ENHANCED EXPRESSION OF ENDOTHELIAL NITRIC OXIDE SYNTHASE IN THE MYOCARDIUM AMELIORATES THE PROGRESSION OF LEFT VENTRICULAR HYPERTROPHY IN L-ARGININE TREATED WISTAR-KYOTO RATS
INTRODUCTION
Left ventricular hypertrophy is characterized by an increase in ventricular mass in response to cardiac stress induced by pressure or volume overload (1). Experimentally, LVH can be induced non-invasively using isoprenaline and caffeine administration (2, 3). A key factor in the genesis of LVH appears to be nitric oxide (NO) deficiency. This is supported by the notion that mice lacking neuronal (nNOS) and endothelial (eNOS) nitric oxide synthase developed spontaneous cardiac hypertrophy (4). Moreover, it has been shown that 12 weeks treatment with L-arginine to enhance NO production in spontaneously hypertensive rats (SHR) attenuated the development of LVH independent of its effect on blood pressure (5). Conversely, Nw-nitro-l-arginine methyl ester (L-NAME) treatment in rats caused LVH to regress in a blood pressure dependent manner when administered with exogenous L-arginine (6). Nonetheless, evidence is also available that NO has antihypertrophic effects in the heart of SHR which are not dependent on its blood pressure lowering ability (5). Although the effect of NO system on the progression of LVH has been extensively studied, the role of eNOS and CSE on the progression of the disease is still enigmatic.
Increased levels of noradrenaline and angiotensin II in this model of LVH (7-9) may explain why it is associated with oxidative stress (10). This results in the excessive generation of reactive oxygen species (ROS) and leads to NO deficiency (11). On another hand, vasoconstriction brought by oxidative stress and elevated angiotensin II and noradrenaline levels can result in stiffer arteries. Therefore, evidence is available that arterial stiffness is reduced when blood vessels are dilated due to NO under physiological conditions (12-14). Similar to NO, hydrogen sulfide (H2S) has important roles in normal physiological states as well as in diseases as a new (15, 16). Both H2S and NO have been reported to have an interdependent production (17-21). Earlier studies reported that H2S was responsible for NO production in smooth muscles (19, 22) while others have shown that NO enhanced the upregulation of H2S production in the plasma (23, 24).
The present study investigated the hypothesis that eNOS down regulation in isoprenaline/caffeine induced LVH is associated with oxidative stress, arterial stiffness and reduced blood perfusion to the kidney in this model. This was evaluated through the chronic administration of L-arginine as an NO donor and studying the molecular expression of eNOS and CSE in the myocardium.
MATERIALS AND METHODS
Animals
The animal handling and the surgical protocol used for this study were approved by the Animal Research and Service Centre (ARASC) at Universiti Sains Malaysia with the approval number 2012/76/364.
Thirty-sixWistar-Kyoto rats were obtained from the local animal care facility at the mentioned university with an initial body weight ranging from 180 to 200 g. The rats were acclimatized for 5 days to the new environment with an ad libitum supply of food and water. Thereafter, animals were divided into two main groups, one was recruited for the acute study (n = 24) while the other group was used to examine the expression of eNOS mRNA in the myocardium (n = 12). The first group was further divided into 4 sub-groups, a control, an LVH, an L-arginine treated control (control-NO) and an L-arginine treated LVH (LVH-NO) group (n = 6 per sub-group). Likewise, the second group was divided into 4 sub-groups of control, LVH, control-NO and LVH-NO groups (n = 3) and used for the eNOS and CSE mRNAs expression studies. LVH was induced by the administration of 5 subcutaneous injections of isoprenaline (5 mg/kg at 72 h interval) and the co-administration of caffeine (62 mg/L in the drinking water) for a period of 2 weeks as previously reported (2, 3). All treated rats received L-arginine (1.25 g/L in the drinking water) for 5 weeks (25). One LVH group (LVH-L-NIO) received L-N5-(1-iminoethyl)-ornithine), a potent inhibitor of endothelial nitric oxide synthase (eNOS) (10 mg/kg I.P.) 15 minutes before the acute experiment (26, 27) and the expression of eNOS protein by Western blotting was compared to a control group (control-L-NIO).
Physiological data collection and measurements
At the end of the treatment period (day 35), the rats were caged separately in specialized metabolic cages and their 24 h water intake and urine output was recorded. Blood samples were withdrawn from the tail vein directly into pre-heparinised tubes and immediately centrifuged at 10,000 g for 5 minutes. Plasma was separated from the samples and used for the measurement of oxidative stress markers and H2S level.
Measurement of electrocardiography (ECG) of anesthetized rats
The food was removed from the cages on the night before the experiment. The rats were anaesthetised with pentobarbitone sodium (60 mg/kg I.P., Nembutal®, CEVA, France). Thereafter, a 3-lead surface ECG recording was taken using gold plated needle electrodes (ADInstrument, Sydney, Australia) inserted underneath the skin as previously reported (28, 29). Recordings were done for 5 min using an amplifier attached to a data acquisition system (PowerLab, ADInstrument, Sydney, Australia). Data for each rat was taken as the average of 15 electrical impulses and the mean was calculated for each group of rats.
Acute experiment
After the completion of ECG recording, the acute experiment was performed based on a previously reported procedure (28-30). The trachea was cannulated to provide ease of ventilation throughout the experiment. The right carotid artery was then cannulated (PP50, Portex, Kent, UK) and the cannula attached to a fluid-filled pressure transducer (model P23 ID Gould, Statham Instruments, UK), which was connected to a data acquisition system (PowerLab®, ADInstrument, Australia) for the continuous monitoring of mean arterial blood pressure (MAP) and heart rate (HR) (36). The left jugular vein was also cannulated (PP50, Portex, Kent, UK) and used for the infusion of maintenance doses of anaesthesia and saline. A mid line abdominal incision was made and the left kidney was exposed and carefully covered with saline soaked cotton pads. The iliac artery was cannulated (PP50, Portex, Kent, UK) and the cannula was attached to a second pressure transducer and connected to the Power Lab system for measurement of iliac artery mean blood pressure. A laser-Doppler flow probe (ADInstruments, Australia) was positioned on the outermost layer of the cortex of the left kidney to record renal cortical blood perfusion (RCBP). After completion of surgery, the rats were allowed to stabilize for 1 hour. The systemic hemodynamic variables, MAP, HR and RCBP, were monitored continuously for 1 hour. The rats were euthanized at the end of the experiment with an over dose of anaesthetic. The pulse wave velocity (PWV) was measured by taking the propagation time of the pulse wave, from carotid to iliac arteries, from the Power Lab trace and the propagation distance was measured manually by putting a cotton thread from insertion point of the carotid to that of the iliac artery (32, 33). The kidneys and heart were harvested post-mortem, dried and weighed for heart, LV and kidney indices. The diameter of LV, wall thickness and internal volume of the LV chamber was measured using a Vernier calliper as reported previously (34). The LV was preserved in 10% formalin for histopathology study of the heart and the kidney.
