INVOLVEMENT OF THE HISTAMINERGIC SYSTEM IN THE RESUSCITATING EFFECT OF CENTRALLY ACTING LEPTIN IN HAEMORRHAGIC SHOCK IN RATS
INTRODUCTION
Haemorrhagic shock is a life-threatening condition resulting from blood loss, and the cardiovascular response to haemorrhage can be divided into three phases. The first one is characterized by an increase of the sympathetic nervous system activity, with tachycardia and a rise in the peripheral resistance. In the second phase, the activation of unmyelinated vagal afferents from the left ventricle initiates a decrease in the sympathetic activity, with a fall in the peripheral resistance and bradycardia. Finally, continuous blood loss leads to a further decrease in blood pressure accompanied by tachycardia (1).
Experimental studies demonstrate an influence of many neuronal systems, including opioidergic (2), melanocortinergic (3), serotonergic (4), cholinergic (5) and histaminergic system (6), on the sympathoinhibitory phase of regulation in critical hypovolaemia. Histaminergic neurons are concentrated at the tuberomammillary nuclei of the hypothalamus and send axons to many parts of the central nervous system influencing circadian rhythms, thermoregulation, learning and memory, nociceptive responses, feeding behaviour, hypothalamic and pituitary hormone secretion and cardiorespiratory control (7). In normotension, histamine acting as a neurotransmitter induces an increase in mean arterial pressure (MAP) with bradycardia in conscious and tachycardia in anaesthetised animals (8). Interestingly, in critical haemorrhagic hypotension, histamine evokes a long-lasting and a few fold higher pressor effect in comparison to normotensive rats, with an improvement in survival (6). We demonstrated an involvement of the sympathetic nervous system, the renin-angiotensin system, as well as of arginine vasopressin (AVP) and proopiomelanocortin (POMC)-derived peptides in histamine-induced resuscitating action (9). Haemodynamic mechanisms responsible include the mobilisation of blood from venous reservoirs leading to a partial normalisation of peripheral blood flows and blood gas and acid-base parameters (10-11).
Leptin is a peptide hormone secreted by adipocytes which decreases energy consumption by reducing appetite and increasing energy expenditure, possibly via increased thermogenesis (12). The former effect is associated with centrally-mediated activation of the sympathetic nervous system, which is not restricted to the adipose tissue, and concerns also the cardiovascular system (13). Experimental studies demonstrate that after administration into the third cerebral ventricle or lateral ventricle (icv.), the peptide evokes a long-lasting increase in MAP in normotensive conscious (13) and anaesthetised rats (14). Moreover, in a rat model of irreversible haemorrhagic shock, leptin induces a long-lasting pressor effect with the increase in survival, and the mechanism includes the activation of the sympathetic nervous system (15). Since leptin, acting as a neuromodulator, is able to enhance the activity of histaminergic neurons (16), and both histamine and leptin have common mechanism involved in the resuscitating action, the purpose of the study was to examine the involvement of the histaminergic system in leptin-mediated cardiovascular effects in haemorrhagic shock.
MATERIAL AND METHODS
Animals
All procedures were performed in accordance to the EU directives and reviewed by the local Ethics Committees (Katowice, Poland and Bursa, Turkey; notifications No 23/2010, 09/2011, 28/2012, 29/2012, 84/2014 and 2009-11/03).
Studies were performed in male Wistar rats weighing 230 – 284 g (5 – 6 months old). The animals were housed in individual cages in the animal colony, under standard, controlled conditions (temperature 20 – 22°C, humidity 60 – 70%, 12 h light/dark cycle) and provided with food and water ad libitum.
Surgical preparation
After induction of general anaesthesia with ketamine/xylazine (100 mg/kg + 10 mg/kg intraperitoneally, supplemented if required), rats were implanted with catheters filled with heparinised saline (300 IU/ml) in the right carotid artery and the right jugular vein. MAP and heart rate (HR) were measured using TAM-A transducer amplifier module and ECGA amplifier (Hugo Sachs Elektronik, Germany), respectively. The electromagnetic perivascular probes (type 1RB and 2.5SB, Hugo Sachs Elektronik, Germany) were implanted around the right renal and superior mesenteric arteries to monitor renal (RBF) and mesenteric (MBF) blood flow and around the distal abdominal aorta, below the ileocaecal artery, to monitor perfusion of the hindquarters (HBF) using TTFM transit time flowmeter module (Transonic Systems Inc., USA). All measurements of blood flow were started after a 30 min adaptation period to avoid influences of probe implantation. Regional vascular resistances were calculated by dividing MAP (mmHg) by regional blood flows (ml/min).
