stress
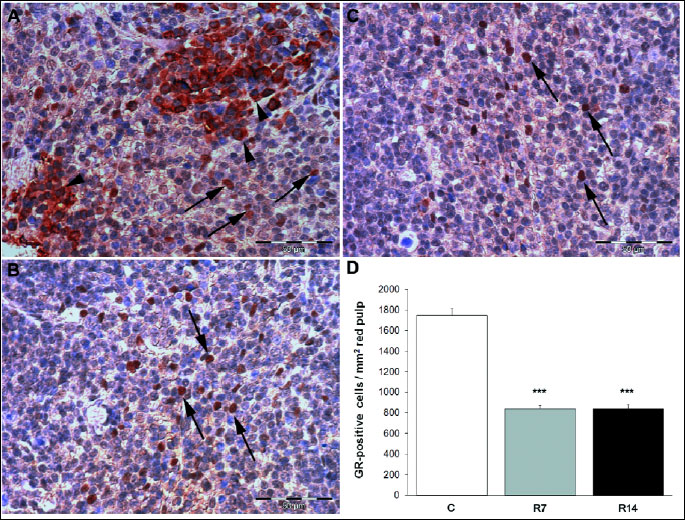
In order to verify that our chronic restraint procedure activated the hypothalamic-pituitary-adrenal axis, as a key endocrine component of stress response, we measured the circulating levels of corticosterone, the major glucocorticoid in mice. Repeated exposure to restraint procedure significantly increased plasma corticosterone concentration [one-way ANOVA F(2,15)=22.834; P<0.001] in stressed animals (Fig. 3). In addition, we used immunohistochemistry to examine whether the exposure to chronic stress alters the number of cells expressing GR in splenic red pulp. Immunohistochemical analysis showed both nuclear and diffuse cytoplasmic staining of GR in the red pulp of control mice (Fig. 4A), while GR-positive cells within the red pulp of chronically stressed mice exhibited predominantly nuclear immunoreactivity (Fig. 4B, C). Furthermore, morphometric analysis revealed markedly decreased the number of GR-expressing cells [one-way ANOVA F(2,12) = 102.765, P<0.001] in the red pulp after 7 and 14 days of stress exposure (Fig. 4D).
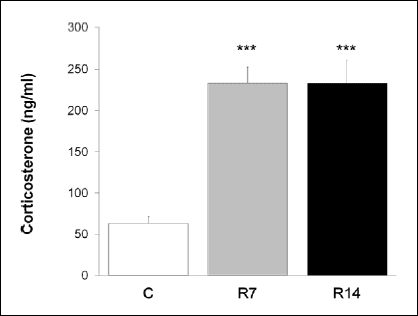 |
Fig. 3. The effects of chronic stress on circulating corticosterone levels. Values are expressed as mean ± S.E.M., n=6 animals per group; ***P<0.001, ANOVA followed by Bonferroni post hoc test. C, control animals; R7, animals stressed for 7 consecutive days; R14, animals stressed for 14 consecutive days. |
Effects of GR blockade on corticosterone levels and spleen index
Blockade of GR with mifepristone was used to examine its role in restraint stress-induced changes. As erythroid response to restraint stress was similar after 7 and 14 consecutive days, we used only mice restrained for 7 days to examine the effects of GR blockade on stress-induced alterations. The results, obtained in this experiment, were analyzed using a 2×2 ANOVA followed by pairwaise comparations with Bonferroni adjustment.
Furthermore, statistical analysis revealed a highly significant difference in corticosterone concentration regarding the main effect for stress [two-way ANOVA F(1,12)=63.271, P<0.001], while there was no significant main effect for GR blockade [two-way ANOVA F(1,12) =3.963, P>0.05], nor significant interaction effect [two-way ANOVA F(1,12) =3.567, P>0.05] (Fig. 5A).
We next examined whether GR blockade affected the spleen weight in stressed mice. The results demonstrated a significant difference in spleen index regarding the main effect for stress [two-way ANOVA F(1,12)=10.698, P<0.05]. Although mifepristone treatment slightly decreased the spleen index in stressed animals, there was no statistically significant main effect for GR blockade [two-way ANOVA F(1,12)=2.922, P>0.05] (Fig. 5B).
