N-ACETYLCYSTEINE EFFECTIVELY DIMINISHED MECONIUM-INDUCED OXIDATIVE STRESS IN ADULT RABBITS
INTRODUCTION
Meconium aspiration syndrome (MAS) is a severe respiratory disorder that occurs in the term and post-term neonates. When meconium, the first faeces of the newborn, is aspirated, it obstructs the peripheral airways, deteriorates the surfactant function, and induces inflammation. Finally, it results in oxidative lung injury, lung edema formation, pulmonary vasoconstriction, and airway hyperresponsiveness (1).
Inflammation and damage of the lung tissue can be caused by both fractions of meconium: hydrophilic (consisting of gastrointestinal enzymes including pancreatic phospholipase A2 or bile acids) and hydrophobic (consisting of cholesterol, free fatty acids etc.). In addition, meconium contains variable amounts of cytokines (IL-1β, IL-6, IL-8, TNFα), and heme (2). Consequently, components of meconium enhance chemotactic activity of polymorphonuclears (PMNs), particularly of neutrophils, and stimulate their leak through the alveolocapillary membrane (3). However, meconium may induce inflammation also indirectly, as it stimulates expression of cytokines (e.g. TNFα, IL-1, IL-6, IL-8), pro-inflammatory enzymes (phospholipase A2, proteases), and other bioactive substances such as derivatives of arachidonic acid, endothelin-1, platelet activating factor, etc. from the activated cells. Furthermore, the cells generate large amounts of reactive oxygen species (ROS) (4, 5). All the mentioned substances dispose to subsequent surfactant dysfunction, parenchymal damage, edema, pulmonary vasoconstriction, and bronchial smooth muscle contraction (1). Furthermore, oxidative injury is partly iatrogenic and results from high concentrations of oxygen used for resuscitation of the patients with MAS.
Considering the role of inflammation in MAS, various anti-inflammatory drugs including antioxidants (6-14) have been used in research studies on MAS. For example, intratracheal administration of recombinant human superoxide dismutase (rhSOD) decreased myeloperoxidase activity, nitric oxide and 8-isoprostane levels and lung injury score in the meconium-instilled rats (9). In the newborn lambs with persistent pulmonary hypertension, rhSOD increased oxygenation, and reduced vasoconstriction and oxidative injury (10).
We have presumed that other representative of antioxidants, N-acetylcysteine (NAC), can be also beneficial in MAS, as it possesses many valuable properties. NAC is N-acetyl derivative of amino acid L-cysteine. Thanks to content of -SH group, NAC acts as a direct scavenger of hydrogen peroxide, hydroxyl radicals, and hypochlorous acid. In addition, NAC is fastly deacetylated to cysteine, a precursor of glutathione synthesis in cells, hence regulating redox status in the cells (15). Furthermore, NAC may reduce generation of pro-inflammatory substances (e.g. TNFα and IL-1) in the cells (16). Alongside potent antioxidant and anti-inflammatory effects, NAC decreases viscosity and elasticity of mucus because of its ability to reduce disulphide bonds (17), what might be useful for meconium clearance from the airways (18). In addition, NAC may partially prevent surfactant dysfunction as it stimulates expression of surfactant protein (SP)-A mRNA (19). Thanks to low bioavailability, NAC is virtually non-toxic. Adverse effects, such as anaphylaxis, tachycardia and hypotension, or reduced chemotaxis and increased cytotoxicity to PMNs are rare and limited to very high concentrations (20). The mentioned favorable properties have designated NAC as potentially beneficial in the treatment of MAS. Its mucolytic effects may improve the removal of highly viscous meconium from the airways and complex antioxidative and anti-inflammatory properties may reduce the meconium-induced lung injury.
As there is no relevant information up to now on the use of NAC in the meconium-induced lung injury, purpose of this pilot study was to determine efficacy of NAC to reduce formation of ROS and to prevent oxidative damage in relation to leukocyte migration into the lung of experimental animals with MAS. The antioxidative action of NAC was detected in the lung tissue homogenate and subsequently in the lung mitochondria fraction, as the oxidation processes are most prominent in the mitochondria. To assess meconium-induced oxidation and antioxidative effect of the treatment on the systemic level, concentrations of TBARS as a marker of lipid peroxidation and total antioxidant status (TAS) were estimated also in the blood plasma.
MATERIAL AND METHODS
General design of experiments
Fresh meconium was collected from 20 healthy neonates, lyophilized and stored at –20°C. Before use, meconium was suspended in 0.9% NaCl at a concentration of 25 mg/ml.