Oxidative stress markers in the plasma and the myocardium
Plasma levels superoxide dismutase activity (SOD), malondialdehyde (MDA), glutathione reductase (GSH), total antioxidant capacity (T-AOC) and nitric oxide activity (NO) were measured in plasma and in heart tissue using laboratory kits (NJJC Bio Inc., Nanjing, China) following the instruction provided by the manufacturer.
Quantification of cystathione g lyase mRNA and endothelial nitric oxide synthase mRNA in the myocardium using RT-PCR
After cervical dislocation of the rats, heart tissue was immediately preserved in RNAlater® Solution (Ambion, Life technologies, USA) in a contamination free area. Total RNA was extracted from the kidney tissues using TRIzole reagent (Ambion, Life technologies, USA) according to the manufacturer guidelines. After the sequential steps of homogenization, washing and elution, total RNA was extracted from the heart tissues. Total RNA was optimized and quantified for purity and yield respectively, using a microplate reader (BioTek Instrument Inc., VT, USA). Total RNA was converted to cDNA using a high capacity RNA-to-cDNA kit (Applied Biosystems, USA) according to the manufacturer’s instruction. In this step, 20 µL aliquots were used for the conversion of RNA to cDNA. The TaqMan primers and probes (TaqMan®-Gene Expression assays, Applied Biosystems, USA) were as follows:
- CSE (Gen Bank accession No. NM_017074.1 and Rn00567128_m1) gene (35).
- eNOS (Gen Bank accession No. NM_021838.2 and Rn02132634_s1) gene (36, 37)
- β-actin (Gen Bank accession No. NM_031144.2 and Rn00667869_m1) gene (38, 39)
The following primers for CSE, eNOS, and internal control beta-actin along with TaqMan chemistry were used (assay ID: Rn00567128_ml, Rn002132634_s1 and Rn00667869_ml respectively) for the gene expression assay.
Quantitative RT-PCR reactions were carried out in triplicate for each rat. Amplification of the housekeeping enzyme (internal control) beta actin was used for sample loading and normalization. The relative quantification of the target gene CSE, eNOS and internal control beta actin was performed using the comparative CT(threshold cycle) method with the formula (2–ΔΔCT) (40).
Nitric oxide synthase protein analysis
Frozen hearts were homogenized in a buffer of 50 mmol/L Tris HCL,Ph 7.4, 1 mmol/L of EGTA, 1 mmol/L dithiothreitol 1mmol/L (DTT), 1 mmol/L pepstatin A, 2 mmol/L leupeptin and 1 mmol/L methane sulfonyl fluoride. All the homogenates were centrifuged at 10,000 g at 4°C for 60 minutes. Supernatants were taken as the cytosolic fractions. All the pellets were solubilised using buffer containing 10% glycerol and 20 mmol/L of 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate and ultracentrifuged to extract the particular fraction.
About 150 mg of protein samples were separated from a particular fraction on 7.5% SDS-polyacrilamide gel and it was transferred to polyvinylidene difluoride membrane. About 5% non fat dry milk was used to block the blots and incubated with rabbit polyclonal anti-bovine eNOS antibody (18). Immunoreactive bands were visualized with horseradish peroxidise conjugated anti-rabbit IgG using an ECL detection kit.
NOS enzyme activity was determined by the conversion of [3H]-L-arginine to [3H]-L-citrulline with saturating concentrations of substrate and cofactors (18). Enzyme activity was expressed as citrulline production in femtomol per milligram of protein per minute.
Determination of concentration of H2S and nitric oxide in the plasma and the myocardium
A blood sample was taken from the tail vein at the end of the treatment period and centrifuged at 5000 rpm for 10 minutes (30). The measurement of H2S in the heart tissue was as reported previously (41). Heart tissue (50 mg) was homogenized in 0.5 ml of zinc acetate (1%) and mixed with 0.5 ml of borate buffer (pH 10.01). After this, a volume of 0.5 ml of N, N-2 dimethyl-p-phenylenediamine (20 mM) and 0.5 ml of FeCL3 (300 mM) were added to tissue homogenate. Reaction tubes were immediately sealed and incubated for 30 min with shaking at 37°C. After incubation, all samples were centrifuged and H2S concentration was measured using the same procedure mentioned for plasma H2S measurement. This method has been used extensively to measure H2S in tissues (41-44).
The concentration of nitric oxide (nitrite/nitrate) in plasma and tissues was determined using a laboratory kit (NJJC Bio Inc., Nanjing, China). The procedure involved preparation of blank, standard and assay solutions. NO concentration was estimated by the procedure stated below while protein quantity was measured using an early reported method (45). For the assay solution, 100 µL of plasma sample was mixed with 400 µL of working solution using a vortex stirrer. The resultant solution was kept at 37°C in a thermostated water bath for 60 minutes. The reagent 3 was added (200 µL) and followed by100 µL of reagent 4. The whole solution was vortexed for 30 seconds and allowed to stand at room temperature for 40 minutes before being centrifuged at 4000 rpm for 10 minutes. About 500 µL of the supernatant was removed carefully without disturbing the precipitate. The developer was added (600 µL), vortexed and then allowed to stand at room temperature for 10 minutes. Absorbance was measured at 550 nm and the following equation was used:
![]()
![]()
OD: optical density D.F: Dilution factor.
Histopathology study of the left ventricle using haematoxylin and eosin staining
The left ventricles (LVs) of all rats were collected after careful isolation from heart. The LV tissue was blotted dry on a filter paper and kept in 10% formalin solution for preservation. After the subsequent steps of embedding, trimming and sectioning, the LV tissue underwent staining with haematoxylin and eosin as reported previously (35).