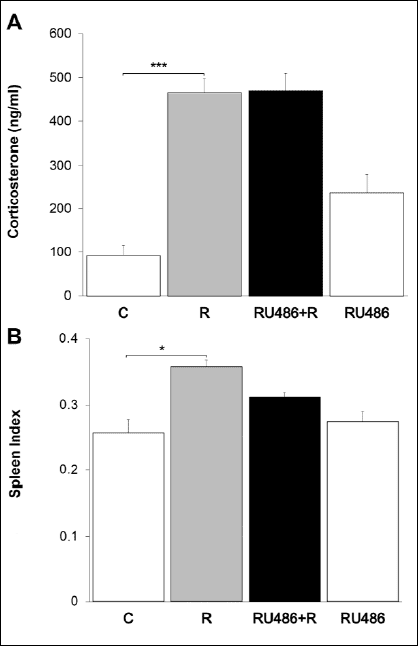 |
Fig. 5. The effects of GR blockade on circulating corticosterone levels and spleen index.There is a highly significant difference in corticosterone levels regarding the main effect for stress, whereas no significant main effect for GR blockade on circulating corticosterone concentration was obtained (A). Statistical analysis reveals a significant difference in spleen index regarding the main effect for stress, but there was no statistically significant main effect for GR blockade (B). Data are expressed as mean ± S.E.M., n=4–6 animals per group; *P<0.05, ***P<0.001, 2×2 two-way ANOVA followed by pairwise comparisons with Bonferroni adjustment. C, control animals; R, chronically stressed animals; RU486+R, animals treated with mifepristone prior to daily restraint; RU486, animals chronically treated with mifepristone alone. |
GR mediates the expansion of CFU-E progenitor cells in the spleen during chronic restraint stress
The role of GR in restraint stress-induced expansion of splenic erythroid progenitors was also tested by mifepristone. Following a 7-day restraint stress protocol, BFU-E progenitors were evidently increased in the spleen [two-way ANOVA, F(1,12)=8.561; Bonferroni adjustment, P<0.05], and pretreatment with mifepristone (50 mg/kg daily) did not significantly change their number [two-way ANOVA, F(1,12)=4.103; Bonferroni adjustment; P>0.05] (Fig. 6A). Furthermore, we have also observed a robust increase in CFU-E progenitors after 7 days of stress [two-way ANOVA, F(1,12)=26.451; Bonferroni adjustment, P<0.001]. However, the administration of mifepristone, 60 min prior to daily stressexposure, completely abolished this effect on CFU-E cells [two-way ANOVA, F(1,12)=26.332; Bonferroni adjustment, P<0.001] (Fig. 6B). In addition, daily dose of mifepristone for 7 consecutive days caused an increase in the number of BFU-E cells, while the number of CFU-E progenitors was not significantly affected in the spleen of non-stressed animals (Fig. 6). Moreover, the number of CFU-E in an additional, vehicle-treated group (DMSO+restraint) was similar to that found in mice restrained for 7 days [321.75±30.8 versus 334.12±40.3] showing no influence of DMSO on erythroid progenitors in our experiment.
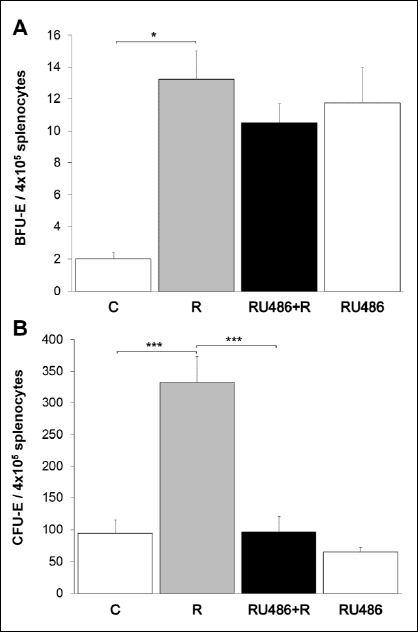 |
Fig. 6. The effects of GR blockade on restraint stress-induced BFU-E and CFU-E growth in the spleen. GR blockade with mifepristone (50 mg/kg daily) did not significantly change stress-induced increased number of BFU-E progenitors (A), while completely prevented the effect of chronic restraint stress on CFU-E cells (B). Data are expressed as mean ± S.E.M., n=4–6 animals per group; *P<0.05, ***P<0.001, 2×2 two-way ANOVA followed by pairwise comparisons with Bonferroni adjustment. C, control animals; R, chronically stressed animals; RU486+R, animals treated with mifepristone prior to daily restraint; RU486, animals chronically treated with mifepristone alone. |
DISCUSSION
We have recently demonstrated the role of chronic psychological stress in prolonged activation of stress erythropoiesis pathway, which implies the involvement of GR, thus leading to an excessive production of immature erythroid cells in the spleen (22). Here, we extend those findings by the experiments showing that GR mediates the expansion of late-stage erythroid progenitors in the spleen during chronic restraint stress.
