CYTOLOGICAL ASSESSMENT OF THE EPITHELIAL CELLS OF THE NASAL MUCOUS MEMBRANE AFTER LOCAL FLUTICASONE THERAPY
INTRODUCTION
Synthetic glucocorticosteroids belong to a group of compounds which are derivatives of adrenal cortex hormones (cortisone, cortisol, corticosterone). Due to their suggested and repeatedly proven strong anti-inflammatory, anti-allergic and immunosuppressive properties (also when compared to natural compounds) (1, 2), these compounds are currently used in nearly all the medical fields and specialties. They form the base of therapy for inflammatory diseases, including, among others, the numerous diseases of the respiratory system, like the allergic, seasonal or perennial rhinitis and nonallergic rhinitis (3-6). In this case, the glucocorticosteroids, such as beclometasone, budesonide, fluticasone propionate or mometasone fuorate are administered locally in the form of nasal aerosols, inhalation powders and water solutions (1, 7). Synthesizing glucocorticosteroids for localized treatment ensured strong, selective and simultaneously safe action, enabling high concentrations of the drug with a minimalized risk of side effects (with the appropriate application technique and proper dosage) even with long-term administration (7-9).
Modern treatment of rhinitis mainly concentrates on actions aiming to reduce the inflammation of nasal mucus. Numerous reports show that the research on topical steroid drugs mainly concentrate on the clinical symptoms related to the nose, such as itching, rhinorrhea, obstruction, burning sensations, etc. (7) and on the reactions of the immunological system (10-12). Cytological assessment is concentrated on exogenous cells which determine the type of the inflammation, such as eosinophils or neutrophils (13-15). Far less attention, however, is being paid to epithelial cells, which can determine the morphological effects of the ongoing inflammation process (16) or the administered treatment (17). It should also be stressed that, depending on their type, glucocorticosteroids can have a diversified influence on the cells of nasal mucous membrane (18). The available literature provides numerous conflicting information on this issue, which causes the mechanism of the anti-inflammatory action of glucocorticosteroids to remain partially unknown. Special attention should be also given to the fact that, despite proper administration, applying such drugs in a localized manner, nasally, together with orally administered drugs, might change the cytological image of the nasal mucous membrane (19).
The goal of the study was to cytologically assess the influence of fluticasone on the cells of nasal mucous membrane, with a special attention to the changes in the morphology of pseudostratified epithelium cells, performed in vivo in standard treatment conditions.
MATERIALS AND METHODS
Patients
The research was performed in cooperation with an allergist from the Allergy Clinic, Military Specialist Medical Clinic SP ZOZ in Kielce. The study was accepted by the Jan Kochanowski University in Kielce Bio-ethical Committee (No. 45/2011).
The study group was 40 patients (17 women and 23 men), aged 5 – 30 (median 12.4) with chronic rhinitis with suspected of allergy. The patients were qualified to participate in the study by a doctor based on long-term, clinically homogenous symptoms, such as runny or stuffy nose, nasal mucosa swelling, itching of nose.
The control group were 10 healthy people (5 women and 5 men) aged 5 – 22 (median 14.20) who were showing no symptoms of rhinitis.
Methods of smear preparation and assessment
Two smears were taken from each patient with the use of a sterile cytology brush, from the surface of the nasal mucous membrane (exfoliative cytology), specifically from the inferior nasal concha (1 cm from its front edge). The cells on the brush were spread by hand on the slide with a single movement parallel to the slide edges. The obtained smears was immediately fixed in 96% ethanol and stained using the methods of Papanicolaou and Pappenheim in order to differentiate morphological changes (20, 21). Afterward the samples were analyzed in a blind test using the Nikon ECLIPSE 80i light microscope with a digital image analysis system Nikon Nis Elements in the magnification of ×400. Changes in the nasal mucous membrane of patients with rhinitis were analyzed based on a control image obtained in smears taken from healthy people. The results of the control group corresponded to the description of healthy nasal mucosa published by Tarchalska-Krynska (20). During the sample assessment, special attention was given to the morphological changes of epithelial cells. These concerned the morphological profile of the nucleus (change of size, karyorrhexis, non-nucleated form), cytoplasm (structure, changes of staining, vacuolation) and cell contour. In case of diagnostic doubt, cells were examined under 1000× magnification. In order to obtain the full picture, an assessment of the percentage of various types of cells in the smears was performed, according to the method of Tarchalska-Krynska (20). The inflammatory cells counted were neutrophils and eosinophils, and the epithelial cells counted were columnar cells, goblet cells and squamous cells. In the microscope field of view, all cell types (epithelial and inflammatory cells), as well as the cells with morphological changes, were counted together, to a total of 500 cells in the preparation. In order to obtain the most reliable results, the cells were counted in three repetitions (total counted: 1500 cells/preparation), and the final result was averaged. Determining cytograms for all patients enabled the emergence of 10 ill people (4 women and 6 men) aged 5 – 20 (median 11.7) with a similar cytological image, which became the basis for their final qualification to the fluticasone treatment group. In this group, there were 7 people with symptoms of perennial rhinitis, accompanied by typical nasal symptoms. Among the persons in 5 cases also demonstrated the profile of atopic diseases such as atopic dermatitis and asthma with symptoms from the conjunctiva (tearing, itching, burning) and the presence of allergen-specific immunoglobulin E for House Dust Mites D.P. (Dermatophagoides pteronyssinus) and D.F. (Dermatophagoides farinae). The remaining 3 people demonstrated characteristics of seasonal allergic rhinitis with typical nasal symptoms as well as the lack of extra-nasal symptoms and diseases associated and the presence of specific IgE to grass, birch, alder and hazel allergens.