Design of the experiments was approved by Local Ethics Committee of Jessenius School of Medicine. Adult rabbits (chinchilla) of 2.5 ± 0.3 kg were anesthetized with intramuscular ketamine (20 mg/kg; Narkamon, Spofa, Czech Republic) and xylazine (5 mg/kg; Rometar, Spofa, Czech Republic) followed by a continuous infusion of ketamine (20 mg/kg/h). A tracheotomy was performed and catheters were inserted into the femoral artery for sampling arterial blood, and into the femoral vein to administer anesthetics. The animals were then paralyzed with pipecuronium bromide (0.3 mg/kg/30 min; Arduan, Gedeon Richter, Hungary) and subjected to a pressure-controlled ventilator (Beat-2, Chirana, Slovakia). Animals were ventilated with a frequency of 30/min, fraction of inspired oxygen (FiO2) of 0.21, inspiration time 50%, peak inspiratory pressure (PIP) of 0.6 kPa to keep a tidal volume (VT) between 7–9 ml/kg, and no positive end-expiratory pressure (PEEP) in this stage of the experiment. After stabilization, ventilatory parameters were recorded and sample of arterial blood was taken for blood gas analysis (RapidlabTM348, Bayer Diagnostics, Germany). Then, 4 ml/kg of saline (Sal group, n=6) or meconium suspension (25 mg/ml) were instilled into the tracheal tube homogenously into the right and left lung lobes during positioning of the animal. From this moment on, settings of ventilation were changed: FiO2 was increased to 1.0, PIP/PEEP to 1.5–1.7/0.3 kPa in meconium-instilled animals and to 1.0/0.2 kPa in saline-instilled animals to keep VT between 7–9 ml/kg and arterial pCO2 in the range of 5.3–7.3 kPa. Within 30 minutes after meconium instillation, respiratory failure developed, defined as >30% decrease in dynamic lung-thorax compliance (Cdyn) and arterial partial pressure of oxygen (PaO2) <10 kPa at FiO2 1.0. Sample of arterial blood was taken for blood gas analysis and parameters were recorded again. Meconium-instilled animals then intravenously received N-acetylcysteine (ACC Injekt, Salutas Pharma GmbH, Germany; Mec + NAC group, n=6) at a dose of 10 mg/kg 30 min after meconium instillation, or were left without treatment (Mec group, n=6). Animals were oxygen-ventilated for additional 5 hours.
Tracheal airflow and VT were measured by a Fleisch head connected to a pneumotachograph. Airway pressure was registered via a pneumatic catheter placed below the end of tracheal tube and connected to an electromanometer. Cdyn was calculated as a ratio between VT adjusted per kg b.w. and airway pressure gradient (PIP-PEEP) (21). Samples of arterial blood were taken at the end of experiment and centrifuged at 3000 rpm for 15 min. Blood plasma was stored at –70°C till the biochemical analyses were performed. Then, the animals were euthanized by an overdose of anesthetics. The trachea and lung were excised. The right lung was cut to small pieces. Pieces from both more and less affected regions were equally used for further investigations, i.e. for estimation of wet/dry weight ratio (see below), or were stored at –70°C for biochemical analyses. Left lung was lavaged with saline (3 × 10 ml/kg, 0.9% NaCl), and bronchoalveolar lavage (BAL) fluid was centrifuged at 1500 rpm for 10 min.
Cells in the bronchoalveolar lavage fluid and blood
Samples of arterial blood were taken before meconium instillation, and at 1, 3, and 5 h of the treatment. Total blood leukocyte count was determined microscopically in a counting chamber after staining by Turck. Differential leukocyte count was estimated microscopically after staining by May-Grunwald/Giemsa-Romanowski.
Total number of cells in the BAL fluid was determined microscopically in a counting chamber. Differential cell count in the sediment was evaluated microscopically after staining by May-Grunwald/Giemsa-Romanowski.
Lung edema formation (wet/dry lung weight ratio)
Strips of the right lung tissue were weighed and dried at 60°C for 24 hours. The ratio between wet and dry weight expressed severity of lung edema.
Biochemical analyses
Lung tissue was washed, minced and homogenized in 50 mM of phosphate buffer (pH=7.4) and 1 mM of butylated hydroxytoluene (BHT) in a ratio 1:5 using homogenizer (Potter, B. Braun Melsungen A.G., Germany) at a temperature 0–4°C. A protein assay was performed by method of Lowry et al. (22). For dilution of the homogenate, 1% sodium dodecyl sulfate (SDS) was used. The protein concentration was calculated using bovine serum albumine (BSA) as a standard.
Mitochondria fraction was prepared from the tissue homogenate by differential centrifugation. Lung homogenate was centrifuged at 400 g for 5 min and supernatant was collected. The supernatant was then centrifuged at 12,000 g for 10 min. The resulting pellet was resuspended in a homogenisation buffer (25 mM 4-morpholinepropanesulfonic acid, 250 mM sucrose, 4 mM MgCl2, 0.05 mM EGTA, pH 7.4) and centrifuged at 12,000 g for 10 min. The final pellet was resuspended in the homogenisation buffer (see above) and stored on ice. The protein concentration was determined by methods of Lowry et al. (22).
Fluorescence measurements were performed in a solution containing 50 µg of membrane protein per ml, 10 mM HEPES, 100 mM KCl, pH 7.0 at 25°C using a spectrofluorimeter (RF-540, Shimadzu, Japan). Fluorescence emission spectra (380–440 nm, slit width 5 nm) of dityrosine, a product of tyrosine oxidation, were measured at an excitation wavelength 325 nm (slit width 5 nm). Emission spectra (from 425 to 480 nm, slit width 5 nm) of lysine conjugates with lipid peroxidation (LPO) products were recovered at an excitation of 365 nm (5 nm slit width). Excitation spectra (from 325 to 380 nm, 5 nm slit width) were measured at 440 nm (5 nm slit width). Fluorescence intensity was expressed in arbitrary units (A.U.) (23).
A determination of thiobarbituric acid-reactive substances (TBARS) formation was performed according to Das (24). TBARS concentrations in the lung homogenate and in the mitochondria were determined from the absorbance at 532 nm and expressed in nM/mg protein, TBARS in the plasma were expressed in nM/ml.
The formation of conjugated dienes in the mitochondria was estimated from the absorbance ratio A233nm/A215nm of mitochondria (0.02 mg protein per ml) dispensed in 10 mM phosphate buffer containing 1% Lubrol (25). The total thiol group content in the mitochondria was determined spectrophotometrically at 412 nm (e=13.6 mM–1cm–1) as described by Hu (26), and expressed in µM/mg protein.
