REGULAR EXERCISE ALLEVIATES RENOVASCULAR HYPERTENSION-INDUCED CARDIAC/ENDOTHELIAL DYSFUNCTION AND OXIDATIVE INJURY IN RATS
INTRODUCTION
Epidemiological studies verify that hypertension is a serious public health problem because it is a major and well-known risk factor for the occurrence of several cardiovascular (peripheral arterial disease, ischemic heart disease, aortic aneurysm) and cerebrovascular diseases resulting in multi-organ damage (1). Renovascular hypertension (RVH), which is a form of secondary hypertension resulting from fibromuscular dysplasia or atherosclerotic renal artery disease, involves the activation of renin-angiotensin-aldosterone (2) and sympathetic nervous systems (3) and the presence of chronic oxidative stress (4). Although RVH constitutes a small percentage of hypertensive patients, it is the most common cause of hypertension that can be surgically corrected (5). During the initial stages of RVH, increased angiotensin II activity, which maintains the high blood pressure, contributes to the progression of atherogenesis and glomerulosclerosis by stimulating vasoconstriction, increasing endothelin release, enhancing extracellular matrix deposition and vascular remodeling (6). Reactive oxygen species (ROS) further trigger the release of vasoconstrictor substances and play an important role in the pathogenesis of hypertension-induced organ failure (7-10). A number of studies have also demonstrated that increased oxidative stress results in endothelial dysfunction, causing reduced bioavailability of nitric oxide (NO), one of the most powerful endothelium-derived vasodilators (11-13).
Apart from various pharmacological treatments that target high blood pressure, physical exercise as a non-pharmacological approach was previously shown to delay the generation of hypertension or to reduce the level of high blood pressure by central and peripheral neurohumoral mechanisms (14-16) and by correcting the imbalance between the production of ROS and the antioxidant defense systems (17). A number of well-controlled epidemiologic studies with large patient groups have reported that the mortality risk of the hypertensive individuals is reduced as their exercise capacity is increased (18-20). Exercise training in hypertensive subjects was also shown to reduce left ventricular hypertrophy or does not worsen the underlying inflammatory burden associated with hypertension (21). Similarly, exercise training in hypertensive animals was shown to decrease blood pressure (22-24) while an increase in vessel compliance (25) was reported.
Although the importance of physical activity in the prevention of cardiovascular and renal diseases has been demonstrated in many studies, the impact of exercise training on RVH-induced vascular dysfunction and oxidative stress with its underlying mechanisms has not been thoroughly elucidated yet. Since hypertension is regarded as a chronic low-grade inflammation with elevated plasma levels of the pro-inflammatory cytokines in hypertensive patients (26, 27), the impact of exercise on RVH-induced oxidative damage of cardiac tissue needs to be investigated. Accordingly, through hemodynamic measurements, biochemical and histological analyses, we aimed to elucidate the therapeutic effect of exercise training when performed after the onset of RVH.
MATERIALS AND METHODS
Animals
Male Wistar albino rats (300 – 350 g, 10 weeks old, n = 40), supplied by the MU Animal Center (DEHAMER), were housed in a humidity (65 – 70 %) and temperature-controlled room (22 ± 2°C) with standardized light/dark (12/12 hour) cycles. Rats were fed with standard rat pellets and tap water ad libitum.
All experimental protocols were approved by the Marmara University (MU) Animal Care and Use Committee.
Surgery and experimental design
One day before the surgery, blood pressures of all rats were measured and transthoracic echocardiography was made to obtain the basal values. Following the anesthesia of the rats with intraperitoneal injection of ketamine (100 mg/kg, i.p.) and chlorpromazine (0.75 mg/kg, i.p.), two-kidney, one-clip (2K1C, Goldblatt) procedure was used to induce RVH (28). Briefly, a silver clip (internal diameter 0.25 mm) was placed around the left renal artery of rats (n = 30), while the right artery has remained untouched. Sham-operated control rats (n = 10) had the similar surgical procedures but the left renal artery was left unclipped.
Exercise was started three weeks after RVH surgery (n = 15) and continued for 9 weeks without a prior training period and no load was applied on the swimming rats. A moderate load swimming exercise model was selected in the exercise groups (29). Swimming sessions of 30-min were made in a cylindrical glass pool (100 × 50 × 50 cm) filled with 35-cm high lukewarm water and were continued 5 days/week for nine consecutive weeks. Sham-operated control rats and half of the RVH group that had surgery on the 3rd week (n = 15) were put on their feet in a separate glass tank filled with only 5-cm water and were left sedentary.
Body weights were monitored, and blood pressures were measured using tail-cuff on a weekly basis throughout the experiment. RVH surgeries of sedentary and exercised groups were performed on the 3rd week after the blood pressure recordings were taken. On the 12th week of the experiment, the second echocardiographic evaluation was performed and the rats were decapitated. Heart and thoracic aorta were carefully removed and blood was collected for the measurement of serum tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2) and IL-6 levels. Thoracic aorta was placed in a dish containing chilled Krebs-Henseleit buffer solution aerated with 95% O2 and 5% CO2, while aortic samples were put in a 10% formaldehyde solution for immunochemistry. Heart weights were measured and cardiac tissue samples obtained from each animal were stored at –80°C until the determination of tissue myeloperoxidase (MPO) and catalase (CAT) activities, malondialdehyde (MDA) and glutathione (GSH) levels.
Blood pressure measurement
Following the swimming sessions that were repeated for 5 days of the week, 2 days of rest period was given. After this interval and before a swimming bout was started, indirect blood pressure measurements were made by the tail-cuff method (Biopac MP35 Systems, Inc. COMMAT Ltd., Ankara, Turkey) on the first day of the week. Rats were placed for 10 min in a chamber heated to 35°C, and then a cuff equipped with a pneumatic pulse sensor was wrapped around the tail of the rat that was placed in an individual plastic restrainer. Blood pressure recorded from each rat during each measurement period was averaged from at least three consecutive readings on that occasion. Blood pressure recordings were made before the first recording of the rats that had a two-day resting.