The assessment of cytological image after fluticasone therapy was performed after 4 weeks of its use and compared to cytology taken from the same patients before treatment.
Applied treatment
Fluticasone propionate in the form of a nasal aerosol was locally applied nasally as two doses of 50 µg to each nostril once per day, in the total daily dose of 200 µg (for adults and children aged 12 or more), while children aged between 4 and 12 were given a single dose of 50 µg to each nostril once per day, in a total daily dose of 100 µg.
Statistical analysis
The statistical analysis of the results was performed using the nonparametric chi2 test (STATISTICA 10.0, StatSoft Poland). Differences were considered statistically significant at P <0.05.
RESULTS
The assessment of the morphology of the nasal mucous membrane of patients with rhinitis symptoms before treatment was performed in reference to the smear from the mucous membrane of patients from the control group, which was picked as correct (Fig. 1a and 1b).
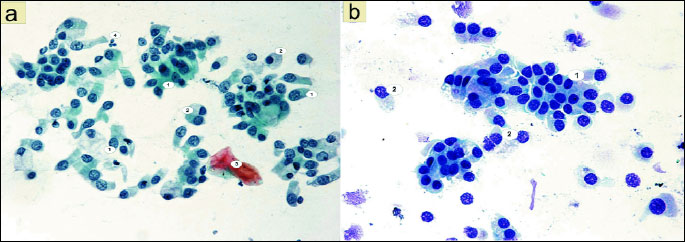
The analysis of smears from the nasal mucous membrane showed significant differences in the cytological image of patients with rhinitis before fluticasone treatment in comparison to the control group results. The statistically significant change was the presence of numerous cells of the stratified squamous epithelium. 37.24 acidophilic cells were present (χ2=35.45; P=0.0000) (Fig. 4). These were large polygonal cells, with translucent cytoplasm and a small pyknotic nucleus without a visible chromatin structure or with nuclear shadows visible in the center (Fig. 2d). Basophilic cells were present in a similar number: 30.80 (χ2=9.94; P=0.0016) (Fig. 4) and were characterized by a large round nucleus with a slightly granular structure (Figs. 2a, 2c and 2d). Some acidophilic cells were characterized by the presence of nuclei with signs of breakdown (Fig. 2d).
Another significant change was the disturbed ratio of columnar to goblet cells, in favor of the goblet cells, caused by a statistically significant decrease in the number of columnar cells to 18.54 (χ2=476.25; P=0.0000) (Figs. 2a, 4 and 5). Furthermore, fairly frequently occurring free nuclei of these cells with very clear nucleoli could be observed (Fig. 2d).
The preparations also contained numerous bacteria, which were loosely distributed or adhered to the surface of squamous epithelial cells (Fig. 2c).
The dominant change was the statistically significant increase in the number of neutrophils to 333.34 (χ2=289.92; P=0.0000) (Figs. 2b and 4). These granulocytes showed the characteristics of poorly differentiated cytoplasm and of cell nuclei contours. A statistically significant number (10.84) among the observed inflammatory cells were also eosinophils (χ2=11.12; P=0.0009) (Figs. 2b and 4), while there were few basophils, lymphocytes and monocytes (Fig. 4).