To investigate role of GR in erythropoiesis, we pretreated the animals with GR antagonist mifepristone prior to restraint. As could be expected, RU486 had no influence on the concentration of corticosterone in chronically stressed mice. In addition, consistent with results of Yarushkina et al. (23) mifepristone alone did not alter corticosterone levels in mice in our study.
Production of red blood cells is essential for the survival and bone marrow is the primary site of adult steady-state erythropoiesis (24). However, even early observations on erythropoiesis showed that this process moves from bone marrow to the spleen during stress conditions (25). The adult mouse and human spleens retain low numbers of hematopoietic stem cells capable of both self-renewal and differentiation into multiple hematopoietic lineages, including erythrocytes (26, 27). Furthermore, erythropoiesis is highly dependent on stromal microenvironment that provide appropriate molecular signals, and spleen-derived stromal cells are superior to cells derived from bone marrow in supporting erythropoiesis under stress conditions (28, 9). Recently, splenic microenvironment has been shown to contain distinct signals that drive the expansion of erythroid progenitors in response to stressful stimuli (15, 13), thereby making spleen the primary site of stress erythropoiesis. In accordance, we have demonstrated that exposure to chronic restraint stress enhances both absolute and relative spleen weights and causes an increase in red pulp cellularity. Furthermore, the spleens of chronically stressed mice exhibited markedly increased number of both early and late erythroid progenitors, BFU-E and CFU-E, respectively. In addition to increased number of erythroid progenitors, chronic psychological stress also induces a significant increase in the number of more mature erythroid cells - nucleated Ter119-positive cells as we have demonstrated recently (29), indicating that increased cellularity of the red pulp is probably due to enhanced extramedullary erythropoiesis in these animals. Interestingly, increased number of CD11b-positive cells has been reported in some models of psychological stress, such as repeated social stress (30), suggesting that these cells may also contribute to increased cellularity and observed splenomegaly. However, a recent study has showed that restraint stress increased CD11b expression on neutrophils from the blood but not in the spleen (31), confirming a stressor-specific alterations in immune response (32). Moreover, using a restraint stress model, Hernandez et al. showed an expansion of the red pulp compartment and involution of splenic white pulp during chronic stress providing additional evidence that splenomegaly in chronically restrained mice is most likely due to an enhanced erythropoiesis (33). Analyzing changes in spleen volume during epinephrine infusion, Bakovic et al. reported a rapid spleen contraction in response to epinephrine (34). Bearing in mind that this influence of epinephrine on the spleen was acute, it was expected to be different from the effect of chronic restraint stress, described in our study.
Efficient erythropoiesis is orchestrated by the integration of multiple regulatory signals (35, 12). Among them, glucocorticoids are known to be potent endogenous enhancers of erythropoiesis, particularly under stress conditions (9). In addition to different endogenous regulators, erythroid cell development is greatly affected by numerous exogenous compounds, such as soy-derived dietary phytoestrogens (36). More recently, Kaminska et al. have demonstrated that phytoestrogens suppress ACTH-stimulated secretion of cortisol and corticosterone, the key mediators of erythropoietic stress response (37). Furthermore, examining a role of glucocorticoids in stress-induced erythropoiesis, Voorhees et al. have shown that psychological stress affects erythroid homeostasis through glucocorticoid stimulation (38). Glucocorticoids play an essential role in mediating a variety of adaptive responses by interacting with specific aspects of the physiology of each tissue (39). In regard to erythropoiesis, glucocorticoids support erythroid colony formation and increase the proliferation of erythroid cells (40). Moreover, treatment with glucocorticoids restores normal erythropoiesis in patients suffering from certain anemias (41). Although the mechanisms by which glucocorticoids regulate stress erythropoiesis are not fully understood, growing evidence suggest that they are evolutionarily conserved, and are possibly part of an ancient glucocorticoid-mediated stress response (9).