Echocardiography
Echocardiographic imaging and calculations were done using a 12-MHz linear transducer and 5 – 8 MHz sector transducer (Vivid 3, General Electric Medical Systems Ultrasound, Tirat Carmel, Israel) according to the guidelines published by the American Society of Echocardiography (30) Under ketamine (50 mg/kg, i.p.) anesthesia, transthoracic echocardiography was made from M-mode, and after observing at least six cardiac cycles, two-dimensional images were obtained in the parasternal long and short axes at the level of the papillary muscles. Interventricular septal thickness (IVS), left ventricular diameter (LVD) and left ventricular posterior wall thickness (LVPW) were measured during systole (s) and diastole (d). Ejection fraction, fractional shortening and left ventricular mass and relative wall thickness were calculated from the M-mode images using the following formulas:
% ejection fraction = (LVDd)3 - (LVDs)3/(LVDd)3 × 100; % fractional shortening = LVDd - LVDs/LVDd × 100; left ventricular mass = 1.04 × ((LVDd + LVPWd + IVSd)3 - (LVDd)3) × 0.8 + 0.14; relative wall thickness = 2 × (LVPWd/LVDd) (30).
Vascular reactivity studies
After removal of the surrounding connective tissue, fresh thoracic aorta was cut transversely into rings approximately 4-mm wide and mounted in an organ bath (Biopac MP35 Systems, Inc. COMMAT Ltd., Ankara, Turkey) containing 20 ml of aerated (95% O2 and 5% CO2). Krebs-Henseleit buffer maintained at 37°C. The rings were placed under a resting tension of 1.0 g. After a 60-min period of equilibration, the rings were exposed to 80 mM KCl. For the measurement of contractile response to phenylephrine (10–9 to 10–3 M), cumulative concentration-response curves were obtained in a stepwise manner, where the consecutive concentration was added after the response has reached the plateau. After completion of phenylephrine concentration curves, tissues were washed 3 times in 30 minutes. Then, on the rings that were pre-contracted with the submaximal dose of phenylephrine (3X10–6 M), the relaxation responses were evaluated by adding increasing cumulative concentrations of carbachol (CCh; 10–9 - 10–3 M). Contractile responses to phenylephrine are expressed as percentages of the maximal contraction induced by 80 mM KCl, and the relaxation responses to CCh are expressed as percentages of the contraction caused by 3X10–6 M phenylephrine.
Measurement of cytokines in the serum
Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2) and IL-6 were quantified according to the manufacturer’s instructions and guidelines (Biosource Europe S.A., Nivelles, Belgium) using enzyme-linked immunosorbent assay (ELISA) kits specific for rat cytokines.
Measurement of cardiac myeloperoxidase activity
Tissue MPO activity, which is frequently utilized to estimate tissue neutrophil accumulation in inflamed tissues, was shown to correlate significantly with the number of neutrophils determined by histochemical analysis (31). The method used to determine MPO activity in the cardiac tissues was similar to that previously described by others (31). The cardiac tissue samples (0.2 – 0.3 g) were homogenized in 10 volumes of ice-cold potassium phosphate buffer (50 mm K2HPO4, pH 6.0) containing hexadecyltrimethylammonium bromide (HETAB; 0.5%, w/v). The homogenate was centrifuged at 41,400 g for 10 min at 4°C, and the supernatant was discarded. The pellet was then re-homogenized with an equivalent volume of 50 mm K2HPO4containing 0.5% (w/v) HETAB and 10 mm EDTA (Sigma). Myeloperoxidase activity was assessed by measuring the H2O2-dependent oxidation of o-dianizidine 2HCl. One unit of enzyme activity was defined as the amount of MPO present per gram of tissue weight that caused a change in absorbance of 1.0 min–1 at 460 nm and 37°C.
Measurement of malondialdehyde (MDA) and glutathione (GSH) levels in the cardiac tissues
Cardiac tissue samples were homogenized in ice-cold trichloracetic acid (1 g tissue plus 10 ml 10% TCA) in an Ultra Turrax tissue homogenizer. Homogenized cardiac samples were centrifuged at 2,000 g for 15 min at 4°C. The supernatant was removed and re-centrifuged at 41,400 g for 8 min. MDA levels were assayed for products of lipid peroxidation by monitoring thiobarbituric acid reactive substance formation as previously described (32). Lipid peroxidation was expressed in terms of MDA equivalents using an extinction coefficient of 1.56 × 10–5 M–1 cm–1 and the results are expressed as nmol MDA/g tissue. Glutathione measurements were performed using a modification of the Ellman procedure (33). Briefly, after centrifugation at 2000 g for 10 min, 0.5 ml of supernatant was added to 2 ml of 0.3 mol/l Na2HPO4·2H2O solution. A 0.2 ml solution of dithiobisnitrobenzoate (0.4 mg/ml 1% sodium citrate) was added and the absorbance at 412 nm was measured immediately after mixing. Glutathione levels were calculated using an extinction coefficient of 1.36 × 105 M–1 cm–1. The results are expressed in µmol GSH/g tissue.
Measurement of catalase (CAT) activity in the cardiac tissues
The method for the measurement of CAT activity is based on the catalytic activity of the enzyme that catalyses the decomposition reaction of H2O2 to give H2O and O2 (34). Briefly, the absorbance of the tissue samples containing 0.4 ml homogenate and 0.2 ml H2O2 was read at 240 nm and 20°C against a blank containing 0.2 ml phosphate buffer and 0.4 ml homogenate for about 1 min.