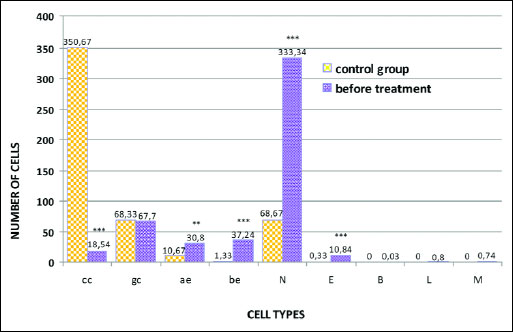 |
Fig. 4. Distribution of epithelial and inflammatory cells in smears of the nasal mucous membrane of patients before fluticasone treatment; *** P<0.001; **P<0.01 compared to control (chi2 test). Abbreviations: cc, columnar cells of pseudostratified epithelium; gc, goblet cells; ae, acidophilic cells of the squamous stratified epithelium; be, basophilic cells of the squamous stratified epithelium; N, neutrophils; E, eosinophils; B, basophils; L, lymphocytes; M, monocytes. |
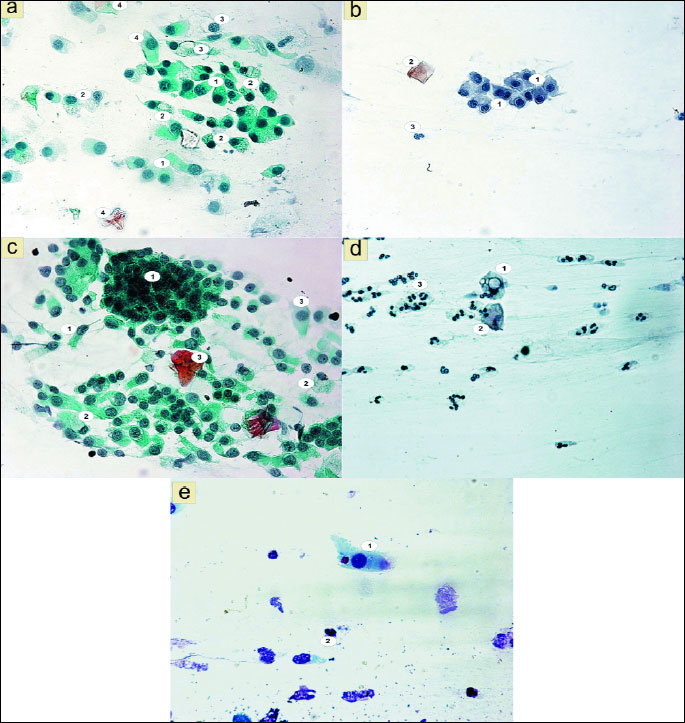
No significant changes in the morphology of epithelial cells were shown, only the presence of 4.7 individual cells with cytoplasmic vacuolation (Figs. 2c, 2d and 8).
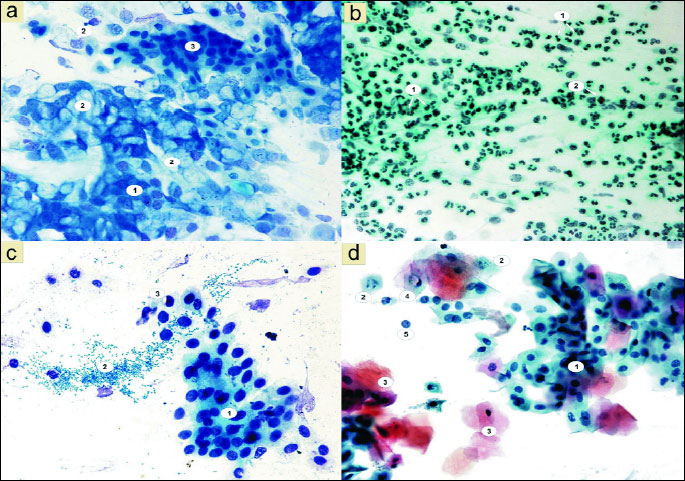
Whereas the analysis of smears taken from the same patients after fluticasone therapy showed the presence of few bacteria, as well as a statistically significant reduction of the number of inflammatory cells such as neutrophils, to 167.17 (χ2=110.22; P=0.0000) and eosinophils to 1.10 (χ2=8.43; P=0.0037) (Fig. 6). The studied granulocytes were showing characteristics of serious breakdown (Figs. 3d, 3e).