Histopathology study of the left ventricle using Picrosirus red stain kit
The same preparative procedure given above was repeated for staining with Picrosirus Red (Polyscience, Inc. Germany). The procedure involved the use of three solutions, solution A, solution B and Solution C. Firstly, the slides were dipped in solution A for 2 min then rinsed well in distilled water; secondly, the slides were placed in solution B for 60 min; thirdly, immersed in solution C for 2 min. Finally, the slides were immersed in 70% ethanol for 45 seconds. Collagen in the LV tissue gives a red colour.
Statistical analysis
The statistical analysis was performed using a one way analysis of variance (6) followed by a Boneferroni post hoc test using GraphPad Prism software (GraphPad Software, San Diego California U.S.A) while gene expression data was analysed using the comparative method (ΔΔCT method) and using the StepOne™ Software (Version 2.1, Applied Biosystem, USA). All data were presented as mean ± S.E.M. with significance at P < 0.05.
RESULTS
Endothelial nitric oxide synthase, cystathione γ lyase mRNAs expression and nitric oxide synthase activity in the myocardium
The expression of eNOS and CSE genes in the myocardium of LVH rats was down regulated by approximately 35% and 68% respectively (all P < 0.05) when compared to the control rats (Fig. 1A and 1B). Treatment of LVH rats with L-arginine restored eNOS (P < 0.05) but not CSE expression when compared to the untreated LVH rats. The treatment of normal rats with L-arginine significantly increased eNOS and CSE gene expression by approximately 85% and 45% respectively (all P < 0.05) compared to the untreated counterparts (Fig. 1A and 1B). Ca++-dependent NOS activity was reduced significantly (all P < 0.05) in LVH when compared to the control group while exogenous administration of L-arginine in LVH significantly increased (all P < 0.05) the NOS activity when compared to LVH as shown in Fig. 1C. Immunoreactive bands were visualized and it was observed that there was a significant increase in eNOS protein expression in LVH-NO when compared to LVH as shown in Fig. 1D.
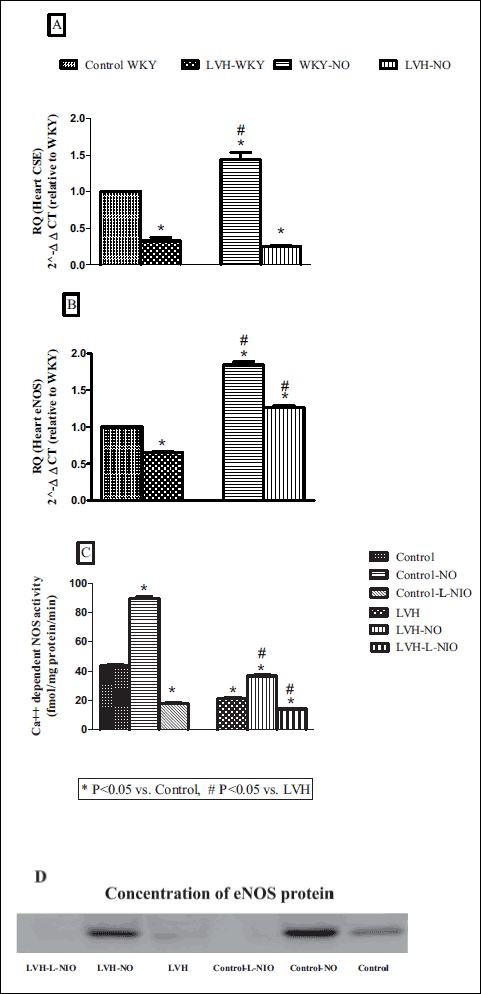 |
Fig. 1. The expression of cystathione γ lyase (CSE) genes (A), endothelial nitric oxide synthase (eNOS) (B), NOS activity (C) and eNOS protein expression in the myocardium (D) of control, LVH, control-NO, LVH-NO, control-L-NIO and LVH-L-NIO rats. Data are expressed as mean ± S.E.M. * represents P < 0.05 compared to control while # represents P < 0.05 compared to LVH group (n = 3). |
Blood pressure, pulse wave velocity and renal cortical blood perfusion data
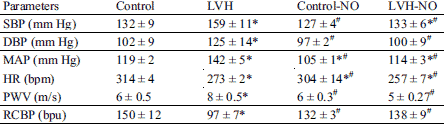
The comparative values of systemic hemodynamic are shown in Table 1. Systolic (SBP), diastolic (DBP), and MAP were higher (P < 0.05) in LVH compared to control rats. However, L-arginine treated LVH rats had lower SBP, DBP and MAP compared to the LVH group (all P < 0.05). In addition, heart rate values in the LVH group as well as in L-arginine treated LVH were lower (P < 0.05) than their control counterparts. The PWV was higher (P < 0.05) in the LVH group as compared to the control and was reduced in the LVH following L-arginine treatment. The renal cortical blood perfusion was lower in the LVH compared to control rats (97 ± 7 vs. 150 ± 12 bpu, P < 0.05). Treatment of LVH rats with L-arginine resulted in a higher renal cortical blood perfusion compared to the untreated counterparts (132 ± 3 vs. 97 ± 7 bpu, P < 0.05) as shown in Table 1.
ECG data
The ECG data are shown in Table 2. The QRS, R-R interval and R-amplitude values were all higher (P < 0.05) in LVH compared to the control group. However, the L-arginine treated LVH group had lower QRS, R-R interval and R-amplitude values compared to LVH rats. Finally, the QTc values in all the groups studied were not different.