For i.c.v. treatment, rats were prepared 5 – 7 days before the experiment by stereotaxic implantation, under ketamine/xylazine anaesthesia, of polyethylene cannulae into the right brain lateral ventricle as previously described (6). All icv. injections were made in the volume of 5.0 µl. Correctness of injections was verified as previously described (6).
Experimental protocol
Severe haemorrhagic shock, according to the method by Guarini et al. (3), was produced by intermittent blood withdrawal from the catheter inserted into the right jugular vein over a period of 15 – 25 min, until MAP decreased to and stabilised at 20 – 25 mmHg.
Immediately after induction of critical MAP, the animals were pre-treated icv. with the histamine H1-H4 receptor antagonists chlorpheniramine (50 nmol), ranitidine (25 nmol), VUF 5681 (25 nmol) and JNJ 10191584 (25 nmol), respectively, or saline. Five min later, rats were injected icv. with leptin (20 µg) or saline. Doses of leptin and histamine H1-H2 receptor ligands were taken from the literature (6, 15, 17). VUF 5681 and JNJ 10191584 were administered at an equimolar dose to previously used thioperamide, H3 receptor inverse agonist/H4 antagonist (18). Animals were continuously monitored for 2 hours after treatment, or until death, if it occurred earlier. Body temperature was monitored by a rectal thermometer and maintained at 37 ± 0.5°C using heating lamps throughout experiment. All the experiments were performed between 8.00 and 14.00.
According to recommendations of the Local Ethics Committee in Katowice, to avoid the duplication of experiments performed at our laboratory with the same rat strain, using the same experimental protocol and with the same H1 and H2 receptor antagonists (6, 17), we did not repeat experiments in the control saline icv.-treated groups and cited and discussed previously published results, especially that the mortality at 2 h in these groups was 100%.
Microdialysis study
Handmade microdialysis probes (by Burcin Altinbas) were used. Anaesthetised and catheterised rats were placed in a stereotaxic frame. The skull was exposed and drilled over the posterior hypothalamus (coordinates: 3.6 mm posterior to bregma, 0.5 mm lateral (right) to the midline and 9.0 mm vertical to the skull). Probes (molecular weight cut-off dialysis membrane was 18.000 Da and length was 2.0 mm) were implanted and then fixed with acrylic cement to the skull.
At the end of the probe placement, the arterial catheter was connected to a transducer for MAP monitoring, and the microdialysis probe was attached to perfusion pump. The dialysis probe was perfused with artificial cerebrospinal fluid (pH 7.4) of the following composition: 120 mmol/l NaCl, 1.3 mmol/l CaCl2, 1.2 mol/l MgSO4, 1.2 mmol/l NaH2PO4, 3.5 mmol/l KCl, 25 mmol/l NaHCO3 and 10 mmol/l glucose. The perfusion rate was 2 µl/min. The dialysis probe was perfused for the first 60 min of the stabilisation period and samples were collected at 10 min intervals. After this period, one more sample was collected before haemorrhage and this sample was measured as basal histamine level. Collection of microdialysis samples was continued 70 min after the start of bleeding. At the end of the experiments, the animals were decapitated, and for the determination of microdialysis probe location, brains were fixed in 4% paraformaldehyde, and 50 micrometer-thick vibratom sections were collected. Sections were stained with haematoxylin-eosin for 15 min. Excess stain was washed off in distilled water and the sections were dehydrated by rinses through graduated ethanol series. Following the cleaning step in xylene, the sections were coverslipped using dispersive pipet extraction. Probe localization was determined and representative pictures (Fig. 1) were taken using an Olympus BX-50 microscope adapted with a CCD digital camera.
High-performance liquid chromatography (HLPC) measurement of histamine levels
Extracellular histamine levels at the posterior hypothalamus were measured by HPLC (Jasco PU-980 Intelligent HPLC pump; Kipp & Zonen printer) and UV detection (Jasco UV-975 Intelligent UV-VIS detector) at a wavelength of 220 nm using a C18 column (Hypersil C18; 5 µm; 25 cm; 4.6 mm ID) with an isocratic system (0.04 mol/l KH2PO4, 0.015 mol/l sodium-1-heptasulfonate and 25% acetonitrile, pH 3.1). Flow rate was 2.0 ml/min. Chromatograms were completed within 10 min.