Activation of GR plays a critical role in the stress response of virtually all cell types (42). Concordantly, GR is expressed by erythroid progenitor cells, and its activation by dexamethasone induces proliferation and expansion of erythroid progenitors in vitro (43). In contrast to these in vitro findings, the examination of GR–/– embryos shows no apparent abnormality in erythropoiesis (11). However, an essential role of GR receptor in the regulation of stress erythroid response has been recognized (44). Thus, using mice deficient in GR dimerization and consecutive DNA-binding activity (GRdim/dim mice), Bauer et al. reported that these mice exhibit no increase in CFU-E progenitors in response to anemia-induced erythropoietic stress (11). Similarly, we used GR blockade with RU486, which induces GR nuclear translocation but not transactivation, to examine the role of this receptor in erythroid progenitor cell response to chronic psychological stress. Acting as a pure GR antagonist in erythroid cells (45), RU486 completely abolished the stimulatory effect of chronic restraint stress on CFU-E formation in the mouse spleen, while had no influence on restraint-induced BFU-E growth.
BFU-E progenitor cells respond to a large number of cytokines that regulate their growth, differentiation and survival (12). In particular, several growth factors and hormones, such as stem cell factor and glucocorticoids, act cooperatively to enhance and sustain proliferation of BFU-E progenitors (43, 46). Thus, erythroid progenitors have a limited capacity for self-renewal, and glucocorticoids are known to promote BFU-E self-renewal rate, ultimately leading to increased production of mature erythrocytes (47). Moreover, Flygare et al. showed that glucocorticoids synergizes with other regulatory factors to increase the number of BFU-E progenitors, suggesting that activation of HIF1alpha may enhance or replace the effect of glucocorticoids on BFU-E self-renewal (48). Accordingly, an increased number of BFU-E progenitors, observed in mice chronically treated with RU486, may be a result of the compensatory mechanisms that are activated to prevent BFU-E exhaustion and provide an optimal erythropoietic output during GR blockade. The existence of such compensatory mechanisms is strongly supported by the fact that mice lacking functional glucocorticoid receptors exhibit normal steady-state erythropoiesis (11). An unaltered CFU-E output in mice treated with RU486 alone, observed in the present study, provides a further endorsement for these findings.
Under stress conditions, however, the additional mechanisms are activated in the spleen to promote the expansion of both BFU-E and CFU-E progenitors (49), thus providing an adequate response to increased erythropoietic demand. Because the vast majority of regulatory factors act cooperatively and directly on BFU-E cells to enhance their expansion in response to stressful stimuli (14, 12), the observed increase in the number of BFU-E progenitors during chronic restraint stress is most likely a result of their synergistic effects. Therefore, despite the prominent role of glucocorticoids in BFU-E self renewal, GR blockade did not significantly decrease the number of these progenitors in chronically stressed mice.
In contrast to BFU-E cells, CFU-E progenitors are mainly controlled by erythropoietin, through the involvement of an EPOR-mediated signaling (50). However, in addition to EPOR, the expansion of erythroid progenitors during stress erythropoiesis also requires the activation of GR, as well as a cooperative signaling between these receptors (46). Accordingly, Bauer et al. reported that, in response to anemia or hypoxia, splenic CFU-E progenitors expand in a GR-dependent manner (11). Consistent with this, we showed that pretreatment with RU486 has totally prevented the stimulatory effect of repeated restraint procedure on late stage erythroid progenitors in the spleen, suggesting a GR-dependent expansion of CFU-E cells during chronic restraint stress. Moreover, markedly decreased number of GR-expressing cells in the red pulp of chronically restrained mice, along with an increase in circulating levels of corticosterone, found in these animals, indicates a ligand-induced activation of GR, confirming the role of this receptor in erythropoietic response to chronic psychological stress. In addition, chronic psychological stress induces glucocorticoid insensitivity in splenic myeloid cells (51, 52), and accordingly, reduced GR expression in the red pulp of chronically restrained animals may represent the beginning of the glucocorticoid resistance in erythroid cells. Furthermore, stress-induced changes in glucocorticoid sensitivity may affect each erythroid cell type differently, thereby causing different BFU-E and CFU-E responses to RU486 treatment in stressed animals. However, further studies on stress-induced glucocorticoid resistance in splenic erythroid cells are needed to test this hypothesis as well as to analyze the glucocorticoid response at the single cell level.
Using in vitro approach, Stellacci et al. demonstrated a direct interaction between GR and EPOR through their physical association in the membrane of human erythroid cells, suggesting a membrane-associated pathway of GR and EPOR signaling (53). Recently, we have showed decreased protein levels of both receptors in the splenic extracts of chronically stressed mice (29). Moreover, analyzing the expression level in each animal, we found that greater decrease in GR was accompanied by greater decrease in EPOR, and vice versa, suggesting an association between GR and EPOR during their activation and a possible cooperative interaction.