Determination of the concentration of nitric oxide and H2S in urine, plasma and myocardium
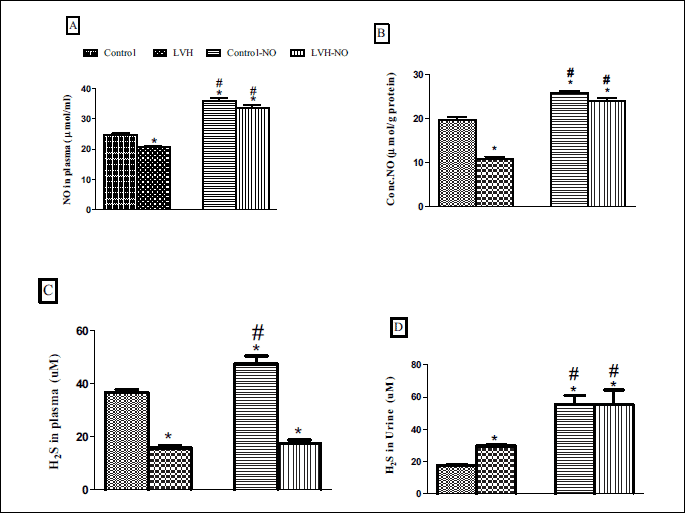
The plasma concentration of NO in LVH rats was lower compared to the control (21 ± 0.5 vs. 36 ± 0.5 µM) but it increased following L-arginine treatment compared to the untreated LVH rats (34 ± 1 µM, P < 0.05) as shown in Fig. 2A. In addition, the concentration of NO of the heart tissues in LVH rats was lower compared to the control (11 ± 1 vs. 20 ± 1mmol/g protein) but it increased following L-arginine treatment compared to the untreated LVH rats (24 ± 1 mmol/g protein, P < 0.05) (Fig. 2B). Similarly, the plasma concentration of H2S was lower in the LVH compared to the control group (16 ± 1 vs. 37 ± 3 µM, P < 0.05). However, the plasma level of H2S was higher (P < 0.05) in the L-arginine treated LVH group compared to the untreated counterparts (18 ± 1 vs. 16 ± 1 µM, P < 0.05) as shown in Fig. 2C. The concentration of H2S in urine was significantly higher in LVH group compared to the control (30 ± 1 vs. 18 ± 0.5 µM, P < 0.05) (Fig. 2D). Finally, the concentration of H2S in the heart was significantly lower (P < 0.05) in the LVH group compared to the control (25 ± 1 vs. 36 ± 1 mmol/mg of protein, P < 0.05) (Fig. 2E).
Measurement of physical indices of the heart
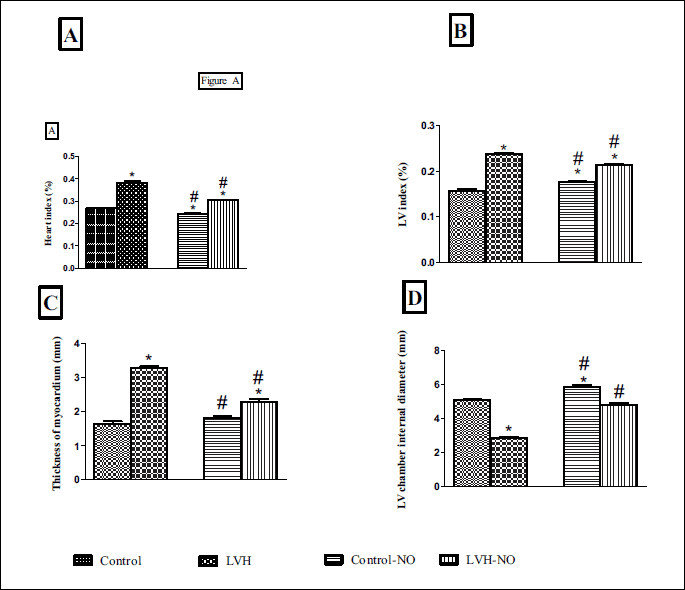
The heart index of the LVH rats was significantly higher than their respective controls (0.38 ± 0.01 vs. 0.26 ± 0.00%, P < 0.05). Treatment of LVH rats with L-arginine resulted in a significantly lower heart index compared to the untreated counterparts (0.30 ± 0.00 vs. 0.38 ± 0.01%, P < 0.05) (Fig. 3A). The LV index was higher in the LVH rats compared to the control rats (0.23 ± 0.00 vs. 0.16 ± 0.00%, P < 0.05) but treatment of LVH rats with L-arginine restored the LV index back to near normal level (0.21 ± 0.00 vs. 0.23 ± 0.00%, P < 0.05) (Fig. 3B). The thickness of the myocardium in the LVH rats was higher than the control animals (3.29 ± 0.04 vs. 1.64 ± 0.07%, P < 0.05). Treatment of the LVH group with L-arginine resulted in a reduced thickness of the myocardium compared to untreated LVH group (2.28 ± 0.07 vs. 3.29 ± 0.04 mm, P < 0.05) (Fig. 3C). Furthermore, the internal diameter of the LV chamber was reduced in LVH rats (2.85 ± 0.04 vs. 5.09 ± 0.02 mm, P < 0.05) compared to the control rats and was restored back to near normal diameter in the L-arginine treated LVH group (4.80 ± 0.11 vs. 5.09 ± 0.019 mm, P < 0.05) (Fig. 3D). Finally, chronic administration of L-arginine in the control group resulted in a significant reduction in the heart index compared to a significant increase in LV index or LV internal diameter (all P < 0.05).
Measurement of oxidative stress parameters in the plasma and the heart tissues
The plasma level of SOD in the LVH group was lower (3.03 ± 0.6 vs. 5.51 ± 0.4 µmol/ml, P < 0.05) than the control group but was restored following L-arginine treatment (8.8 ± 0.18 µmol/ml, P < 0.05) as shown in Fig. 4A. Conversely, the plasma level of MDA in the LVH group was higher than in the control rats (36 ± 0.3vs. 21 ± 2 nmol/ml, P < 0.05) but this value was reduced following L-arginine treatment (30 ± 0.52 nmol/ml, P < 0.05) (Fig. 4B). The plasma level of GSH in the LVH group was lower than in the control group (134 ± 19 vs. 542 ± 17 µmol/ml, P < 0.05) but was higher following L-arginine treatment (610 ± 68 µmol/ml, P < 0.05) (Fig. 4C). Finally, the total antioxidant capacity in the LVH rats was lower than the control (10 ± 0.22 vs. 18 ± 1 U/ml, P < 0.05) and remained the same following the treatment of LVH rats with L-arginine (10 ± 0.6 U/ml) (Fig. 4D).