Drugs
The following drugs were used: leptin (rat, recombinant) (Sigma-Aldrich, USA), chlorpheniramine maleate, ranitidine hydrochloride (Research Biochemicals Incorporated, USA), JNJ 10191584, VUF 5681 (Tocris Bioscience, UK), ketamine hydrochloride, xylazine (Biowet Sp. z o. o., Poland), heparin (Polfa, Poland). All drug solutions were prepared freshly on the day of the experiment.
Statistical analysis
All values are given as means ± standard deviation, with P < 0.05 considered as the level of significance. The Fisher’s exact test was used to examine statistical differences in survival percentage. Histamine levels were compared by Kruskal-Wallis test, whereas statistical evaluation of the other results was performed using analysis of variance (ANOVA) and the post-ANOVA test of Student-Newman-Keuls.
RESULTS
The initial pre-bleeding values of MAP, pulse pressure (PP), HR and peripheral blood flows in all groups did not reveal significant differences.
The total bleeding volume necessary for the induction of critical MAP in all animals was 2.43 ± 0.35 ml/100 g body weight. In the control saline icv. injected group, bleeding from MAP 85.3 ± 6.56 mmHg to 23.11 ± 1.12 mmHg was associated with a decrease in PP from 43.76 ± 5.85 mmHg 15.32 ± 3.7 mmHg and HR from 324 ± 18 beats/min to 210 ± 23 beats/min (Fig. 2). Furthermore, haemorrhage led to a decrease in RBF from 4.97 ± 0.78 ml/min to 0.87 ± 0.22 ml/min, HBF from 10.84 ± 1.48 ml/min to 2.35 ± 0.65 ml/min and MBF from 6.75 ± 1.29 ml/min to 1.29 ± 0.12 ml/min (Fig. 3). Initial renal (RVR), hindquarters (HVR) and mesenteric vascular resistance (MVR) in the control saline-injected group were: 17.35 ± 2.0 mmHg/ml/min, 7.95 ± 1.33 mmHg/ml/min and 12.2 ± 1.64 mmHg/ml/min, respectively (Fig. 4). After shock induction, vascular resistances were increased to 27.5 ± 3.6 mmHg min/ml/ml, 11.0 ± 3.0 mmHg/ml/min and 22.8 ± 4.9 mmHg/ml/min, respectively (Fig. 4).
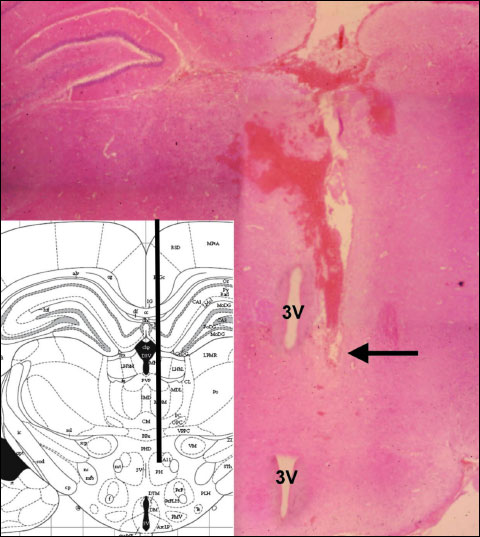 |
Fig. 1. Photomicrograph of a typical placement of microdialysis probe in the posterior hypothalamus; the arrow indicates the tip of the microdialysis probe; 3V - third ventricle; haematoxylin-eosin staining. |
Influence of histamine receptor ligands on haemodynamic effects of leptin in haemorrhage-shocked rats
Leptin administered to haemorrhage-shocked rats induced an increase in MAP, PP and HR (Fig. 2), which were accompanied by a rise in peripheral blood flows (Fig. 3). The effects started within 10 – 15 min after leptin injection, were long-lasting and associated with 100% survival rate at 2 h (P < 0.05 versus control saline-treated animals, Fisher’s exact test). Since the bleeding termination to the end of the observation period peripheral vascular resistances were increased (Fig. 4). In the saline-pre-treated group, 20 min after leptin injection RVR, HVR and MVR were still increased by 56.4%, 35.9% and 115.3%, respectively, in comparison with the pre-bleeding values (Fig. 4).