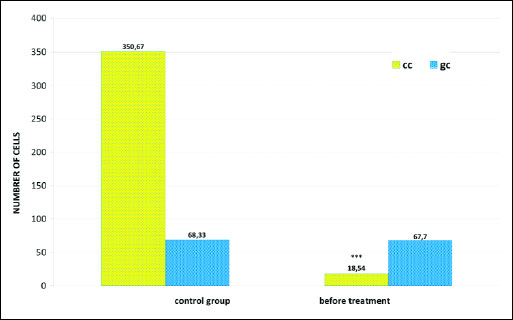 |
Fig. 5. Distribution of columnar cells (cc) and goblet cells (gc) in smears of the nasal mucous membrane of patients before fluticasone treatment; *** P<0.001 compared to control (chi2 test). |
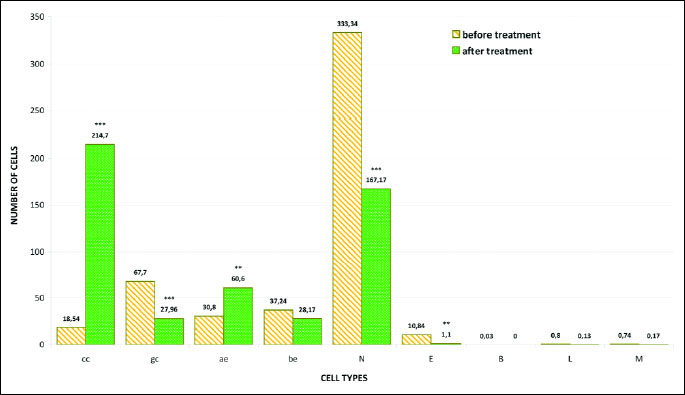
At the same time, changes in the ratio of cells of pseudostratified epithelial cells were observed, in the form of a statistically significant increase in the number of columnar cells to 214.70 (χ2=217.16; P=0.0000) (Figs. 3c, 6 and 7) and the equally statistically significant decrease in the number of goblet cells to 27.96 (χ2=18.44; P=0.0000) (Figs. 3c, 6 and 7).
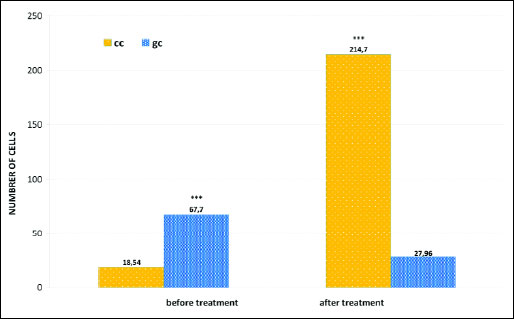 |
Fig. 7. Distribution of columnar cells (cc) and goblet cells (gc) in smears of the nasal mucous membrane of patients before and after fluticasone treatment; *** P<0.001, compared to before treatment (chi2 test). |
A statistically significant increase in the number of basophilic cells of the stratified squamous epithelium was also shown, up to 60.60 (χ2=10.77; P=0.0010) (Fig. 6), with a statistically insignificant decrease of the number of acidophilic cells to 28.17 (χ2=1.33; P=0.2483) (Fig. 6). No significant changes were noted in relation to the number of basophils, lymphocytes and monocytes (Fig. 6).
Special attention was however drawn by the vacuolation changes in the form of one large vacuole or numerous small vacuoles in the cytoplasm of both columnar cells (Fig. 3a), and squamous epithelial cells (Fig. 3d and 3e). A significant increase in the number of vacuolated cells can be seen, up to 40.03, while the smears of patients before treatment only 4.70 were shown (χ2=28.50; P=0.0000) (Fig. 8). Among the cells with vacuolation changes, revealed the presence of forms with a perinuclear halo (Fig. 3b).
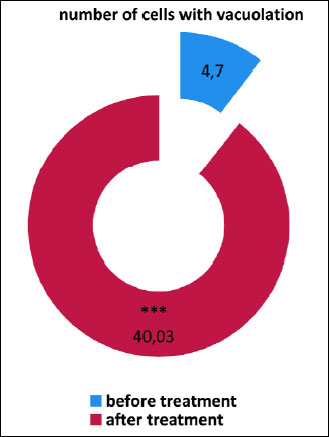 |
Fig. 8. The number of cells with cytoplasm vacuolation in smears from nasal mucous membrane taken from patients before and after fluticasone treatment; *** P<0.001 compared to before treatment (chi2 test). |
DISCUSSION
Progress in diagnosing and treating allergic diseases is based on the improved understanding of the mechanisms of these diseases, the development of new drugs, as well as on the improvement and broadening of indications regarding drugs which are already in use (22). The pathomechanism of inflammatory rhinitis is related to the inflow and activation of cells - mastocytes, basophils, eosinophils, T lymphocytes and neutrophils in the area of the inflammatory process (23, 24), and its understanding allows us to correctly and justifiably use specific drugs and therapeutic methods (25).