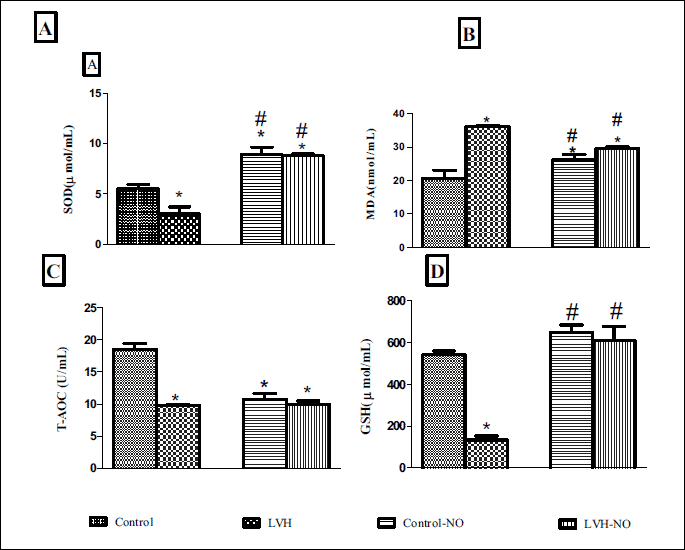
The SOD level in the myocardium of LVH group was lower (10 ± 1 vs. 23 ± 1 U/mg of protein, P < 0.05) than the control group and was restored following L-arginine treatment (23 ± 1 U/mg of protein, P < 0.05) as shown in Fig. 5A. Conversely, myocardial MDA in the LVH group was higher than in the control rats (22 ± 1 vs. 9 ± 1 nmol/mg of protein, P < 0.05) but this was reduced following L-arginine treatment (14 ± 1 nmol/mg of protein, P < 0.05) (Fig. 5B). Finally, myocardial GSH in the LVH group was lower than in the control group (17 ± 1 vs. 29 ± 1 U/mg of protein P < 0.05) but was higher following L-arginine treatment (26 ± 1 U/mg of protein, P < 0.05) as shown in Fig. 5C.
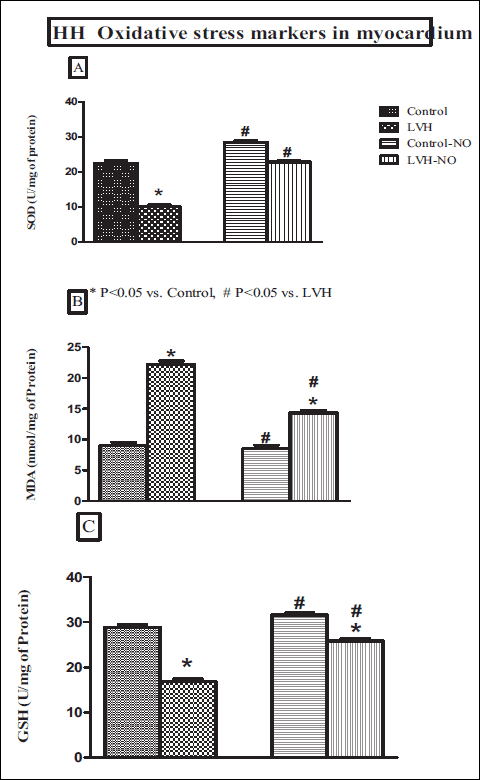 |
Fig. 5. Myocardium levels of SOD (A), MDA (B), and GSH (C) of control, LVH, control-NO and LVH-NO rats. Data are expressed as mean ± S E M. * represents P < 0.05 compared to control while # represents P < 0.05 compared to LVH group (n = 6). |
Histopathology using haematoxylin and eosin staining and Picrosirus red staining
The histopathological analysis of the heart tissues showed thick bands of collagen on the hypertrophied heart muscle of the LVH rats compared to the control. These bands of collagen were fused and had a thin thread like deposition indicating that L-arginine treatment reduced collagen content of heart tissue as shown in Fig. 6.
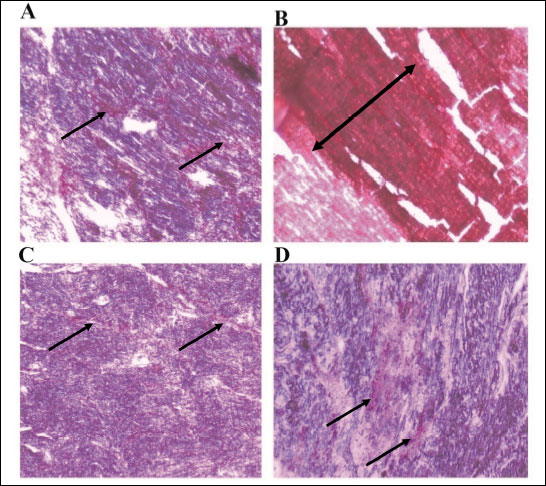 |
Fig. 6. The collagen deposition in the myocardium of controls (A), control-NO (B), LVH (C) and LVH-NO (D) rats. All sections were stained with Picrosirius red stain. (Magnification, ×100). |
The haematoxylin and eosin staining showed normal striations of the heart muscle in control rats while fibroblast and inflammatory cells were seen in the perimysium of the LVH rats. The treatment of LVH rats with L-arginine reduced the number of fibroblast as these were converted to collagen and inflammatory cells in the perimysium and the striations are partially restored. Fibrosis was also observed and the cardiomyocytes had degenerated in the LVH L-arginine treated group as shown in Fig. 7.