The activity of cytochrome c oxidase (COX) in the mitochondria was measured by monitoring of ascorbate reduced cytochrome c. Membrane proteins were resuspended in a reaction buffer (50 mM Tris/HCl (pH 8.0, 0.01% n-dodecyl-β-D-maltoside). The reaction was initialized by addition of reduced cytochrome c to the final concentration of 0.05 mM. Oxidation of cytochrome c was monitored spectrophotometrically at 550 nm by Ultrospec III (Pharmacia-Amersham) and a rate of oxidation was calculated from the slope of absorbance dependence on the incubation time using the value of 19.6 M–1cm–1 as a molar extinction coefficient of oxidized cytochrome c at 550 nm. Cytochrome c was reduced with ascorbic acid and separated on a Sephadex G-25 column. The concentration of reduced cytochrome c was controlled by the measurement of the absorbance ratio A550nm/A565nm. COX activity was expressed in nM of oxidized cytochrome c per mg of protein per min (27).
Quantification of total antioxidant status (TAS) in the lung homogenate and in the blood plasma was carried out by ELISA kit using ABTS (2,2’-azino-di-[3-ethylbenzthiazoline sulphonate]) radical formation kinetics (Randox TAS kit, Randox Laboratories Ltd., UK) and expressed in mM/l. Eosinophil cationic protein (ECP) in the plasma and lung homogenate was detected by ELISA kits (Diagnostics Development, Sweden) and were expressed in µg/l.
Statistical analysis
Between-group differences were evaluated by one-way analysis of variance (ANOVA) with the post-hoc Fisher’s LSD test, within-group differences were evaluated by Wilcoxon’s test. Strength of association between the markers was expressed by Pearson’s correlation coefficient (r) and Bonferroni probability (P). P<0.05 was considered statistically significant. Data were expressed as means ± S.E.M.
RESULTS
Cell counts in the arterial blood
The total leukocyte count in the blood gradually decreased after meconium instillation and was lower in Mec vs. Sal group at 1 and 3 h (P<0.01) and at 5 h of the treatment (P<0.001). NAC increased total circulating leukocytes compared to Mec group at the end of experiment (P<0.05; Fig. 1).
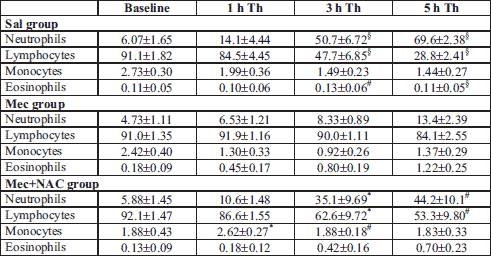
At 5 h of the treatment, relative number of neutrophils was lower and numbers of lymphocytes and eosinophils were higher in the Mec vs. Sal group (all P<0.001). NAC elevated circulating neutrophils and decreased lymphocytes (both P<0.001). In addition, NAC increased monocytes (P<0.05 or 0.01) during the experiment and showed a tendency to reduce eosinophils (P<0.05) compared to the Mec group (Table 1).
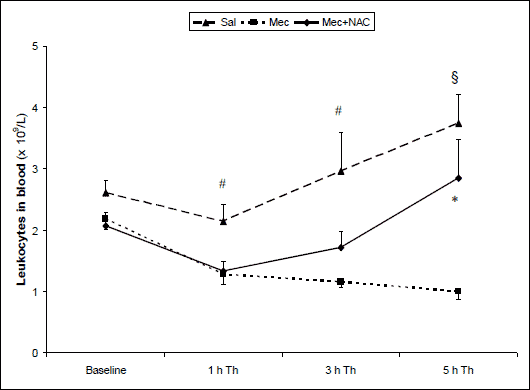 |
Fig. 1. Total leukocyte count in the arterial blood (×109/l) at the end of experiments in the saline-instilled group (Sal), in the meconium-instilled non-treated (Mec) group, and in the meconium-instilled and N-acetylcysteine-treated group (Mec + NAC). For comparisons vs. Mec: *P<0.05, #P<0.01, §P<0.001. |
Cell counts in the bronchoalveolar lavage fluid
Total count of cells in the BAL fluid was higher in the Mec vs. Sal group (1842 ± 358×103/µl in Mec group vs. 155 ± 45 × 103/µl in Sal group; P<0.001). NAC decreased the number of cells migrating into the alveolar space compared with Mec group (913 ± 217 × 103/µl in Mec+NAC group; P<0.05).
The presence of meconium in the lungs elevated the relative numbers of neutrophils (P<0.001) and eosinophils (P<0.01) and decreased the monocyte-macrophages (P<0.001) in the BAL fluid compared to the Sal group. NAC decreased neutrophils (P<0.001) and eosinophils (P<0.01) and elevated the relative number of monocyte-macrophages (P<0.001) compared to the Mec group (Fig. 2).