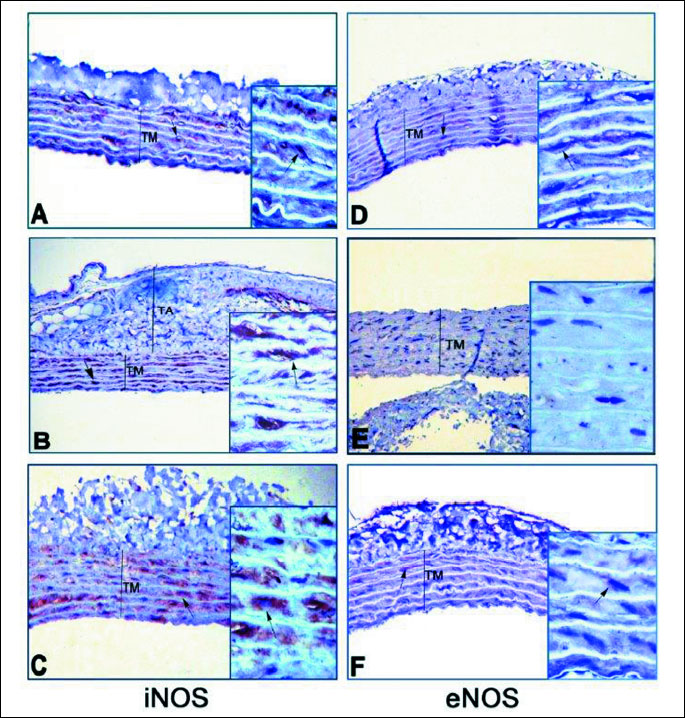
Immunohistochemical analysis of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) protein distributions in the aorta
Aortae from all groups were fixed in a 10 % formaldehyde solution and washed in tap water for 2 hours. Then the tissues were dehydrated with increasing concentrations (70, 90, 96 and 100%) of ethanol and cleared with xylene. Paraffin-embedded sections that were cut at 3-µm thickness were de-paraffinized with xylene, and rehydrated with ethanol and water. Antigen retrieval was accomplished by Decloacking chamber (Bicare Medical DC2008) in a citrate diva buffer (DV Sitogen 2004 LX, MX pH 6.2) for 40 min at 110°C. The endogenous peroxidase activity was blocked with 3% H2O2 (ScyTek ACA 125) for 15 min at room temperature and later rinsed with phosphate buffered saline (PBS). Blocking reagent (UV Blocking, SycTek AA125) was applied to each slide followed by 5 min incubation at room temperature in a humid chamber. Sections were incubated for 1 hour at room temperature with rabbit polyclonal eNOS antibody (1:100, eNOS Rabbit PAb, RB-9279-P) or iNOS antibody (1:50, iNOS Ab-1, Rabbit PAb, RB-9242-P1). Antibodies were diluted in a large volume of UltrAb Diluent (TA-125-UD). The sections were biotinylated goat anti-rabbit antibodies (TS-060-HR, SycTek ABF125). After slides were washed in PBS, the streptavidin peroxidase label reagent (TS-060-HR, HRP SycTek ABG125) was applied for 20 min at room temperature in a humid chamber. The colored product was developed by incubation with 3,3’-diaminobenzidine tetrahydrochloride dihydrate (DAB) (Thermo Scientific RTU TA-060-HD). The slides were counterstained with Mayer’s hematoxylin (LabVision, TA-125-MH) and mounted in entellan (Shandon Ref: 999040). Immunohistochemical staining was photographed with light microscope (Leica 390-CU, Germany).
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 6.0 (GraphPad Software, Inc. La Jolla, CA, USA). All data are expressed as means ± S.E.M. Groups of data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test or Student’s t test where appropriate. Values of P < 0.05 were regarded as significant.
RESULTS
Effect of exercise performed after the onset of renovascular hypertension on blood pressure
The basal systolic blood pressures that were recorded before starting the experiments were not different among three groups (Fig. 1). As expected in the current RVH model, mean systolic blood pressures measured on the 3rd week of clip placement were elevated in the sedentary RVH and the exercised RVH groups significantly as compared with the blood pressures of the control group (P < 0.001), indicating the onset of hypertension. Even on the 12th week, the high blood pressure of the RVH group that has exercised for 9 weeks was not significantly different than that of the sedentary RVH group.
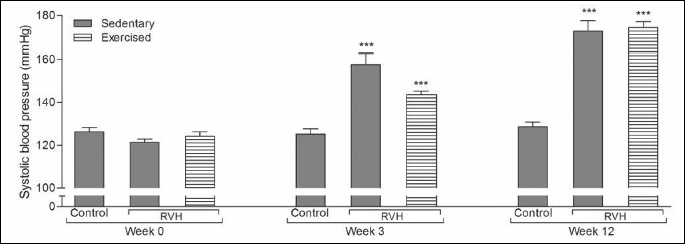
***P < 0.001: compared to control group.
Effect of exercise performed after the onset of renovascular hypertension on cardiac hypertrophy and renal atrophy
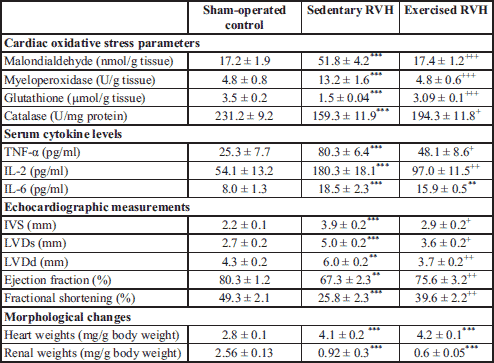
In order to assess cardiac hypertrophy, the ratio of the heart weight (mg) to the body weight (g) (HW/BW) measured on the day of decapitation was used. The body weights of the rats were increased gradually throughout the 12-week experimental period without any significant differences among the experimental groups. However, the HW/BW ratios calculated in the sedentary and exercised RVH groups were significantly increased when compared with the ratio of the control group (P < 0.001, Table 1), suggesting eccentric cardiac hypertrophy. Renal atrophy index, which is the ratio of the clipped left kidney weight (mg) to the body weight (g), was measured on the day of decapitation. Renal atrophy indices of both the sedentary and exercised RVH groups were decreased with respect to sham-operated control group (P < 0.001, Table 1), confirming the experimental model of hypertension.