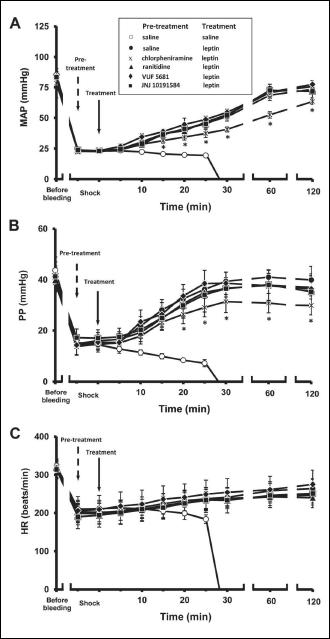 |
Fig. 2. Influence of icv. pre-treatment with chlorpheniramine (50 nmol), ranitidine (25 nmol), VUF 5681 (25 nmol), JNJ 10191584 (25 nmol) and saline (5 µl) on MAP (A), PP (B) and HR (C) after leptin (20 µg, icv.) administration in rats subjected to critical haemorrhagic hypotension. Means ± S.D., 6 animals per group. In all leptin-treated groups, since 10 min (MAP, PP) and 25 min (HR) till the death of control animals P < 0.05 versus the saline-treated control group; *P < 0.05 versus the saline-pre-treated leptin-injected group; previous (6, 17) and present studies with histamine receptor ligands (data not shown) did not evoke MAP, PP and HR changes after their administration in the control groups. |
In the saline-treated control group, there we no increases in MAP, PP, HR (Fig. 2) and peripheral blood flows (Fig. 3), and all animals died within 30 min.
Chlorpheniramine inhibited MAP, PP and regional haemodynamic effects of leptin (Figs. 2-4), however, without influence on HR and survival rate at 2 h. The antagonist given alone, as we showed previously (6), had no influence on haemodynamic parameters in the icv. saline-treated control group.
On the other hand, H2, H3 and H4 receptor blockers ranitidine, VUF 5681 and JNJ 10191584 did not influence measured cardiovascular parameters (Figs. 2-3) and calculated peripheral vascular resistances (Fig. 4) in leptin-treated group. The antagonists given alone in the control groups, as we reported previously with ranitidine (17) and studied here with VUF 5681 and JNJ 10191584 (data not shown), had no influence on measured haemodynamic parameters and calculated peripheral vascular resistances.
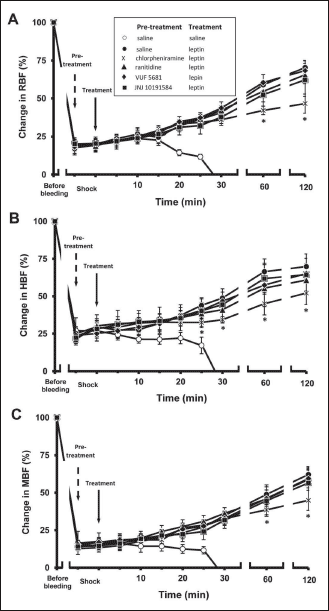 |
Fig. 3. Influence of icv. pre-treatment with chlorpheniramine (50 nmol), ranitidine (25 nmol), VUF 5681 (25 nmol), JNJ 10191584 (25 nmol) and saline (5 µl) on RBF (A), HBF (B) and MBF changes (C) after leptin (20 µg, icv.) administration in rats subjected to critical haemorrhagic hypotension. Means ± S.D., 6 animals per group. Pre-bleeding values of RBF, HBF and MBF in the control group were: 4.97 ± 0.78 ml/min, 10.84 ± 1.48 ml/min and 6.75 ± 1.29 ml/min, respectively. In all leptin-treated groups, since 10 min (HBF), 15 min (MBF) and 20 min (RBF) till the death of control animals P < 0.05 versus the saline-treated control group; *P < 0.05 versus the saline-pre-treated leptin-injected group; previous (6, 17) and present studies with histamine receptor ligands (data not shown) did not reveal peripheral blood flow changes after their administration in the control groups. |
Influence of leptin on histamine release in the posterior hypothalamus in critically hypotensive rats
In vivo microdialysis studies showed that the basal extracellular histamine concentrations at the posterior hypothalamus in control and leptin-treated groups were 0.14 ± 0.03 pmol/10 min and 0.133 ± 0.08 pmol/10 min, respectively (Fig. 5). Induction of critical haemorrhagic hypotension did not significantly influence histamine concentrations (Fig. 5).