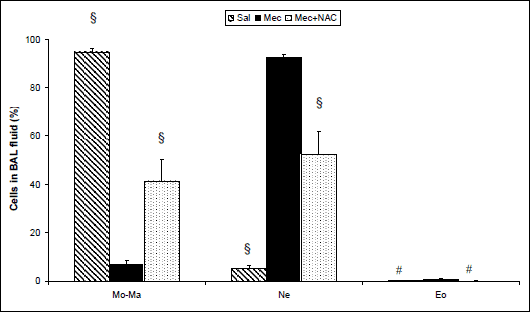 |
Fig. 2. Differential leukocyte count (Mo-Ma; monocyte-macrophages, Ne; neutrophils, Eo; eosinophils) in the BAL fluid (%) at the end of experiments in the saline-instilled group (Sal), in the meconium-instilled non-treated (Mec) group, and in the meconium-instilled and N-acetylcysteine-treated group (Mec + NAC). For comparisons vs. Mec: #P<0.01, §P<0.001. |
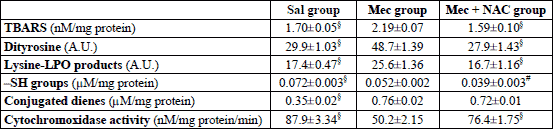
Oxidation markers in the lung homogenate
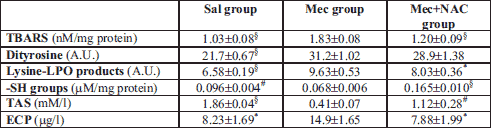
Meconium aspiration caused significant oxidative stress in the lung, as indicated by increased TBARS, a representative of lipid oxidation, and by increased concentrations of dityrosines and lysine-LPO products, markers of protein oxidation (all P<0.001 in Mec vs. Sal group). Higher production of ROS was additionally proven by decreased content of -SH groups (P<0.01) and lower total antioxidant status (TAS) (P<0.001). Activation of PMNs was expressed also by higher ECP in the lung homogenate (P<0.05; Table 2). NAC decreased formation of TBARS (P<0.001) and lysine-LPO products (P<0.05) and showed a trend to decrease dityrosines (P>0.05). NAC elevated the total content of thiol groups (P<0.001 vs. Mec group) and prevented a decrease in TAS (P<0.01; Table 2). In addition, NAC decreased ECP in the lung tissue (P<0.05; Table 2).
Oxidation markers in the lung mitochondria
To elucidate the oxidative processes in the meconium-injured lungs more in detail, oxidative stress was determined also in the isolated lung mitochondria. In the Mec group, higher concentrations of conjugated dienes and TBARS, indicators of lipid peroxidation, as well as higher concentrations of –SH groups, dityrosines and lysine-LPO products were found in comparison to Sal group (all P<0.001). In addition, activity of cytochrome c oxidase in the mitochondria decreased after meconium instillation compared to the saline-instilled animals (P<0.001; Table 3). NAC prevented a decrease in cytochrome c oxidase activity compared to the non-treated Mec group (P<0.001) and significantly reduced the concentrations of TBARS and fluorescence intensity of dityrosines and lysine-LPO products (all P<0.001). The conjugated dienes slightly decreased (P>0.05) and total content of –SH groups was lower than in the Mec group (P<0.01; Table 3).
Oxidation markers in the plasma
To estimate impact of inflammation and oxidative changes in the lungs on the systemic level, some markers were determined also in the blood plasma taken at the end of experiments. Concentrations of TBARS (P<0.001; Fig. 3) and ECP (P<0.05; Fig. 4) increased and TAS decreased (P<0.05; Fig. 5) in the Mec vs. Sal group. NAC administration decreased TBARS (P<0.001; Fig. 3) and ECP (P<0.05; Fig. 4) and prevented elevation in TAS (P<0.05; Fig. 5) in comparison to Mec group.
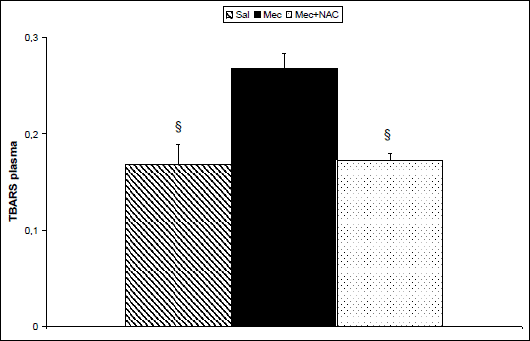 |
Fig. 3. Thiobarbituric acid-reactive substances (TBARS, in nM/ml) in the plasma at the end of experiments in the saline-instilled group (Sal), in the meconium-instilled non-treated (Mec) group, and in the meconium-instilled and N-acetylcysteine-treated group (Mec + NAC). For comparisons vs. Mec: §P<0.001. |
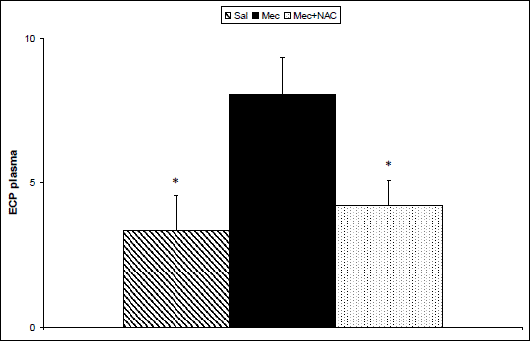 |
Fig. 4. Eosinophil cationic protein (ECP, in µg/l) in the plasma at the end of experiments in the saline-instilled group (Sal), in the meconium-instilled non-treated (Mec) group, and in the meconium-instilled and N-acetylcysteine-treated group (Mec + NAC). For comparisons vs. Mec: *P<0.05. |
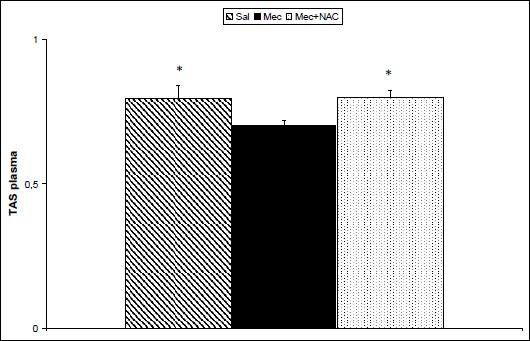 |
Fig. 5. Total antioxidant status (TAS, in mM/l) in the plasma at the end of experiments in the saline-instilled group (Sal), in the meconium-instilled non-treated (Mec) group, and in the meconium-instilled and N-acetylcysteine-treated group (Mec + NAC). For comparisons vs. Mec: *P<0.05. |
Lung edema formation
Meconium instillation increased edema formation expressed as a wet/dry lung weight ratio (W/D ratio) compared to saline instillation in the Sal group (P<0.001). NAC diminished the wet-dry ratio in comparison to the non-treated Mec group (P<0.001; Fig. 6).