The simultaneous and cooperative action between glucocorticoids and other factors, such as: erythropoietin, bone morphogenetic protein 4 and stem cell factor, promotes extramedullary erythropoiesis during chronic psychological stress (29). In accordance, pretreatment with RU486, the GR antagonist, caused only a slight decrease in spleen weight of stressed animals. However, since impaired GR signaling is unifying mechanism for development of erythrocytosis in syndromes associates with chronic exposure to high levels of glucocorticoids, such as Cushing syndrome and immunosuppressive treatments as well as in patients with polycythemia vera (54), GR may represent a diagnostic tool to predict enhanced erythroid cell development in subjects chronically exposed to high concentrations of glucocorticoids.
In conclusion, we have demonstrated for the first time that blockade of GR completely abolishes the stimulatory effect of chronic psychological stress on CFU-E formation in the mouse spleen, suggesting an indispensable role for GR in the expansion of erythropoietin-dependent CFU-E progenitors under chronic stress conditions. Further studies are needed to investigate molecular background with a possible involvement of GR/EPOR crosstalk signaling in restraint stress-induced erythropoietic response.
Acknowledgments: This work was supported by a grant (175053) from the Ministry of Education, Science and Technological Development of Republic of Serbia. We would like to thank Professor Vesna Koko for advice on histological analysis and continuous support. Technical help of Mrs. Snezana Markovic and Mrs. Leposava Jovanovic is appreciated.
Conflict of interests: None declared.
REFERENCES
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol 2003; 463: 235-272.
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci 2009; 10: 459-466.
- Patchev VK, Patchev AV. Experimental models of stress. Dialogues Clin Neurosci 2006; 8: 417-432.
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev 2009; 33: 1089-1098.
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55-89.
- Riedemann T, Patchev AV, Cho K, Almeida OF. Corticosteroids: way upstream. Mol Brain 2010; 3: 2.
- Chen W, Dang T, Blind RD, et al. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol 2008; 22: 1754-1766.
- Gursoy A, Dogruk Unal A, Ayturk S, et al. Polycythemia as the first manifestation of Cushing’s disease. J Endocrinol Invest 2006; 29: 742-744.
- Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 2011; 118: 6258-6268.
- Socolovsky M. Molecular insights into stress erythropoiesis. Curr Opin Hematol 2007; 14: 215-224.
- Bauer A, Tronche F, Wessely O, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev 1999; 13: 2996-3002.
- Lodish H, Flygare J, Chou S. From stem cell to erythroblast: regulation of red cell production at multiple levels by multiple hormones. IUBMB Life 2010; 62: 492-496.
- Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol 2011; 18: 139-145.
- Perry JM, Harandi OF, Paulson RF. BMP4, SCF and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood 2007; 109: 4494-4502.
- Perry JM, Harandi OF, Porayette P, Hegde S, Kannan AK, Paulson RF. Maintenance of the BMP4-dependent stress erythropoiesis pathway in the murine spleen requires hedgehog signaling. Blood 2009; 113: 911-918.
- O’Neill HC. Niches for extramedullary hematopoiesis in the spleen. Niche 2012; 1: 12-16.
- Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 2005; 105: 2741-2748.
- Dumitriu B, Bhattaram P, Dy P, et al. Sox6 is necessary for efficient erythropoiesis in adult mice under physiological and anemia-induced stress conditions. PLoS One 2010; 5: e12088.
- Ottenweller JE, Servatius RJ, Natelson BH. Repeated stress persistently elevates morning, but not evening, plasma corticosterone levels in male rats. Physiol Behav 1994; 55: 337-340.
- Branch DR, Calderwood S, Cecutti MA, Herst R, Solh H. Hematopoietic progenitor cells are resistant to dimethyl sulfoxide toxicity. Transfusion 1994; 34: 887-890.
- Kleinschnitz C, Blecharz K, Kahles T, et al. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 2011; 42: 1081-1089.
- Budec M, Vignjevic S, Markovic D, et al. Effects of chronic psychological stress on extramedullary erythropoiesis: involvement of EPOR, GR, c-kit and BMP4 signaling. Haematologica 2013; 98(s1): 418.
- Yarushkina NI, Bagaeva TR, Filaretova LP. Central corticotropin-releasing factor (CRF) may attenuate somatic pain sensitivity through involvement of glucocorticoids. J Physiol Pharmacol 2011; 62: 541-548.
- Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life 2009; 61: 800-830.