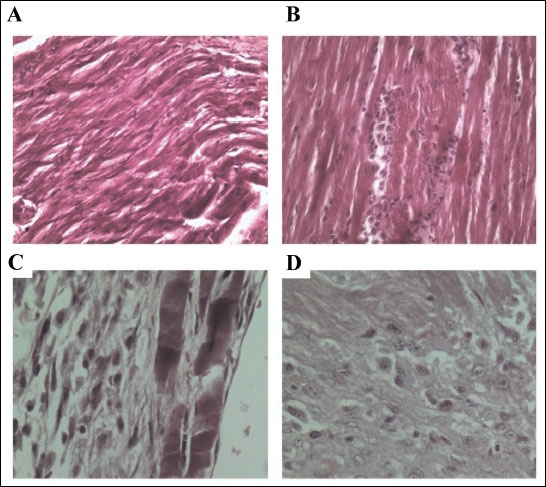 |
Fig. 7. Haematoxylin and eosin (H&E) staining of the myocardium of control (A), control-NO (B), LVH (C) and LVH-NO (D) rats. (Magnification, ×100). |
DISCUSSION
The present study showed that in LVH eNOS is down regulated and causes an imbalance in the oxidative status of the body and this leads to arterial dysfunction by increasing arterial stiffness. Increased oxidative stress together with increased arterial stiffness and NO deficiency impacts on the kidney to reduce renal cortical blood perfusion. Interestingly, the chronic administration of L-arginine up regulated eNOS mRNA in the myocardium and resulted in increased heart tissue and plasma NO levels and a regression of LVH. It was also shown that L-arginine would suppress myocardial CSE mRNA and hence H2S levels in the plasma in the LVH model. There were a number of novel findings. The first is the observation that there was a down regulation of the eNOS/NO-cGMP regulatory pathway locally in the myocardium during LVH. This down regulation may be attributed to a higher sympathetic activity and adrenergic stimulation in this model which could also contribute to the increased oxidative stress, endothelial dysfunction and subsequently lead to a lower renal cortical blood perfusion. The chronic administration of L-arginine ameliorated the development of LVH by enhancing the molecular expression of eNOS and CSE mRNAs in the myocardium in addition to improving systemic hemodynamic parameters, heart geometry, antioxidative capacity, arterial stiffness and renal cortex blood perfusion. The second observation was that under normal conditions, L-arginine enhances H2S production in the myocardium by up regulating CSE mRNA. Contrariwise, L-arginine down regulates CSE mRNA in the myocardium of the LVH group and therefore inhibits H2S production.
The present study demonstrated that eNOS mRNA was down regulated in the myocardium of LVH rats. In addition, there was a reduction in local as well as systemic NO production as reflected by the tissue and circulating levels of NO. In relation to this, previous studies demonstrated that chronic inhibition of NO synthesis resulted in the development of LVH (46) and L-NAME chronic administration resulted in LVH by the inhibition of eNOS (47) in mice. What remained unclear was the impact of chronic treatment with L-arginine, to restore NO production on the expression of eNOS in the myocardium in the caffeine/isoprenaline induced model of LVH. The eNOS is responsible for the local generation of NO in the heart with a resultant enhancement of guanylyl cyclase activity which in turn generates cyclic guanosine monophosphate (cGMP) (48). The findings from the present study showed that tissue levels of NO increased in L-arginine treated LVH rats. Indeed, this took place with a concomitant up regulation of eNOS in the myocardium indicating that the activation of the heart tissue eNOS/NO system ameliorated LVH in these rats. This suggestion is in agreement with the notion that localized up regulation eNOS/NO-cGMP pathway is responsible for an anti-hypertrophic action by exploiting the NO-cGMP pathway counter regulatory system (49). Besides this localised effect, systemic plasma levels of NO were also increased in the L-arginine treated LVH group indicating not only a compartmental but also a systemic up regulation of the eNOS/NO pathway. This global up regulation of the eNOS/NO-cGMP pathway may be a secondary mechanism responsible for regression of the cardiac hypertrophy in these rats. This mechanism may operate by promoting vasodilation and blood pressure reduction and ultimately reducing overload on the heart (50). This increased bioavailability and reduction of blood pressure is in line with previous study reported on diabetic spontaneously hypertensive rats (51).
Another factor for the enhanced expression of eNOS/NO is the decreased plasma level of H2S in LVH rats. This possibility is in line with a previous report which showed that NO was essential for enhancing the production of H2S (23). However, the treatment of LVH rats with L-arginine in the present study had no effect on the plasma level of H2S rather it increased its excretion in urine.
The lower plasma levels of H2S in LVH rats is consistent with the observation of the down regulation of cystathione γ lyase (CSE) enzyme in the myocardium of LVH compared to the control rats as shown in this study. Conversely, L-arginine treatment in control rats resulted in higher expression of CSE enzyme in the myocardium and increased plasma level of H2S. These observations indicate that the normal relationship between NO and H2S regulation is disrupted in this model of LVH.
The present study demonstrated that in this model of LVH, there is an increased SBP, DBP and MAP values. Indeed, the spontaneously hypertensive rat model has been used to study LVH (52, 53). The increased peripheral vasoconstriction due to elevated blood pressure either in LVH rats or SHR may result from a higher after load which ultimately leads to adaptive changes in the heart. In the L-arginine treated LVH group, there was a lower SBP, DBP and MAP compared to the untreated animals. These findings could be due to the potential vasodilator and antihypertensive role of increased NO, as reflected by the increased eNOS mRNA and plasma levels of NO (54-56). It is not clear if this effect of NO on blood pressure in LVH rats is related to the amelioration of hypertrophy in these rats as data from the SHR indicated that the myocardial hypertrophy was independent of the blood pressure lowering mechanism (5, 57).
Arterial stiffness was also reduced by chronic treatment with L-arginine and is in line with previous reports using other models of ventricular hypertrophy (12-14). In the isoprenaline/caffeine model of LVH, angiotensin II level is elevated (9) which is associated with an imbalance in the NO/cGMP pathway leading to endothelial dysfunction (58). In the present study, up regulation of the eNOS/NO-cGMP pathway improved endothelial function as indicated by the reduced pulse wave velocity in the L-arginine treated LVH rats. The up regulation of eNOS/NO-cGMP is an indirect evidence of decreased local and systemic angiotensin II as NO down regulates the synthesis of ACE (59) and angiotensin II type-1 receptors (AT1) in the vasculature (60).
The QRS complex voltage or duration can be used as an effective tool for the detection of LVH (61). The present study demonstrated a significantly elevated QRS complex, an increased R-R interval and a rise in R amplitude in LVH group which was due to increased ventricular size. These ECG tracing changes were all improved in the L-arginine treated LVH group compared to the untreated rats indicating an improvement in the conduction mechanism in the heart of these rats.
The present study demonstrated that LV and heart indices were restored after chronic treatment with L-arginine in LVH rats. These observations would be consistent with a reduction in the mechanical stress imposed on the heart (62) and reflected by lower blood pressure in these rats. On another hand, this also signifies the anti-hypertrophic role of NO in this model of LVH (63). The increase in NO generation per se is an indicator of the up regulation of the eNOS/NO-cGMP pathway.