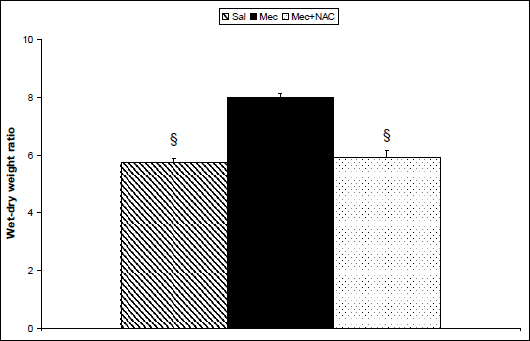 |
Fig. 6. Formation of lung edema expressed by wet/dry lung weight ratio in the saline-instilled group (Sal), in the meconium-instilled non-treated (Mec) group, and in the meconium-instilled and N-acetylcysteine-treated group (Mec + NAC). For comparisons vs. Mec: §P<0.001. |
Correlations between the measured parameters
Pearson’s evaluation showed significant correlations between counts of total cells, neutrophils and eosinophils in the BAL fluid vs. biochemical markers in the lung homogenate, mitochondria, or in the plasma. Clear correlations were also found between wet/dry lung ratio and cells in the BAL fluid and in the blood at the end of experiment; and between wet/dry ratio vs. almost all biochemical markers in the lung homogenate, in the mitochondria, and in the plasma (all P<0.05).
DISCUSSION
Inflammation and oxidative stress play a key role in the development of severe MAS. In the meconium-instilled rabbits, NAC decreased number of cells migrating into the alveolar space, particularly of neutrophils. In addition, NAC reduced formation of oxidation products in the lung homogenate and isolated mitochondria, prevented a meconium-induced decrease in total antioxidant status and diminished lung edema formation.
Meconium acts as a potent chemoattractant for PMNs. Higher counts of neutrophils may be observed in the lung within several hours after meconium instillation (11). In this study, relative numbers of neutrophils and eosinophils in the BAL fluid increased after meconium instillation compared to saline-instilled controls, presumably due to their demargination and migration through the capillary wall into the interstitium, and further into the alveolar spaces. Subsequently, decreased neutrophils in the blood were detected, while eosinophils remained increased. This discrepancy may be explained by different mechanisms regulating the influx of these two types of leukocytes into the alveolar space (28, 29).
Decrease in circulating PMNs and extent of their accumulation in the lung may be proportional to severity of the lung injury (30). In this study, clear correlations were found between the lung edema formation and cells in the BAL fluid and cells circulating in the blood, respectively. NAC reduced both neutrophils and eosinophils in the BAL fluid in the treated animals in comparison to the non-treated meconium-instilled rabbits. Similarly, decreased neutrophils in the alveolar space after NAC were found in other models of acute lung injury (31-33). On the other hand, NAC showed no effect on neutrophil population in the BAL fluid in rats with lung contusion (34), or LPS-induced lung injury (35).
The influx of PMNs into the meconium-exposed lung is associated with production of a wide range of bioactive substances. To estimate participation of eosinophils in the meconium-induced lung injury, ECP as a marker of eosinophil activation was detected (36). In both the lung homogenate and plasma, concentrations of ECP increased after meconium instillation and decreased after NAC. ECP in the lung homogenate significantly correlated with the number of eosinophils in the BAL fluid, while the relationship to the number of BAL neutrophils was non-significant (P = 0.078, r = 0.426). On the other hand, plasma ECP correlated significantly with neutrophils in the BAL fluid (P = 0.019, r = 0.563) and non-significantly with their decrease in the blood (P = 0.084, r = –0.431). These findings might be partially explained by the fact that ECP may be produced in lower amounts also by neutrophils (37).
Activated leukocytes generate also ROS, which may directly injure the lung tissue. In these experiments, meconium instillation increased markers of protein and lipid oxidation in the lung homogenate and isolated lung mitochondria compared to saline instillation.
Lipoperoxidation (LPO) of membrane lipids was expressed by the production of conjugated dienes (CD) and malonyldialdehyde (MDA). CD are products of interaction of ROS with unsaturated bonds of lipids (24). Free MDA molecules react with free thiol (–SH) groups of cysteine or amino (–NH2) groups of lysine and generate complexes reacting with thiobarbituric acid (TBA), which create thiobarbituric acid-reactive substances (TBARS) (24). Thus, an obvious increase in TBARS and CD in this study clearly showed overproduction of ROS after meconium instillation.
Intravenous NAC significantly reduced TBARS in both the lung homogenate and mitochondria, while CD in the mitochondria decreased just slightly. Similarly, in various models of acute lung injury, NAC reduced the generation of MDA in the lung tissue and serum of rats (32, 34, 38) and lowered LPO-markers in the lungs of rats with LPS-induced lung injury (35) and in mice with hyperoxic lung injury (33). However, up to this moment, there are no studies evaluating antioxidative effects of NAC in MAS.