The cytological assessment of the smear from the surface of the mucous membrane is, unlike the biopsy, a completely noninvasive method, which can often be used in routine ambulatory diagnostic. It can be performed in every situation, in every patient of every age, and can be repeated numerous times in the same person (26). The safety and lack of age limits are confirmed by cytological studies in infants (27, 28). Since the picture obtained in cytological studies is a reflection of the processes taking place in the nasal mucous membrane, studies of this type provide many new and significant information about the type of the inflammatory reaction, as well allowing the monitoring of the effectiveness of the implemented treatment (24, 29 – 31). It should also be noted that the smear technique obtains the highest total number of inflammatory cells (approximately 3 – 4 times higher than in lavages and scrapings) and epithelial cells (in this case almost 70%, while in lavages 42%), which was proven in studies comparing all three methods of obtaining material for cytology (31, 32).
Without glucocorticosteroid drugs it would certainly be difficult to imagine modern therapy of these diseases, the pathogenesis of which is associated with the activation of various inflammatory cells (33). Besides nasal mucosa diseases they are commonly used in the treatment of asthma, Crohn's Disease or infant meconium aspiration syndrome (MAS), where they, among other effects, significantly limit the influx of inflammatory cells (34-36).
It should be remembered that these drugs control numerous physiological processes in various organs and tissues and on many levels, from the proliferation and differentiation of cells, to the regulation of their metabolic activity and programmed death (37, 38). The cellular reaction to glucocorticosteroids is very diverse and shows great variability in its specificity and sensitivity (39).
Glucocorticosteroids, independent on the form of administration, can change the cytological image of the mucous membrane, it should however be noted that these changes can be beneficial or undesirable (19).
The cytological studies preformed on a group of patients with rhinitis symptoms before their treatment, in comparison to the control smears, clearly show a disturbance in the biocenosis of the nasal mucous membrane. The presence of numerous bacteria has been shown (Fig. 2c), as well as of granulocytes with the predominance of neutrophils (Figs. 2b and 4). With the assumption that a cytogram described as correct should contain single neutrophils (13, 20, 25), the presented change suggests a pathological state. Furthermore, the observed granulocytes display the characteristics of poorly differentiated cytoplasm, as well as contours of nuclei, which suggests a chronic state. In acute inflammations granulocytes, existing as young forms, have clear and intensively staining nuclei and well differentiated cytoplasm (40).
Changes in the content of the cellular population of the nasal mucous membrane are also a confirmation of the pathological state. A disturbance in the ratio of columnar cells to goblet cells has been noted, to the benefit of the goblet cells (Figs. 2a, 4, and 5), while in proper cytograms the columnar cells should outnumber goblet cells by a ratio of 5 (25, 41, 42). The mechanism contributing to the increase of goblet cells is their over-proliferation, known as hyperplasia, which often accompanies inflammation in the respiratory epithelium (43, 44). According to some authors, the hyperplasia of goblet cells can accompany inflammation in which the dominant cell is the neutrophil, like in the chronic inflammation of nasal mucous membrane and rhinosinusitis with the exclusion of nasal polyps (45). It should also be stressed that the studied material showed the presence of free nuclei of columnar cells (Fig. 2d), which was a result of intensive cytolitic processes, and is also an example of changes commonly associated with a disturbed biocenosis (40, 46).
The presence of very numerous cells of the squamous stratified epithelium has also been confirmed (Figs. 2a, 2c, 2d and 4). The prevalence of these cells, in different stages of development, with the assumption that the material was properly obtained, suggests a pathological state (41). Since the analyzed smears showed a prevalence of cells originating from the deeper strata of the epithelium (basophilic with a large round nucleus with a mildly granular structure), it suggests a damage to the upper layers of the epithelium (47). The breakdown of the nucleus (karyorrhexis) observed in the basophilic cells is also, together with an enlargement and a dissolution (karyolysis) of this structure, a common occurrence in inflammation (40).
The obtained changes, especially the neutrophilic reaction, indicate a chronic infectious rhinitis, in which neutrophils can often be the only cells in the smear (48).
A comparison of the cytological image of patients with rhinitis before treatment and the same patients after treatment with fluticasone enabled the demonstration of significant differences in epithelial reaction and number of inflammatory cells.
The consequence of the drug activity was the decrease of inflammation of nasal mucosa, confirmed mainly through the reduction of the number of neutrophils (Fig. 6), which can be related to results of similar cytological studies with the use of another glucocorticosteroid-mometasone in allergic rhinitis (19).