The chronic treatment of LVH rats with L-arginine up regulated the vasodilatory mechanism which would be expected to reduce the load on the heart and ultimately ameliorate the thickening of the myocardium as well as other histological alterations. This resulted in an increase in the internal volume of the LV thus providing better mechanical contraction of the ventricle. Our study is in line with previous findings which reported that NO and cGMP reduce the growth of the myocardium brought about by higher noradrenaline levels (64). Another contributory mechanism for the reduction in the thickness of the myocardium may be the lower level of oxidative stress in the myocardium and increased bioavailability of NO (62, 65). This increased bioavailability of NO and reduced oxidative stress is in coherence with previous study (66) where salidroside (primary active component of Rhodiola rosea) improve the guanylyl cyclase (GC) activity and reduced the oxidative stress by increasing the NO bioavailability in diabetic rat model. Finally, the reduced collagen deposition in the myocardium may also be a factor for the improved thickness of the myocardium as well as the physical indices of heart.
The renal cortical blood perfusion in LVH rats in the present study was also enhanced following L-arginine treatment. Reduced blood perfusion in the kidney is possibly related to the increased circulating or kidney levels of noradrenaline and angiotensin II (7-9, 67, 68). This is supported by the view that angiotensin II suppresses NO-cGMP production (58). Therefore, chronic administrations of L-arginine up regulate NO-cGMP production which ultimately leads to increased renal cortical blood perfusion. In relation to this, previous studies indicated that NO increases renal papillary blood flow (69, 70). Finally, reduced arterial stiffness due to the chronic administration of L-arginine is another reason for improved renal cortical blood perfusion by increasing arterial distensibility (12).
In conclusion, the present findings showed that the eNOS/NO pathway was responsible for the maintenance of the myocardial architecture and physiology. Furthermore, there was an increase in oxidative and mechanical stress on the myocardium as well as a decrease in the bioavailability of NO. It is possible that the up regulation of the eNOS/NO pathway ameliorated the oxidative stress status in the myocardium of L-arginine treated LVH rats and reversed the elevated mechanical stress.
Acknowledgment: The Institute of Postgraduate Studies (IPS) is acknowledged for the provision of a USM fellowship (Teaching) to Ashfaq Ahmad (APEX (1002/JHEA/ATSG4001) and research grant no. 304/PFARMASI/6313109 to Hassaan A. Rathore.
Conflict of interests: None declared.
REFERENCES
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 1997; 59: 551-571.
- Flanagan ET, Buckley MM, Aherne CM, Lainis F, Sattar M, Johns EJ. Impact of cardiac hypertrophy on arterial and cardiopulmonary baroreflex control of renal sympathetic nerve activity in anaesthetized rats. Exp Physiol 2008; 93: 1058-10 64.
- Buckley MM, Johns EJ. Impact of L-NAME on the cardiopulmonary reflex in cardiac hypertrophy. Am J Physiol Regul Integr Comp Physiol 2011; 301: R1549-R1556.
- Barouch LA, Cappola TP, Harrison RW, et al. Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mol Cell Cardiol 2003; 35: 637-6 44.
- Matsuoka H, Nakata M, Kohno K, et al. Chronic L-arginine administration attenuates cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 1996; 27: 14-18.
- Paulis L, Matuskova J, Adamcova M, et al. Regression of left ventricular hypertrophy and aortic remodelling in NO-deficient hypertensive rats: effect of l-arginine and spironolactone. Acta Physiol 2008; 194: 45-55.
- Bell DG, Jacobs I, Ellerington K. Effect of caffeine and ephedrine ingestion on anaerobic exercise performance. Med Sci Sports Exerc 2001; 33: 1399-1403.
- Collomp K, Ahmaidi S, Audran M, Chanal JL, Prefaut C. Effects of caffeine ingestion on performance and anaerobic metabolism during the Wingate test. Int J Sports Med 1991; 12: 439-443.
- Crowley SD, Gurley SB, Herrera MJ, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 2006; 103: 17985-17990.
- Romero JC, Reckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999; 34: 943-949.
- Harrison D. Endothelial function and oxidant stress. Clin Cardiol 1997; 20 (11 Suppl. 2): II-11-7.
- Wilkinson IB, Qasem A, McEniery CM, et al. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002; 105: 213-217.
- Schulman SP, Becker LC, Kass DA, et al. L-arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (vintage mi) randomized clinical trial. JAMA 2006; 295: 58-64.
- Rector TS, Bank AJ, Mullen KA, et al. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation 1996; 93: 2135-2141.
- Wang R. Hydrogen sulfide: a new EDRF. Kidney Int 2009; 76: 700-704.
- Sanderson K. Physiology: emissions control. Nature 2009; 459: 500-502.
- Ali M, Ping C, Mok YY, et al. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 2006; 149: 625-634.
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 1997; 237: 527-531.
- Grossi L. Hydrogen sulfide induces nitric oxide release from nitrite. Bioorg Med Chem Lett 2009; 19: 6092-6094.
- Yong QC, Cheong JL, Hua F, et al. Regulation of heart function by endogenous gaseous mediators - crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Signal 2011; 14: 2081-2091.
- Whiteman M, Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med 2009; 13: 488-507.
- Hosoki R. Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 1997; 237: 527-531.
- Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 2003; 81: 848-853.
- Wang R. Two’s company, three’sa crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002; 16: 1792-1798.
- Ye S, Nosrati S, Campese VM. Nitric oxide (NO) modulates the neurogenic control of blood pressure in rats with chronic renal failure (CRF). J Clin Invest 1997; 99: 540-548.
- Shen J, Ma S, Chan P, et al. Nitric oxide down-regulates caveolin-1 expression in rat brains during focal cerebral ischemia and reperfusion injury. J Neurochem 2006; 96: 1078-1089.
- Tsai SK, Hung LM, Fu YT, et al. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg 2007; 46: 346-353.
- McLerie M, Lopatin A. Dominant-negative suppression of I (K1) in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol 2003; 35: 367-378.
- Lopez-Santiago LF, Meadows LS, Ernst SJ, et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol 2007; 43: 636-647.
- Ahmad A, Sattar MA, Rathore HA, et al. Functional contribution of a1D-adrenoceptors in the renal vasculature of left ventricular hypertrophy induced with isoprenaline and caffeine in Wistar-Kyoto rats. Can J Physiol Pharmacol 2014; 92: 1029-1035.
- Johren O, Imboden H, Hauser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-converting enzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res 1997; 757: 218-227.