Effect of exercise performed after the onset of renovascular hypertension on echocardiographic measurements
Transthoracic echocardiography measurements (Fig. 2) monitored on the 12th week are summarized in Table 1. When compared to sham-operated control group, RVH caused significant increases in IVS, LV end-diastolic and LV end-systolic dimensions of the sedentary group (P < 0.01 – 0.001), while these parameters were reduced in the exercised RVH group (P < 0.05 – 0.01; Table 1). Furthermore, reductions in ejection fraction and fractional shortening observed in the sedentary RVH group (P < 0.01 – 0.001) were found to be elevated in the exercised RVH group (P < 0.01).
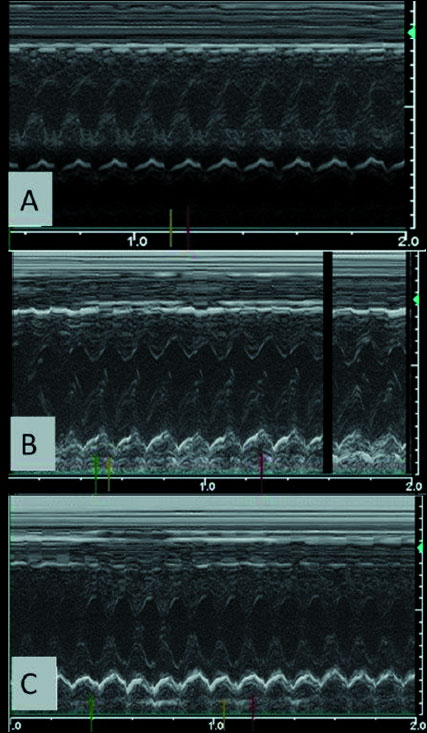 |
Fig. 2. Representative echocardiographic scans of (A) the sedentary sham-operated control group with normal M-mode view; (B) sedentary renovascular hypertension (RVH) group with increased interventricular septum and left ventricular posterior wall thickness; and (C) exercised RVH group, demonstrating normal M-mode view as the sham-operated group. |
Effect of exercise performed after the onset of renovascular hypertension on the contraction and relaxation of the aortic rings
In the aortic rings of the sham-operated control rats, addition of 10–9 to10–3 M phenylephrine cumulatively into the organ bath caused a concentration-dependent contraction, reaching the 50% maximal response (EC50) at the 7.83 × 10–7 M concentration (Fig. 3A). Both in the sedentary RVH or exercised RVH groups that had a 9-week duration of hypertension after its onset, the contractile responses of the aortic rings to phenylephrine were significantly amplified, demonstrating reduced EC50 values (6.87 × 10–7 M and 5.9 × 10–7 M, respectively).
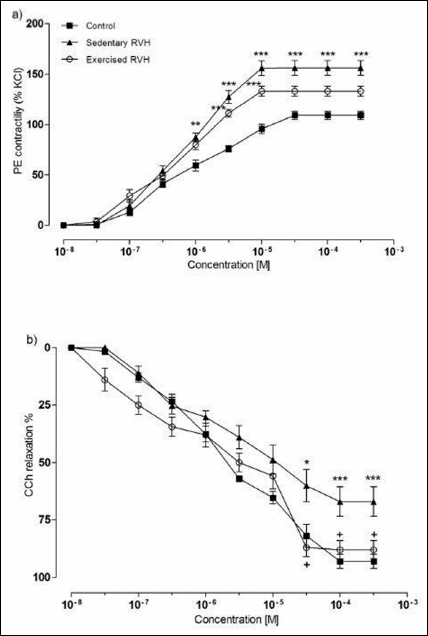 |
Fig. 3. (a) Concentration-response curves obtained by cumulative addition of phenylephrine (PE) to rat thoracic aorta. RVH increased contractile activity of sedentary and exercised RVH groups in comparison to sedentary sham-operated control group (*P < 0.05). Points indicate percentage of contraction induced by 124 mM KCl. (b) Concentration-response curves obtained by cumulative addition of carbachol (CCh) to rat thoracic aorta strips pre-contracted with 30 mM phenylephrine. RVH impaired relaxation response in the sedentary group with respect to sedentary sham-operated control group (*P < 0.05), and the relaxation response in the exercised RVH group was higher than that of the sedentary RVH group (+P < 0.05). Values are shown as mean ± S.E.M. of eight experiments. |
CCh added cumulatively at doses of 10–9 to 10–3 M to aortic rings of sham-operated control group, which were pre-contracted with the submaximal (60 – 70% of maximal contraction) dose of phenylephrine (3 × 10–5 M), caused a dose-dependent relaxation response with an EC50 value of 1.84 × 10–6 M (Fig. 3B). EC50 value in the sedentary RVH (1.42 × 10–6 M) was not different than that of the control group, while in the exercised RVH groups EC50 value of the relaxation responses was significantly higher (3.62 × 10–6 M; P < 0.05) as compared to the control group, indicating a decrease in the CCh-induced aortic relaxation.
Effect of exercise performed after the onset of renovascular hypertension on the serum levels of pro-inflammatory cytokines
Serum levels of pro-inflammatory cytokines TNF-α, IL-2 and IL-6 were increased in the sedentary RVH group as compared to the control group (P < 0.001; Table 1). In the exercised RVH group, TNF-α (P < 0.05) and IL-2 (P < 0.01) levels, but not IL-6 levels, were significantly depressed.