The observed decrease of goblet cells, with a simultaneous, also statistically significant increase of the number of columnar cells (the proper ratio of columnar to goblet cells) shows a gradual regeneration of the nasal mucous membrane (Figs. 3c, 6 and 7). The confirmation of the results obtained in relation to goblet cells were the cytological studies of Lin et al. (18) on glucocorticosteroids used in people with rhinitis and the long-standing research of Mygind (49) and Sorensen (50) on the activity of budesonide based on the assessment of nasal mucous membrane segments under an electron microscope. Whereas the stimulating effect of glucocorticosteroids on columnar cells was shown in the research on mometasone performed by Tarchalska-Krynska (19) and Meltzer (51). Another confirmation of the beneficial effect of topical glucocorticosteroid therapy on the proper proportion of columnar to goblet cells comes from the results of our own cytological studies on the use of budesonide (52, 53).
In comparison to the state before treatment, fluticasone therapy did not influence the general number of squamous stratified epithelial cells (Fig. 6), because an increase in basophilic cells was obtained, together with a simultaneous decrease in the number of acidophilic cells, which suggests a low effectiveness of the drug in relation to cells of this type, which had also been confirmed in other studies (20).
However, special attention has been drawn by the increase of vacuolated epithelial cells (Fig. 8), mainly columnar. Some cells were characterized by the presence of a single vacuole (Fig. 3e) or numerous small vacuoles in the cytoplasm (Figs. 3a and 3d), others were characterized by perinuclear vacuolation, a clear nuclear halo (Fig. 3b). The intensified vacuolation of the cell cytoplasm might suggest an induction of autophagic processes. According to numerous reports, autophagy plays a significant role in numerous aspects of inborn and adaptive immunity, including, among others, the activation of the immunological system, survival of infected cells (54) or the elimination of intracellular pathogens, including bacteria and viruses (55, 56). It should be stressed that according to numerous literature data, autophagy can be associated not only with macrophages, but also with cells not belonging to the immunological system (57). Whereas the so called perinuclear halo effect, according to the available literature, appears because of karyoplasm shrinkage and can be the result of reversible cytoskeleton damage (58). In our previous research we had confirmed the effectiveness of topical glucocorticosteroid therapy in the treatment of nasal mucosa diseases, however we have also revealed changes which require further study. As described in professional literature (59), the noninvasive study of nasal nitric oxide (NO) levels can be an excellent supplementation of the cytological studies both in the assessment of the inflammatory reaction of the nasal mucous membrane and the reaction to anti-inflammatory treatment. As shown in professional literature (60), the study design should also include the fact that the very use of hypertonic saline solution in nasal aerosol form can have a significant effect on the nasal NO levels.
In summary, the obtained results of the cytological study of nasal mucosa shows that therapy with fluticasone propionate used in the dose of 100 µg to 200 µg in patients with diagnosed chronic infectious reaction of nasal mucosa caused a significant decrease of inflammation visible in the cytological image in the form of reduction of bacteria and the accompanying nasal neutrophilia. Simultaneously, no harmful action of the applied glucocorticosteroid was observed, which was confirmed by the lack of significant changes in the number of squamous epithelial cells with a clearly increased number of columnar cells. Whereas the observed escalation in vacuolation of these cells, with a simultaneous lack of apoptosis characteristics, doesn't show the characteristics of degenerative changes, but might suggest an additional mechanism of glucocorticosteroids in nasal mucosa inflammation.
Conflict of interests: None declared.
REFERENCES
- Wu X, Adedoyin OO, Mansour HM. Pulmonary and nasal anti-inflammatory and anti-allergy inhalation aerosol delivery systems. Antiinflamm Antiallergy Agents Med Chem 2011; 10: 215-229.
- Mygind N, Nielsen LP, Hoffmann HJ, et al. Mode of action of intranasal corticosteroids. J Allergy Clin Immunol 2001; 108: 16-25.
- Corren J. Intranasal corticosteroids for allergic rhinitis: How do different agents compare? J Allergy Clin Immunol 1999; 104: 144-149.
- Zawisza E, Bardadin J. Topical corticotherapy in the treatment of seasonal and perennial allergic rhinitis. Allergy 2005; 1: 26-29.
- Berlucchi M, Pedruzzi B. Intranasal mometasone furoate for treatment of allergic rhinitis. Therapeutics 2010; 2: 761-769.
- Greiner AN, Meltzer EO. Pharmacologic rationale for treating allergic and nonallergic rhinitis. J Allergy Clin Immunol 2006; 118: 985-996.