As overproduction of ROS caused by meconium aspiration may subsequently oxidize proteins, several indicators of protein oxidation were determined, too. Content of thiol groups, components of cysteine and glutathione, i.e. amino acids important in the antioxidant protection system, is a sensitive marker of oxidative injury. In the presence of ROS, free -SH groups are easily oxidized and make disulfide bonds, what decreases their concentration (26). In our study, thiol groups significantly decreased after meconium instillation in both the lung homogenate and mitochondria. Administration of NAC increased the content of –SH groups in the homogenate, presumably because NAC itself contains –SH group in the molecule. However, we may just speculate on the mechanisms responsible for a slight decrease in content of –SH groups in the mitochondria compared to the Mec group. Probably, the concentration of free radicals in the mitochondria, site of the pronounced oxidation, was so high due to inflammation and oxygen ventilation that free –SH groups were occupied, and that process was irreversible during the time of observation. Furthermore, –SH groups originated from NAC may be utilized sooner than they reach mitochondria.
Protein oxidation by ROS may cause modification of amino acid side chains and formation of new groups, cleavage of peptide bonds, or formation of covalent protein-protein cross bonds. Products of oxidative modification of aromatic amino acids (e.g. dityrosines or lysine-LPO products) may be easily detected (23, 39). In this study, the fluorescence intensity of dityrosine, a result of tyrosine oxidation, and lysine conjugates with LPO products increased after meconium instillation and diminished after NAC treatment.
Metabolic functions of mitochondria in the presence of ROS may be determined by estimation of activity of Krebs cycle enzymes participating in the production of ROS or individual enzymatic complexes of the Krebs cycle. Cytochrome c oxidase (COX) is the last enzyme in the respiratory electron transport chain of mitochondria located in the membrane. As COX is highly sensitive to oxidative stress (27), decreased activity of COX after meconium instillation indirectly indicates inhibition of its enzymatic activity due to accumulation of ROS, which may cause the post-translational modification of proteins. NAC treatment partially prevented a decrease in COX activity. To estimate the antioxidant capacity of the lung tissue and blood plasma, total antioxidant status (TAS) was measured. TAS includes various antioxidants, such as glutathione, vitamins C, A, and E, and enzymes (e.g. catalase, superoxide dismutase and peroxidases). Low concentrations of antioxidants or inhibition of the antioxidant enzymes cause oxidative stress and cell damage or death (40). In this study, meconium instillation decreased TAS in both lung homogenate and plasma, while NAC increased the antioxidant capacity, probably due to promoting cellular glutathione production (20, 41), and/or due to reduced production of oxidants. In addition to TAS, concentration of TBARS in the plasma was determined to evaluate the systemic effects of meconium instillation. High concentrations of TBARS detected not only in the lungs, but also in the plasma after the meconium instillation support the previous observations that the distant organs and tissues (e.g. hippocampus) may be damaged by oxidation, as well (42). Similarly to our results, NAC increased TAS and decreased serum MDA in rat models of oxidative lung injury (38, 43).
In addition, ROS can activate several signaling pathways including mitogen-activated protein kinase (MAPK) signaling, which may ultimately promote inflammation (44). Furthermore, end-products of lipid peroxidation activate extracellular signal-regulated kinase p44/42 (Erk1/2), c-Jun N terminal kinase, and p38MAPK, whereas this activation may be blocked by NAC (45, 46). Activation of the mentioned kinases is accompanied by increased activity of various transcription factors including activating protein (AP)-1 and nuclear factor (NF)-κB (47, 48). Supporting this hypothesis, NAC decreased concentration of NF-κB in the lung tissue in a recent study (34). In addition, NAC is known to inhibit activity of proteolytic enzymes (e.g. matrix metalloproteinases or urokinase-type plasminogen activator) (49, 50). As endogenous and meconium-derived proteases have been suggested as playing part in the pathogenesis of MAS, some of the protective effects offered by NAC in these experiments might be partially explained also by inhibition of these enzymes.
Reduced generation of ROS and other pro-inflammatory substances following NAC treatment finally resulted in decreased formation of lung edema. However, diminished lung edema is also attributed to other effects of NAC, particularly to the ability to reduce pulmonary vasoconstriction and right-to-left pulmonary shunts (34, 51). In the models of acute lung injury, NAC decreased pulmonary vascular resistance and lung edema in dogs with oxygen-induced lung injury (51), reduced alveolar disruption and edema in rats with lung contusion (34), and decreased edema formation in LPS-induced lung injury in rats (35).
NAC exerted complex antioxidative action in the meconium-induced acute lung injury on all measured levels: mitochondrial, organ, and systemic. As preventing mitochondrial oxidative stress (52) has become fundamental for effective therapeutic strategy in disorders with mitochondrial dysfunction, NAC acting as a direct radical scavenger and a stimulator of mitochondrial glutathione and other thiol-based antioxidants can be useful in a wide spectrum of diseases including COPD, interstitial lung diseases, hyperoxic lung injury, fever, cardiovascular, oncological or neurodegenerative diseases (50, 53-59). In this moment, we can just hypothesize which of the mentioned mechanisms of NAC protects the lung from oxidative stress and in what extent, or whether NAC protects from ventilation-induced oxidative stress rather than from meconium-induced oxidative stress etc. Nevertheless, antioxidative and anti-inflammatory effects of NAC appeared to be significant in animal model of MAS, therefore we can suggest that it might be useful also in the newborns suffering from MAS.
Concluding, instillation of meconium caused significant lung inflammation, oxidative stress, and lung edema. Intravenous NAC reduced the influx of PMNs into the lung and decreased concentrations of markers of oxidative stress in the lung homogenate, in isolated mitochondria, as well as in the plasma. Thus, our results suggest that NAC may be perspectively used also in the treatment of MAS.