- Hara H, Ogawa M. Erythropoietic precursors in mice with phenylhydrazine-induced anemia. Am J Hematol 1976, 1: 453-458.
- Dor FJ, Ramirez ML, Parmar K, et al. Primitive hematopoietic cell populations reside in the spleen: studies in the pig, baboon, and human. Exp Hematol 2006; 34: 1573-1582.
- Morita Y, Iseki A, Okamura S, Suzuki S, Nakauchi H, Ema H. Functional characterization of he matopoietic stem cells in the spleen. Exp Hematol 2011; 39: 351-359.
- Yanai N, Matsuya Y, Obinata M. Spleen stromal cell lines selectively support erythroid colony formation. Blood 1989; 74: 2391-2397.
- Vignjevic S, Budec M, Markovic D, et al. Chronic psychological stress activates BMP4-dependent extramedullary erythropoiesis. J Cell Mol Med 2014; 18: 91-103.
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol 2004; 148: 106-115.
- Stiner-Jones L, Reader B, Minnillo J, Sheridan J. Restraint stress alters innate immunity. Brain Behav Immun 2012; 26 (s1): S41-S42.
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses inmice. Brain Behav Immun 2008; 22: 105-113.
- Hernandez ME, Martinez-Mota L, Salinas C, et al. Chronic stress induces structural alterations in splenic lymphoid tissue that are associated with changes in corticosterone levels in Wistar-Kyoto rats. Biomed Res Int 2013; 2013: 868742.
- Bakovic D, Pivac N, Zubin Maslov P, Breskovic T, Damonja G, Dujic Z. Spleen volume changes during adrenergic stimulation with low doses of epinephrine. J Physiol Pharmacol 2013; 64: 649-655.
- Kerenyi MA, Orkin SH. Networking erythropoiesis. J Exp Med 2010; 207: 2537-2541.
- Vanhees K, Coort S, Ruijters EJ, et al. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J 2011; 25: 797-807.
- Kaminska B, Ciereszko R, Kiezun M, Dusza L. in vitro effects of genistein and daidzein on the activity of adrenocortical steroidogenic enzymes in mature female pigs. J Physiol Pharmacol 2013; 64: 103-108.
- Voorhees JL, Powell ND, Moldovan L, Mo X, Eubank TD, Marsh CB. Chronic restraint stress upregulates erythropoiesis through glucocorticoid stimulation. PloS One 2013; 8: e77935.
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000; 886: 172-189.
- Udupa KB, Crabtree HM, Lipschitz DA. in vitroculture of proerythroblasts: characterization of proliferative response to erythropoietin and steroids. Br J Haematol 1986; 62: 705-714.
- Liang R, Chan TK, Todd D. Childhood acute lymphoblastic leukaemia and aplastic anaemia. Leuk Lymphoma 1994; 13: 411-415.
- Necela BM,Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc Am Thorac Soc 2004; 1: 239-246.
- von Lindern M, Zauner W, Mellitzer G, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood 1999; 94: 550-559.
- Chute JP, Ross JR, McDonnell DP. Minireview: Nuclear receptors, hematopoiesis, and stem cells. Mol Endocrinol 2010; 24: 1-10.
- Wessely O, Deiner EM, Beug H, von Lindern M. The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. EMBO J 1997; 16: 267-280.
- Kolbus A, Blazquez-Domingo M, Carotta S et al. Cooperative signaling between cytokine receptorsand the glucocorticoid receptorin the expansion of erythroid progenitors: molecular analysis by expression profiling. Blood 2003; 102: 3136-3146.
- Zhang L, Prak L, Rayon-Estrada V, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature 2013; 499: 92-96.
- Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood 2011; 117: 3435-3444.
- Millot S, Andrieu V, Letteron P, et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation. Blood 2010; 116: 6072-6081.
- Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol 2005; 15: 146-155.
- Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology 2008; 33: 108-117.
- Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptorresistance, inflammation, anddiseaserisk. Proc Natl Acad Sci USA 2012; 109: 5995-5999.
- Stellacci E, Di Noia A, Di Baldassarre A, Migliaccio G, Battistini A, Migliaccio AR. Interaction between the glucocorticoid and erythropoietin receptors in human erythroid cells. Exp Hematol 2009; 37: 559-572.
- Varricchio L, Masselli E, Alfani E, et al. The dominant negative b isoform of the glucocorticoid receptor is uniquely expressed in erythroid cells expanded from polycythemia vera patients. Blood 2011; 118: 425-436.
A c c e p t e d : November 17, 2014