- van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy 2000; 55: 116-134.
- Sastre R, Mosges R. Local and systemic safety of intranasal corticosteroids. J Investig Allergol Clin Immunol 2012; 22: 1-12.
- Kariyawasam HH, Scadding GK. Seasonal allergic rhinitis: fluticasone propionate and fluticasone furoate therapy evaluated. J Asthma Allergy 2010; 3: 19-28.
- Bellodi S, Tosca M.A, Pulvirenti G, et al. Activity of budesonide on nasal neutrophilic inflammation and obstruction in children with recurrent upper airway infections. A preliminary investigation. Int J Pediatr Otorhinolaryngol 2006; 70: 445-452.
- Stanaland BE. Once-daily budesonide aqueous nasal spray for allergic rhinitis: A review. Clin Ther 2004; 26: 473-492.
- Xaubet A, Mullol J, Roca-Ferrer J, et al. Effect of budesonide and nedocromil sodium on IL-6 and IL-8 release from human nasal mucosa and polyp epithelial cells. Respir Med 2001; 95: 408-414.
- Provero MC, Macchi A, Antognazza S, et al. Allergic and nonallergic rhinitis in children: the role of nasal cytology. Open J Pediatr 2013; 3: 133-138.
- Meltzer EO, Orgel HA, Rogenes PR, Field EA. Nasal cytology in patients with allergic rhinitis: effects of intranasal fluticasone propionate. J Allergy Clin Immunol 1994; 94: 708-715.
- Nowacki Z, Neuberg J, Strzalka K, Szczepanik M, Szczepanik R, Mazurek H. Is prediction of the allergic march possible on the basis of nasal cytology? Pneumonol Alergol Pol 2010; 78: 263-270.
- Makowska W. Cytological picture of the damage and regeneration of the nasal epithelium. Otolaryngol Pol 1991; 45: 445-451.
- Tarchalska-Krynska B, Samolinski B, Bialek S, Zawisza E. Corticosteroids and surgical therapies of the nasal polyps in cytological evaluation. Int Rev Allergol Immunol 1999; 5: 21-24.
- Lin RY, Clarin E, Lee M, Menikoff H. Decreased nasal goblet cells in patients reporting topical corticosteroid use. J Allergy Clin Immunol 1997; 99: 265-266.
- Tarchalska-Krynska B, Zawisza E, Chustecki A. Mometazon - nowy glikokortykosteroid w terapii sezonowego alergicznego niezytu nosa. Ocena skutecznosci klinicznej leku i cytologiczna ocena blony sluzowej nosa. Alerg Astma Immun 1998; 3: 161-166.
- Tarchalska-Krynska B. Non-allergic rhinitis in cytological examination of nasal mucosa. Otolaryngol Pol 2007; 6: 83-87.
- Anniko M, Bernal-Sprekelsen M, Bonkowsky V, Bradley P, Iurato S. Otorhinolaryngology, Head and Neck Surgery. Berlin, Springer Science & Business Media, 2010.
- Rosenwasser LJ. Treatment of allergic rhinitis. Am J Med 2002; 113: 17-24.
- Patkowski J, Wytrychowski K. Pathophysiology of apoptosis and its role in allergic inflammation and allergic diseases. Adv Clin Exp Med 2006; 15: 321-328.
- Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 2004; 114: 155-212.
- Gelardi M, Marseglia GL, Licari A, et al. Nasal cytology in children: recent advances. Ital J Pediatr 2012; 38: 1-7.
- Gelardi M, Incorvaia C, Passalacqua G, Quaranta N, Frati F. The classification of allergic rhinitis and its cytological correlate. Allergy 2011; 66: 1624-1625.
- Tarchalska-Krynska B. Cytological evaluation of nasal mucosa in upper respiratory diseases. Cytological evaluation of the nasal mucosa in healthy new-born children. New Medicine 1999; 3: 68-69.
- Tupieka-Kolodziejska A, Gawecka A, Tarchalska-Krynska B, Kornacka MK. Nasal cytology of newborn children with gastro-esophageal reflux disease. Otolaryngologia 2006; 5: 119-121.
- Meltzer EO. Evaluating rhinitis: clinical, rhinomanometric and cytologic assessments. J Allergy Clin Immunol 1988; 82: 900-908.
- Ferrara L, Naviglio D, Caruso AA. Cytological Aspects on the effects of a nasal spray consisting of standardized extract of citrus lemon and essential oils in allergic rhinopathy. ISRN Pharm 2012; 2012: 1-6.