Acknowledgements: Authors thank D. Kuliskova, Z. Remisova, M. Petraskova and M. Hutko for technical assistance. Study was supported by Projects BioMed (ITMS 26220220187), APVV-0435-11, and by Grants VEGA No. 1/0291/12 and 1/0305/14.
Conflict of interests: None declared.
REFERENCES
- Mokra D, Mokry J. Meconium aspiration syndrome: From pathomechanisms to treatment. New York, Nova Science Publishers, 2010.
- de Beaufort AJ, Bakker AC, van Tol MJD, Poorthius BJ, Schrama AJ, Berger HM. Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured A549 epithelial cells. Pediatr Res 2003; 54: 491-495.
- Yamada T, Minakami H, Matsubara S, Yatsuda T, Kohmura Y, Sato I. Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol 2000; 46: 21-30.
- Soukka HR, Ahotupa M, Ruutu M, Kaapa PO. Meconium stimulates neutrophil oxidative burst. Am J Perinatol 2002; 19: 279-284.
- Craig S, Lopez A, Hoskin D, Markham F. Meconium inhibits phagocytosis and stimulates respiratory burst in alveolar macrophages. Pediatr Res 2005; 57: 813-818.
- Holopainen R, Laine J, Halkola L, Aho H, Kaapa P. Dexamethasone treatment attenuates pulmonary injury in piglet meconium aspiration. Pediatr Res 2001; 49: 162-168.
- Lukkarinen H, Laine J, Lehtonen J, et al. Angiotensin II receptor blockade inhibits pneumocyte apoptosis in experimental meconium aspiration. Pediatr Res 2004; 55: 326-333.
- Korhonen K, Kiuru A, Svedstrom E, Kaapa P. Pentoxifylline reduces regional inflammatory and ventilatory disturbances in meconium-exposed piglet lungs. Pediatr Res 2004; 56: 901-906.
- Lu MP, Du LZ, Gu WZ, Yu ZZ, Chen XX, Yu ZS. Anti-inflammation and anti-oxidation effects of recombinant human superoxide dismutase on acute lung injury induced by meconium aspiration in infant rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 2005; 34: 55-59.
- Lakshminrusimha S, Russell JA, Wedgwood S, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1370-1377.
- Mokra D, Mokry J, Drgova A, Bulikova J, Petraskova M, Calkovska A. Single-dose versus two-dose dexamethasone effects on lung inflammation and airway reactivity in meconium-instilled rabbits. J Physiol Pharmacol 2007; 58 (Suppl. 5): 379-387.
- Mokra D, Mokry J, Drgova A, Petraskova M, Bulikova J, Calkovska A. Intratracheally administered corticosteroids improve lung function in meconium-instilled rabbits. J Physiol Pharmacol 2007; 58 (Suppl. 5): 389-398.
- Tripathi S, Saili A, Dutta R. Inflammatory markers in meconium induced lung injury in neonates and effect of steroids on their levels: a randomized controlled trial. Indian J Med Microbiol 2007; 25: 103-107.
- Mokra D, Drgova A, Mokry J, et al. Comparison of the effects of low-dose vs. high-dose aminophylline on lung function in experimental meconium aspiration syndrome. J Physiol Pharmacol 2008; 59 (Suppl. 6): 449-459.
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 1989; 6: 593-597.
- Haddad JJ. A redox microenvironment is essential for MAPK-dependent secretion of pro-inflammatory cytokines: modulation by glutathione (GSH/GSSG) biosynthesis and equilibrium in the alveolar epithelium. Cell Immunol 2011; 270: 53-61.
- Sadowska AM, Verbraecken J, Darquennes K, De Backer WA. Role of N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis 2006; 1: 425-434.
- Ivanov VA. Meconium aspiration syndrome treatment - new approaches using old drugs. Med Hypotheses 2006; 66: 808-810.
- Fu Z, Yang Z, Li A. The effects of NAC on the expression and activity of SPA in rats inflicted by smoke inhalation injury. Zhonghua Shao Shang Za Zhi 2000; 16: 173-176.
- Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med 1998; 92: 609-623.
- Mokra D, Tonhajzerova I, Pistekova H, et al. Short-term cardiovascular effects of selective phosphodiesterase 3 inhibitor olprinone versus non-selective phosphodiesterase inhibitor aminophylline in a meconium-induced acute lung injury. J Physiol Pharmacol 2013; 64: 751-759.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Dousset N, Ferretti G, Taus M, Valdiguie P, Curatola G. Fluorescence analysis of lipoprotein peroxidation. Methods Enzymol 1994; 233: 459-469.
- Das DK. Cellular, biochemical and molecular aspects of reperfusion injury. Ann NY Acad Sci 1994; 723: XIII-VXI.
- Braughler JM, Duncan LA, Chase RL. The involment of iron in lipid peroxidation. Importance of ferric to ferrous ration in initiation. J Biol Chem 1986; 261: 10282-10289.
- Hu ML. Measurement of protein thiol groups and gluthatione in plasma. Methods Enzymol 1994; 233: 380-385.
- Capaldi RA, Marusich MF, Taanman JW. Mammalian cytochrome c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cell. Methods Enzymol 1995; 260: 117-132.
- Sriramarao P, Norton CR, Borgstrom P, DiScipio RG, Wolitzky BA, Broide DH. E-selectin preferentially supports neutrophil but not eosinophil rolling under conditions of flow in vitro and in vivo. J Immunol 1996; 157: 4672-4680.