- Mitchell GF, Pfeffer MA, Finn PV, Pfeffer JM. Comparison of techniques for measuring pulse-wave velocity in the rat. J Appl Physiol 1997; 82: 203-210.
- Anand Swarup KR, Sattar MA, Abdullah NA, et al. Effect of dragon fruit extract on oxidative stress and aortic stiffness in streptozotocin-induced diabetes in rats. Pharmacognosy Res 2010; 2: 31-35.
- Gwathmey JK, Kim CS, Hajjar RJ, et al. Cellular and molecular remodeling in a heart failure model treated with the beta-blocker carteolol. Am J Physiol 1999; 276: H1678-H1690.
- Hassan MI, Boosen M, Schaefer L, et al. Platelet-derived growth factor-BB induces cystathionine g-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br J Pharmacol 2012; 166: 2231-2242.
- Xu S, Zhou X, Yuan D, Xu Y, He P. Caveolin-1 scaffolding domain promotes leukocyte adhesion by reduced basal endothelial nitric oxide-mediated ICAM-1 phosphorylation in rat mesenteric venules. Am J Physiol Heart Circ Physiol 2013; 305: H1484-H1493.
- Lee DY, Wauquier F, Eid AA, et al. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species. J Biol Chem 2013; 288: 28668-28686.
- Santha P, Pakaski M, Fazekas O, et al. Acute and chronic stress induced changes in gene transcriptions related to Alzheimer’s disease. [in Hungarian] Ideggyogyaszati Sz 2012; 65: 195-200.
- Cannino G, Ferruggia E, Rinaldi AM. Proteins participating to the post-transcriptional regulation of the mitochondrial cytochrome c oxidase subunit IV via elements located in the 3’ UTR. Mitochondrion 2009; 9: 471-480.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–DDCT method. Methods 2001; 25: 402-408.
- Xia M, Chen L, Muh RW, Li P-L, Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther 2009; 329: 1056-1062.
- Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 1982; 206: 267-277.
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 2004; 313: 22-27.
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 2004; 287: H2316-H2323.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
- Anderson NH, Devlin AM, Graham D, et al. Telemetry for cardiovascular monitoring in a pharmacological study new approaches to data analysis. Hypertension 1999; 33: 248-255.
- Ocsan RJ, Lai YN, Prabhu KV, Hambly BD, McLachlan CS. Chronic NG-nitro-l-argininr methyl ester (L-NAME) administration in C57BL/6J mice induces a sustained decrease in c-kit positive cells during development of cardiac hypertrophy. J Physiol Pharmacol 2013; 64: 727-736.
- Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther 2000; 86: 49-86.
- Booz GW. Putting the brakes on cardiac hypertrophy exploiting the NO-cGMP counter-regulatory system. Hypertension 2005; 45: 341-346.
- Panidis IP, Kotler MN, Ren JF, Mintz GS, Ross J, Kalman P. Development and regression of left ventricular hypertrophy. J Am Coll Cardiol 1984; 3: 1309-1320.
- Mason R, Corbalan J, Jacob R, Dawoud H, Malinski T. Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and rantes levels in hypertensive rats with diabetes. J Physiol Pharmacol 2015; 66: 65-72.
- Yeh JL, Liu CP, Hsu JH, et al. KMUP-1 inhibits hypertension-induced left ventricular hypertrophy through regulation of nitric oxide synthases, ERK1/2, and calcineurin. Kaohsiung J Med Sci 2012; 28: 567-576.
- Bing OH, Brooks WW, Robinson KG, et al. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J Mol Cell Cardiol 1995; 27: 383-396.
- Ignarro LJ, Lippton H, Edwards JC, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther 1981; 218: 739-749.
- Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation 1993; 87: 1468-1474.
- Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation b-blockers stimulate nitric oxide release from endothelial cells through ATP efflux. A novel mechanism for antihypertensive action. Circulation 2003; 107: 2747-2752.
- Kristek F. Long-term administration of L-arginine did not influence blood pressure, heart rate, cardiac hypertrophy or arterial wall thickness of spontaneously hypertensive rats. Exp Physiol 1998; 83: 595-603.
- Mollnau H, Wendt M, Szocs K, et al. Effects of angiotensin II infusion on the expression and function of NAD (P) H oxidase and components of nitric oxide/cGMP signaling. Circ Res 2002; 90: e58-e65.
- Higashi Y, Oshima T, Ono N, et al. Intravenous administration of L-arginine inhibits angiotensin-converting enzyme in humans. J Clin Endocrinol Metab 1995; 80: 2198-2202.
- Ichiki T, Usui M, Kato M, et al. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension 1998; 31: 342-326.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20: 1180-1186.
- Aikawa R, Nagai T, Tanaka M, et al. Reactive oxygen species in mechanical stress-induced cardiac hypertrophy. Biochem Biophys Res Commun 2001; 289: 901-907.
- Simko F, Simko J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiol Res 2000; 49: 37-46.
- Calderone A, Thaik CM, Takahashi N, Chang D, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest 1998; 101: 812-818.
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 2004; 287: R1014-R1030.
- Alameddine A, Fajloun Z, Bourreau J, et al. The cardiovascular effects of salidroside in the Goto-Kakizaki diabetic rat model. J Physiol Pharmacol 2015; 66: 249-257.
- Leenen FH, White R, Yuan B. Isoproterenol-induced cardiac hypertrophy: role of circulatory versus cardiac renin-angiotensin system. Am J Physiol Heart Circ Physiol 2001; 281: H2410-H2416.
- Madrid MI, Garcia-Salom M, Tornel J, de Gasparo M, Fenoy FJ. Interactions between nitric oxide and angiotensin II on renal cortical and papillary blood flow. Hypertension 1997; 30: 1175-1182.
- Mattson DL, Roman RJ, Cowley A. Role of nitric oxide in renal papillary blood flow and sodium excretion. Hypertension 1992; 19: 766-769.
- Fenoy FJ, Ferrer P, Carbonell L, Garcia-Salom M. Role of nitric oxide on papillary blood flow and pressure natriuresis. Hypertension 1995; 25: 408-414.
A c c e p t e d : January 19, 2016
Dr. Ashfaq Ahmad, Cardiovascular and Renal Laboratory of Physiology, School of Pharmaceutical Sciences, Universiti Sains Malaysia. e-mail: raza_chohan487@hotmail.com