- Howarth PH, Persson CG, Meltzer EO, Jacobson MR, Durham SR, Silkoff PE. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol 2005; 115: 414-441.
- Woszczek G, Kowalski ML, Majkowska-Wojciechowska B, et al. Porownanie obrazu cytologicznego blony sluzowej nosa w badaniach wymazow, wyskrobin i popluczyn nosa. Alerg Astma Immun 1996; 1: 47-51.
- Cosio BG, Torrego A, Adcock IM. Molecular mechanisms of glucocorticoids. Arch Bronconeumol 2005; 41: 34-41.
- Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol 2011; 163: 29-43.
- Irving PM, Gearry RB, Sparrow MP, Gibson PR. Review article: appropriate use of corticosteroids in Crohn's disease. Aliment Pharmacol Ther 2007; 26: 313-329.
- Mokra D, Tonhajzerova I, Pistekova H, et al. Short-term cardiovascular effects of selective phosphodiesterase 3 inhibitor olprinone versus non-selective phosphodiesterase inhibitor aminophylline in ameconium-induced acute lung injury. J Physiol Pharmacol 2013; 64: 751-759.
- Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions an introduction. Ann NY Acad Sci 2004; 1024: 1-8.
- Amsterdam A, Sasson R. The anti-inflammatory action of glucocorticoids is mediated by cell type specific regulation of apoptosis. Mol Cell Endocrinol 2002; 189: 1-9.
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 2013; 132: 1033-1044.
- Malarewicz A. Ilustrowana cytodiagnostyka ginekologiczna. Cytologia zmian szyjki macicy. Warszawa, BGW, 1994.
- Tarchalska-Krynska B. Diagnostyka cytologiczna blony sluzowej nosa. Kiedy i jak ja wykonac? Alergia 2003; 2: 33-35.
- Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol Head Neck Surg 2010; 9: 1-24.
- Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol 2010; 42: 268-275.
- Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical researchand patient care. Otolaryngol Head Neck Surg 2004; 131: S1-S61.
- Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 2003; 129: 1-32.
- Hans Friedrich N. Gynecological Cytology. New York, Thieme, 2007.
- Chosia M, Domagala W. Cytodiagnostyka szyjki macicy. Warszawa, Fundacja Pro Pharmacia Futura, 2010.
- Gershin ME, Incando G. Diseases of the Sinuses: a Comprehensive Textbook of Diagnosis and Treatment. New Jersey, Humana Press, 1996.
- Mygind N, Sorensen H, Pedersen CB. The nasal mucosa during long-term treatment with beclomethasone dipropionate aerosol. A light and scanning electron microscopic study of nasal polyps. Acta Otolaryngol 1978; 85: 437-443.
- Sorensen H, Mygind N, Pedersen CB, Prytz S. Long-term treatment of nasal polyps with beclomethasone dipropionate aerosol. III. Morphological studies and conclusions. Acta Otolaryngol 1976; 82: 3-4, 260.
- Meltzer EO, Jalowayski AA, Orgel HA, Harris AG. Subjective and objective assessments in patients with seasonal allergic rhinitis: effect of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol 1998; 102: 39-49.
- Trybus E, Staszczyk K, Trybus W, Kopacz-Bednarska A, Mycinska M, Krol T. The changes in nasal mucous membrane after topical glicocorticosteroidotherapy application. Acta Biochim Pol 2008; 55: 315.
- Trybus E, Kopacz-Bednarska A, Krol T, et al. The influence of budesonide on nasal cytology. Materials of 17th International Symposium ,,Molecular and Physiological Aspects of Regulatory Processes of the Organism" Cracow, 2008; 556-557.
- Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ 2009; 16: 57-69.
- Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Curr Biol 2012; 22: 540-545.
- Paludan C, Schmid D, Landthaler M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005; 307: 593-596.
- Pyo JO, Jang MH, Kwon YK, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem 2005; 280: 20722-20729.
- Florczak K, Gross M, Kaluzna M, et al. Cytologic features of cervicitis and vaginitis in phase-contrast microscopy. Ginekologia Prakt 2007; 3: 2-10.
- Kharitonov S, Rajaulasingam K, O'Connor B, Durham SR, Barnes PJ. Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal corticosteroids. J Allergy Clin Immunol 1997; 99: 58-64.
- Bencova A, Vidan J, Rozborilova E, Kocan I. The impact of hypertonic saline inhalation on mucociliary clearance and nasal nitric oxide. J Physiol Pharmacol 2012; 63: 309-313.
A c c e p t e d : November 3, 2014