Effect of exercise performed after the onset of renovascular hypertension on the cardiac malondialdehyde and glutathione levels, myeloperoxidase and catalase activities
In the sedentary RVH group, cardiac MDA level was significantly higher than that of the sham-operated control group, indicating increased lipid peroxidation (P < 0.001; Table 1). Exercise performed after the onset of RVH abolished this increase and returned the cardiac MDA levels back to control levels (P < 0.001). Similarly, MPO activity, which is accepted as an indicator of neutrophil infiltration, was significantly higher in the cardiac tissues of the sedentary RVH group (P < 0.001) as compared to the control group (Table 1). However, exercise significantly decreased the cardiac MPO activity when it was performed after the onset of RVH (P < 0.001).
In accordance with increased MDA level and MPO activity, induction of RVH in the sedentary rats caused significant reductions in cardiac GSH levels and CAT activities, when compared to those of the control rats (P < 0.001, Table 1). However, in the exercised RVH group, GSH levels and CAT activities were replenished significantly (P < 0.001 and P < 0.05).
Effect of exercise performed after the onset of renovascular hypertension on the immunohistochemical staining of inducible nitric oxide synthase and endothelial nitric oxide synthase in the aorta
Qualitative immunohistochemical analysis on the aortic tissues of the sham-operated control rats revealed that the iNOS immunoreactivity was evident in tunica media layer (Fig. 4A). However, the iNOS immunostaining (dark brown) of aortic wall layers appeared to be more intense in the sedentary RVH (Fig. 4B) and exercised RVH (Fig. 4C) groups as compared to the control group. Normal eNOS immunoreactivity was observed in the tunica media layer of the sham-operated control group (Fig. 4D). The dark brown immunostaining indicating eNOS activity was more abundant in the exercised RVH group (Fig. 4F) compared to the sham-operated control group. However, in the sedentary group, the immunostaining of eNOS was not observed in none of the layers of the vessel wall (Fig. 4E).
DISCUSSION
The present results demonstrate that blood pressure, aortic contractile response, left ventricular dimensions, cardiac oxidative damage and aortic iNOS staining were increased in the sedentary rats, while eNOS staining in the aorta and ejection fraction were decreased. Exercise after RVH reversed the echocardiographic alterations and oxidative parameters and increased the staining of aortic eNOS, indicating the positive impact of exercise on RVH-induced oxidative damage and cardiac dysfunction. The reversal of RVH-induced increases in the pro-inflammatory cytokines and the oxidant/antioxidant imbalance in the cardiac tissue implicate the therapeutic effects of exercise on hypertension-induced oxidative injury.
It has been shown that physical exercise delays the generation of hypertension and reduces the level of high blood pressure (16) when exercise is started before the development of hypertension. However, our data suggests that implementing exercise sessions after the development of hypertension - starting by the third week of RVH surgery - had no effect on high blood pressure, but improved oxidative damage and cardiac dysfunction. This anti-oxidant effect of exercise was reached even no load adjustments were made throughout the swimming sessions. These data implicate that starting exercise as a life style change following the development of hypertension cannot reduce blood pressure, but can be of benefit in controlling the oxidant/antioxidant balance in the cardiac tissue.
In the long term, hypertension often yields left ventricular hypertrophy, which is a major risk factor for the development of coronary heart disease, ventricular arrhythmia, and sudden cardiac death (35). Despite any changes in the heart weight, our data revealed that induction of RVH in sedentary rats resulted in increased left ventricular end-diastolic/end-systolic dimensions and thickened left interventricular septum with a concomitant reduction in ejection fraction. The occurrence of hypertensive vascular disease involves several hemodynamic mechanisms including myocyte hypertrophy, collagen deposition, increased ratio of arteriolar wall thickness/lumen and coronary arteriolar compression by the left ventricle (36). In addition, increased oxidative stress was shown to play an important part in the pathogenesis of experimental renovascular hypertension (37). in vivo and in vitro studies demonstrated that vascular endothelial cells release cyclooxygenase-derived endothelium-dependent contracting factors and ROS, resulting in facilitated vasoconstriction along with impaired endothelium-dependent relaxation mediated by the NO production (12, 38-42). Since eNOS has a major role in determining blood flow and resistance (43, 44), impairment in its expression or its increased phosphorylation, as well as the presence of oxidative stress, may result in endothelial dysfunction (45). Our results revealed that decreased eNOS and increased iNOS staining were observed in the aorta of sedentary rats with RVH. In accordance with these results, we also demonstrated that aortic contractile response was exaggerated along with a diminished relaxation response to CCh, showing that altered iNOS/eNOS activities result in aortic dysfunction. Moreover, exercise reversed the contraction/relaxation responses in parallel with a possible improvement in endothelial function.
Evidence obtained from animal (46, 47) and human studies (48, 49) indicate that oxidative stress induced by hypertension cause damage in several target organs (50). On the other hand, several antioxidants, including GSH, vitamin C, and superoxide dismutase were reported to improve blood pressure control and vascular function in animals with hypertension and atherosclerosis (51, 52). As with the antioxidants, acute/chronic, low/moderate-intensity exercise is well known to decrease the elevated blood pressure and delay the onset of hypertension (53-59) with a significant improvement in arterial and cardiac remodeling (57, 60). Accordingly, when added to many pharmaceutical antihypertensive therapies, exercise is widely recommended to reduce high blood pressure (58, 61). However, it has been also reported that the best strategies to enhance endogenous antioxidant levels may themselves result in oxidative stress (62), and high intensity exercise may induce oxidative stress due to the generation of ROS exceeding the defense capacity of the tissues (63, 64). On the other hand, high levels of antioxidant enzymes with a greater resistance to exercise-induced oxidative stress have been observed in individuals undergoing exercise (65, 66). In spontaneously hypertensive rats, exercise has improved endothelium function with increased elastin, fibrillin and eNOS content in the aortic wall (67). In keeping with these data, the current findings revealed that oxidative stress due to RVH was ameliorated by physical exercise, while cardiac dysfunction was significantly improved, suggesting that exercise-induced improvements of cardiac function in RVH may be associated with reduced ROS production. Despite the beneficial effects of exercise on RVH-induced oxidative stress, RVH-induced hypertension was not affected. In the 2K1C renin-dependent RVH rats, oxidative stress is not the only involved pathophysiological mechanism. It was shown that depression of the central atrial natriuretic factor in 2K1C hypertension may further promote the elevation of blood pressure (68). Thus, the central adaptations following the induction of RVH that result in the hyperactivity of the arteriolar smooth muscle could not be reversed by exercise.