- Sheikh Bahaie N, Rao SP, Massoud A, Sriramarao P. GM-CSF differentially regulates eosinophil and neutrophil adhesive interactions with vascular endothelium in vivo. Iran J Allergy Asthma Immunol 2010; 9: 207-217.
- Ferreira PJ, Bunch TJ, Albertine KH, Carlton DP. Circulating neutrophil concentration and respiratory distress in premature infants. J Pediatr 2000; 136: 466-472.
- van Helden HP, van de Meent D, Oostdijk JP, et al. Protection of rats against perfluoroisobutene (PFIB)-induced pulmonary edema by curosurf and N-acetylcysteine. Inhal Toxicol 2004; 16: 549-564.
- Yeh ST, Guo HR, Su YS, et al. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology 2006; 223: 181-190.
- Nagata K, Iwasaki Y, Yamada T, et al. Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir Med 2007; 101: 800-807.
- Turut H, Ciralik H, Kilinc M, Ozbag D, Imrek SS. Effects of early administration of dexamethasone, N-acetylcysteine and aprotinin on inflammatory and oxidant-antioxidant status after lung contusion in rats. Injury 2009; 40: 521-527.
- Choi JS, Lee HS, Seo KH, et al. The effect of post-treatment N-acetylcysteine in LPS-induced acute lung injury of rats. Tuberc Respir Dis (Seoul) 2012; 73: 22-31.
- Venge P, Bystrom J, Carlson M, et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy 1999; 29: 1172-1186.
- Monteseirin J, Vega A, Chacon P, et al. Neutrophils as a novel source of eosinophil cationic protein in IgE-mediated processes. J Immunol 2007; 179: 2634-2641.
- Akca T, Canbaz H, Tataroglu C, et al. The effect of N-acetylcysteine on pulmonary lipid peroxidation and tissue damage. J Surg Res 2005; 129: 38-45.
- Giulivi C, Davies KJ. Dityrosine: a marker for oxidatively modified proteins and selective proteolysis. Methods Enzymol 1994; 233: 363-371.
- van Eijl S, Mortaz E, Versluis C, Nijkamp FP, Folkerts G, Bloksma N. A low vitamin A status increases the susceptibility to cigarette smoke-induced lung emphysema in C57BL/6J mice. J Physiol Pharmacol 2011; 62: 175-182.
- Cantin AM, Begin R. Glutathione and inflammatory disorders of the lung. Lung 1991; 169: 123-138.
- Aaltonen M, Soukka H, Halkola L, Jalonen J, Holopainen IE, Kaapa PO. Meconium aspiration induces oxidative injury in the hippocampus of newborn piglets. Early Hum Dev 2005; 81: 439-447.
- Skrzydlewska E, Farbiszewski R. Protective effect of N-acetylcysteine on reduced glutathione, reduced glutathione-related enzymes and lipid peroxidation in methanol intoxication. Drug Alcohol Depend 1999; 57: 61-67.
- Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 2008; 122: 456-468.
- Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem 1999; 274: 2234-2242.
- Tsukagoshi H, Kawata T, Shimizu Y, Ishizuka T, Dobashi K, Mori M. 4-Hydroxy-2-nonenal enhances fibronectin production by IMR-90 human lung fibroblasts partly via activation of epidermal growth factor receptor-linked extracellular signal-regulated kinase p44/42 pathway. Toxicol Appl Pharmacol 2002; 184: 127-135.
- Antonicelli F, Parmentier M, Drost EM, et al. Nacystelyn inhibits oxidant-mediated interleukin-8 expression and NF-kappaB nuclear binding in alveolar epithelial cells. Free Radic Biol Med 2002; 32: 492-502.
- Zhang H, Liu H, Iles KE, et al. 4-Hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated electrophile response element/nuclear factor erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol 2006; 34: 174-181.
- Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab 2003; 88: 1723-1729.
- Radomska-Lesniewska DM, Skopinska-Rozewska E, Jankowska-Steifer E, et al. N-acetylcysteine inhibits IL-8 and MMP-9 release and ICAM-1 expression by bronchoalveolar cells from interstitial lung disease patients. Pharmacol Rep 2010; 62: 131-138.
- Wagner PD, Mathieu-Costello O, Bebout DE, Gray AT, Natterson PD, Glennow C. Protection against pulmonary O2 toxicity by N-acetylcysteine. Eur Respir J 1989; 2: 116-126.
- Frantz MC, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen 2010; 51: 462-475.
- van Overveld FJ, Demkow U, Gorecka D, de Backer WA, Zielinski J. New developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteine. J Physiol Pharmacol 2005; 56 (Suppl. 4): 135-142.
- Radomska-Lesniewska DM, Sadowska AM, Van Overveld FJ, Demkow U, Zielinski J, De Backer WA. Influence of N-acetylcysteine on ICAM-1 expression and IL-8 release from endothelial and epithelial cells. J Physiol Pharmacol 2006; 57 (Suppl. 4): 325-334.
- Brozmanova M, Plevkova J, Bartos V, et al. Oral N-acetylcysteine reverses hyperoxia-related cough suppression in guinea pigs. J Physiol Pharmacol 2007; 58 (Suppl. 5): 75-84.
- Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician 2009; 80: 265-269.
- Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst 2010; 102: 14-25.
- Wrotek S, Jedrzejewski T, Potera-Kram E, Kozak W. Antipyretic activity of N-acetylcysteine. J Physiol Pharmacol 2011; 62: 669-675.
- Johnson WM, Wilson-Delfosse AL, Mieyal JJ. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 2012; 4: 1399-1440.
A c c e p t e d : January 23, 2015