Recent data have suggested that one of the 3 polymorphisms of endothelial NOS genotype (NOS3), which control the synthesis of NO, could be associated with an enhanced risk for essential hypertension in adults (69). Experimental studies have also shown that endothelial injury aggravates the inflammatory response through the loss of normal production of eNOS, while its induction improves the injurious effects of oxidative stress (70). In association with these data, the present study reveals that the protection against oxidative stress afforded by regular exercise in RVH involves eNOS upregulation and iNOS suppression. Previously, different exercise protocols were shown to reduce increased lipid peroxidation, xanthine oxidase activity, nitrotyrosine and O2– levels, iNOS expression in various models of hypertension (71-75). Although our present qualitative data on the eNOS and iNOS staining do not provide concrete evidence that could be confirmed by Western blotting, the alterations in the overall staining of the tunica media along with the contractility data suggest that both eNOS and iNOS activities are modified by exercise in rats with RVH. Accordingly, decreased iNOS staining and increased eNOS staining in the aorta of exercised rats were accompanied with reductions in neutrophil accumulation and lipid peroxidation, while GSH and catalase were replenished in accomplishing the anti-oxidative effect of exercise. In parallel to our results, a recent study has shown that agents restoring vascular NO via the augmentation of cytoprotective NO release were proved to reduce both inflammation and hypertension in hypertensive rats with diabetes (76).
In hypertensive patients, the plasma levels of the pro-inflammatory cytokines were reported to increase, verifying that hypertension is a chronic low-grade inflammation (26, 27). Inflammatory active state in hypertension induces the activation of numerous pro-inflammatory genes (TNF-α, IL-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1) (77). Pro-inflammatory cytokines have been found to activate ROS, which in turn can activate various intracellular signaling pathways (78). In the current study, exercise depressed RVH-induced elevations in TNF-α and IL-2, suggesting the inhibitory effect of exercise on pro-inflammatory cytokine production. However, RVH-induced elevations in IL-6 were not significantly reduced by exercise, which may be explained by the direct stimulatory effect of muscle contraction on the generation of IL-6 as a myokine (79). Blunted endothelium-dependent relaxation of pre-contracted aortic rings and decreased eNOS expression observed as indicators of endothelial dysfunction in the current study may be associated with the overproduction of ROS, which have a major role in the development and progression of hypertension (78, 79). Thus, the decreased bioavailability of NO, as well as increased ROS production, accompanied with the generation of inflammatory cytokines (82-84) could be contributing to blood pressure elevation and the progression of renal disease by facilitating the remodeling of the vascular wall. Although the effects of exercise as a protective and therapeutic tool in improving antioxidant capacity and reducing oxidative tissue damage were extensively studied (85-91), impact of regular moderate exercise on renovascular hypertension-induced oxidative cardiac injury was not elucidated thoroughly yet. A recent study has shown that exercise reversed the RVH-induced blockade of endogenous NO generation within the paraventricular nucleus (92). The present results indicate an increment in cytokine levels in sedentary RVH (93), while exercise exerted a protective effect on cardiac tissue concomitant with a reduction in the levels of the pro-inflammatory cytokines. In accordance with the pronounced activation of the inflammatory markers, RVH in the sedentary rats elevated the cardiac MDA levels and MPO activity and depleted the antioxidant GSH and catalase contents of the cardiac tissue, while regular swimming exercise performed after the onset of RVH attenuated oxidative damage by up-regulating the cardiac antioxidant defense system. Similarly, treadmill exercise has resulted in the up-regulation of aortic and cardiac antioxidant defense systems of hypertensive rats (72, 73). Meta-analysis of randomized controlled intervention trials have shown that exercise has more pronounced effects on the blood pressure of hypertensive patients as compared to normotensive subjects that have followed the similar exercise programs (55), and it is widely recommended to all hypertensive patients (1, 94, 95).
Based on our results, it can be concluded that moderate exercise ameliorates endothelium dysfunction and cardiac oxidative stress even after emergence of hypertension, and merits consideration as a preventive and a therapeutic tool for renovascular hypertension. Physical activity, as a part of lifestyle modification, should be considered as a common therapeutic intervention for the control of renovascular hypertension and associated cardiovascular outcomes.
Acknowledgement: The study was partially presented at the The Physiological Society Meeting in Oxford and was supported by Marmara University Research Fund (SAG-C-YLP-211009-0305). The authors are grateful to Prof. Feriha Ercan for her critical evaluation and re-organization of the histopathological data.
Conflict of interests: None declared.
REFERENCES
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560-2572.
- Garovic V, Textor SC. Renovascular hypertension: current concepts. Semin Nephrol 2005; 25: 261-271.
- Campese VM, Ku E, Park J. Sympathetic renal innervation and resistant hypertension. Int J Hypertens 2011; 2011: 814354. doi: 10.4061/2011/814354
- Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT. The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol 2011; 38: 144-152.
- Vertes V, Ghose MK. The pathophysiology of renovascular hypertension. Urol Clin North Am 1975; 2: 227-236.
- Boissiere J, Eder V, Machet MC, Courteix D, Bonnet P. Moderate exercise training does not worsen left ventricle remodeling and function in untreated severe hypertensive rats. J Appl Physiol (1985) 2008; 104: 321-327.
- Ersahin M, Sehirli O, Toklu HZ, et al. Melatonin improves cardiovascular function and ameliorates renal, cardiac and cerebral damage in rats with renovascular hypertension. J Pineal Res 2009; 47: 97-106.
- Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 2012; 44 (Suppl. 1): S2-S16.
- Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 2011; 34: 431-440.
- Toklu HZ, Sehirli O, Ersahin M, et al. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J Pharm Pharmacol 2010; 62: 1784-1793.
- Hooper WC, Catravas JD, Heistad DD, Sessa WC, Mensah GA. Vascular endothelium summary statement I: Health promotion and chronic disease prevention. Vascul Pharmacol 2007; 46: 315-317.
- Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 2003; 284: R893-R912.
- Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol 2006; 45: 268-276.
- Rodrigues MC, Campagnole-Santos MJ, Machado RP, et al. Evidence for a role of AT(2) receptors at the CVLM in the cardiovascular changes induced by low-intensity physical activity in renovascular hypertensive rats. Peptides 2007; 28: 1375-1382.
- Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med 2013; 43: 1009-1023.
- Soares ER, Lima WG, Machado RP, et al. Cardiac and renal effects induced by different exercise workloads in renovascular hypertensive rats. Braz J Med Biol Res 2011; 44: 573-582.
- Parker L, McGuckin TA, Leicht AS. Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin Physiol Funct Imaging 2014; 34: 377-383.
- Blair SN, Kohl HW, III, Barlow CE, Gibbons LW. Physical fitness and all-cause mortality in hypertensive men. Ann Med 1991; 23: 307-312.
- Kokkinos P, Manolis A, Pittaras A, et al. Exercise capacity and mortality in hypertensive men with and without additional risk factors. Hypertension 2009; 53: 494-499.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346: 793-801.
- Rossoni LV, Oliveira RA, Caffaro RR, et al. Cardiac benefits of exercise training in aging spontaneously hypertensive rats. J Hypertens 2011; 29: 2349-2358.
- Evenwel R, Struyker-Boudier H. Effect of physical training on the development of hypertension in the spontaneously hypertensive rat. Pflugers Arch 1979; 381: 19-24.
- Hayashi A, Kobayashi A, Takahashi R, Suzuki F, Nakagawa T, Kimotro K. Effects of voluntary running exercise on blood pressure and renin-angiotensin system in spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J Nutr Sci Vitaminol (Tokyo) 2000; 46: 165-170.
- Melo RM, Martinho E, Jr, Michelini LC. Training-induced, pressure-lowering effect in SHR: wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension 2003; 42: 851-857.
- Hagg U, Andersson I, Naylor AS, et al. Voluntary physical exercise-induced vascular effects in spontaneously hypertensive rats. Clin Sci (Lond) 2004; 107: 571-581.
- Dorffel Y, Latsch C, Stuhlmuller B, et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 1999; 34: 113-117.
- Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest 2001; 31: 31-36.
- Navar LG, Zou L, Von Thun A, Tarng Wang C, Imig JD, Mitchell KD. Unraveling the mystery of goldblatt hypertension. News Physiol Sci 1998; 13:170-176.
- Cakir B, Kasimay O, Kolgazi M, Ersoy Y, Ercan F, Yegen BC. Stress-induced multiple organ damage in rats is ameliorated by the antioxidant and anxiolytic effects of regular exercise. Cell Biochem Funct 2010; 28: 469-479.
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358-367.
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78: 206-209.
- Casini AF, Ferrali M, Pompella A, Maellaro E, Comporti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene-intoxicated mice. Am J Pathol 1986; 123: 520-531.
- Aykac G, Uysal M, Yalcin AS, Kocak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology 1985; 36: 71-76.
- Aebi H. Catalase in vitro. Methods Enzymol 1984; 105: 121-126.
- Frohlich ED. State of the Art lecture. Risk mechanisms in hypertensive heart disease. Hypertension 1999; 34: 782-9.
- Frohlich ED. Ischemia and fibrosis: the risk mechanisms of hypertensive heart disease. Braz J Med Biol Res 2000; 33: 693-700.
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 2002; 346: 1954-1962.
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 2001; 37: 529-534.
- Hilgers RH, Xing D, Gong K, Chen YF, Chatham JC, Oparil S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol 2012; 303: H513-H522.
- Kerr S, Brosnan MJ, McIntyre M, et al. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 1999; 33:1353-8.
- Patzak A, Kleinmann F, Lai EY, Kupsch E, Skelweit A, Mrowka R. Nitric oxide counteracts angiotensin II induced contraction in efferent arterioles in mice. Acta Physiol Scand 2004; 181: 439-444.
- Taddei S, Salvetti A. Endothelial dysfunction in essential hypertension: clinical implications. J Hypertens 2002; 20: 1671-1674.
- Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H. Protective role of endothelial nitric oxide synthase. J Pathol 2003; 199: 8-17.
- Huang PL. Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep 2003; 5: 473-480.
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4: 181-189.
- Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension 1991; 17: 707-719.
- Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 2002; 82: S12-S22.
- Minuz P, Patrignani P, Gaino S, et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation 2002; 106: 2800-2805.
- Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant 2006; 21: 850-853.
- Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol Ther 2006; 111: 81-98.
- Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 2008; 31 (Suppl. 2): S181-S184.
- Sekiguchi F, Yanamoto A, Sunano S. Superoxide dismutase reduces the impairment of endothelium-dependent relaxation in the spontaneously hypertensive rat aorta. J Smooth Muscle Res 2004; 40: 65-74.
- Chen HH, Chiang IP, Jen CJ. Exercise training increases acetylcholine-stimulated endothelium-derived nitric oxide release in spontaneously hypertensive rats. J Biomed Sci 1996; 3: 454-460.
- Cheng L, Yang C, Hsu L, Lin MT, Jen CJ, Chen H. Acute exercise enhances receptor-mediated endothelium-dependent vasodilation by receptor upregulation. J Biomed Sci 1999; 6: 22-27.
- Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc 2001; 33(Suppl. 6): S484-S492.
- Graham DA, Rush JW. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol (1985) 2004; 96: 2088-2096.
- Maiorana A, O’Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med 2003; 33: 1013-1035.
- Pescatello LS, Franklin BA, Fagard R, et al. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 2004; 36: 533-553.
- Yen MH, Yang JH, Sheu JR, Lee YM, Ding YA. Chronic exercise enhances endothelium-mediated dilation in spontaneously hypertensive rats. Life Sci 1995; 57: 2205-2213.
- Locatelli J, Monteiro de Assis LV, Morais Araujo C, et al. Swimming training promotes cardiac remodeling and alters the expression of mRNA and protein levels involved in calcium handling in hypertensive rats. Life Sci 2014; 117: 67-74.
- Agarwal D, Elks CM, Reed SD, Mariappan N, Majid DS, Francis J. Chronic exercise preserves renal structure and hemodynamics in spontaneously hypertensive rats. Antioxid Redox Signal 2012; 16: 139-152.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408: 239-247.
- McArdle A, Jackson MJ. Exercise, oxidative stress and ageing. J Anat 2000; 197: 539-541.
- McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 2001; 280: C621-C627.
- Ji LL. Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 1995; 18: 1079-1086.
- Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol (1985) 1995; 79: 675-686.
- Moraes-Teixeira JA, Felix A, Fernandes-Santos C, Moura AS, Mandarim-de-Lacerda CA, de Carvalho JJ. Exercise training enhances elastin, fibrillin and nitric oxide in the aorta wall of spontaneously hypertensive rats. Exp Mol Pathol 2010; 89: 351-357.
- Bahner U, Geiger H, Palkovits M, Ganten D, Klotz B, Heidland A. Changes in the central ANF-system of renovascular hypertensive rats. Kidney Int 1991; 39: 33-38.
- Wrzosek M, Sokal M, Sawicka A, et al. Impact of obesity and nitric oxide synthase gene G894T polymorphism on essential hypertension. J Physiol Pharmacol 2015; 66: 681-689.
- Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int 2002; 61: 855-861.
- Bertagnolli M, Schenkel PC, Campos C, et al. Exercise training reduces sympathetic modulation on cardiovascular system and cardiac oxidative stress in spontaneously hypertensive rats. Am J Hypertens 2008; 21: 1188-1193.
- Fernandes T, Nakamuta JS, Magalhaes FC, et al. Exercise training restores the endothelial progenitor cells number and function in hypertension: implications for angiogenesis. J Hypertens 2012; 30: 2133-2143.
- Husain K, Hazelrigg SR. Oxidative injury due to chronic nitric oxide synthase inhibition in rat: effect of regular exercise on the heart. Biochim Biophys Acta 2002; 1587: 75-82.
- Kimura H, Kon N, Furukawa S, et al. Effect of endurance exercise training on oxidative stress in spontaneously hypertensive rats (SHR) after emergence of hypertension. Clin Exp Hypertens 2010; 32: 407-415.
- Roque FR, Hernanz R, Salaices M, Briones AM. Exercise training and cardiometabolic diseases: focus on the vascular system. Curr Hypertens Rep 2013; 15: 204-214.
- Mason RP, Corbalan JJ, Jacob RF, Dawoud H, Malinski T. Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and rantes levels in hypertensive rats with diabetes. J Physiol Pharmacol 2015; 66: 65-72.
- Skurk T, van Harmelen V, Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-kappaB. Arterioscler Thromb Vasc Biol 2004; 24: 1199-1203.
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840-844.
- Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 2013; 280: 4131-4148.
- Toblli JE, DiGennaro F, Giani JF, Dominici FP. Nebivolol: impact on cardiac and endothelial function and clinical utility. Vasc Health Risk Manag 2012; 8: 151-160.
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol 2009; 157: 527-536.
- Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol 2004; 24: 101-114.
- Noiri E, Peresleni T, Miller F, Goligorsky MS. in vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest 1996; 97: 2377-2383.
- Ruiz-Ortega M, Lorenzo O, Suzuki Y, Ruperez M, Egido J. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens 2001; 10: 321-329.
- Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 2005; 111: 1763-1770.
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005; 98: 1154-1162.
- Pinho RA, Andrades ME, Oliveira MR, et al. Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol Int 2006; 30: 848-853.
- Radak Z, Kaneko T, Tahara S, et al. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med 1999; 27: 69-74.
- Radak Z, Sasvari M, Nyakas C, Pucsok J, Nakamoto H, Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys 2000; 376: 248-251.
- Starnes JW, Barnes BD, Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol (1985) 2007; 102: 1793-1798.
- Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev 2011; 17: 6-63.
- Rossi NF, Chen H, Maliszewska-Scislo M. Paraventricular nucleus control of blood pressure in two-kidney, one-clip rats: effects of exercise training and resting blood pressure. Am J Physiol Regul Integr Comp Physiol 2013; 305: R1390-R1400.
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007; 112: 375-384.
- Fagard RH, Bjornstad HH, Borjesson M, et al. ESC Study Group of Sports Cardiology recommendations for participation in leisure-time physical activities and competitive sports for patients with hypertension. Eur J Cardiovasc Prev Rehabil 2005; 12: 326-331.
- O’Connor FG, Meyering CD, Patel R, Oriscello RP, Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension, athletes, and the sports physician: implications of JNC VII, the Fourth Report, and the 36th Bethesda Conference Guidelines. Curr Sports Med Rep 2007; 6: 80-84.
A c c e p t e d : January 19, 2016