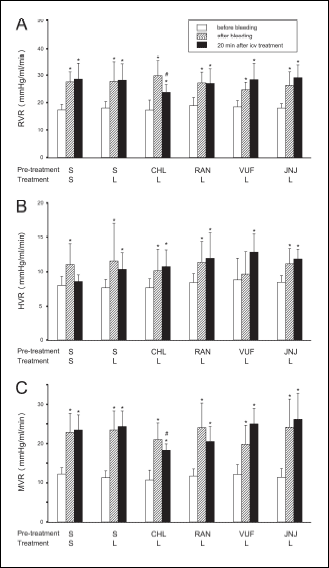 |
Fig. 4. RVR (A), HVR (B) and MVR (C) before and after bleeding termination, and 20 min after icv. injection with leptin (L, 20 µg) in groups pre-treated with chlorpheniramine (CHL, 50 nmol), ranitidine (RAN, 25 nmol), VUF 5681 (VUF, 25 nmol), JNJ 10191584 (JNJ, 25 nmol), and in the control saline-injected (S) group. Means ± S.D., 6 animals per group. *P < 0.05 versus pre-bleeding value; #P < 0.05 versus corresponding value in saline-pre-treated leptin injected group. |
Intracerebroventricular administration of leptin (20 µg) evoked 181.5% increase in extracellular histamine levels at the posterior hypothalamus during the first 10 min after injection (Fig. 5). On the contrary, in the control group there were no significant changes in the posterior hypothalamic histamine levels within 10 min after saline treatment (Fig. 5).
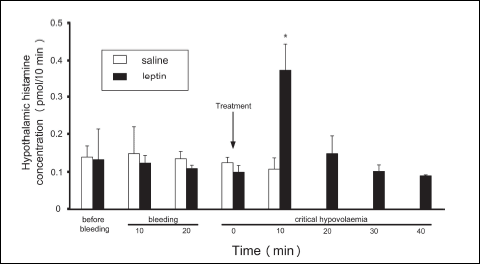 |
Fig. 5. Hypothalamic extracellular histamine concentrations before bleeding, 10 and 20 min after the start of bleeding and 10 – 40 min after icv. leptin (20 µg) or saline injections. Means ± S.D., 4 animals per group. *P < 0.05 versus saline-treated group. There are no results of histamine levels in the control group at 20, 30 and 40 min of critical hypovolaemia, because all animals died before the collections. |
DISCUSSION
Centrally acting leptin influences cardiovascular regulation both in normotension (13, 19) and critical haemorrhagic hypotension (15). Present results extend previous studies and show for the first time the influence of leptin on the activity of histaminergic neurones in critical hypovolaemia. However, the main novel finding in this study is the direct demonstration of the involvement of the histaminergic system in leptin-mediated resuscitating effect in haemorrhage-shocked rats.
Due to a low permeability of the blood-brain barrier, similarly to previous studies (15), we administered leptin centrally, although the influence of circulating leptin on the central cardiovascular regulation in haemorrhagic shock cannot be excluded, since i) in focal and global cerebral hypoxia/traumatic brain injury the blood-brain permeability increases (20), ii) the effect is potentiated during oxidative stress (21) which characterises the reperfusion phase of haemorrhagic shock resuscitation and iii) peripherally administered leptin induces neuroprotective effects on focal cerebral ischemia/reperfusion injury (22).
As we confirmed here, leptin given at a dose 20 µg icv. to rats subjected to critical haemorrhagic hypotension induces a long-lasting and significantly higher increase in MAP, relative to normotensive animals (15) with a reversal of bradycardia and improvement of the survival rate at 2 h, which is considered as a long-lasting survival. The action of leptin is accompanied by the rise in RBF, MBF and HBF, as demonstrated since 10 min after treatment (15). We hypothesized that the haemodynamic mechanism responsible for shock reversal is related to the increase in the circulating blood volume, as a consequence of blood mobilisation from venous reservoirs, that in turn leads to the increase in peripheral blood flows. This hypothesis is supported by the finding of a long-lasting vasoconstriction, especially in the mesenteric region (15). The differences among RVR, MVR and HVR can be explained by the specificity of the sympathetic innervations of these vascular beds, as well as the different vascular density of receptors for endogenous vasoconstrictors. Similar differences in regional peripheral blood flows and vascular resistances were described earlier in studies regarding cardiovascular effects of the histaminergic system activation (9) and the treatment with AVP (23) in haemorrhagic shock.
Our data may suggest a potential direction of a new adjunctive pharmacological intervention in haemorrhagic shock, however, it is difficult to assess their clinical significance, especially that i) leptin was given centrally, ii) we used a model of isolated haemorrhagic shock, whereas in humans it is often accompanied by polytrauma and iii) anaesthesia, volume replacement and other standard therapeutics used to treat haemorrhagic shock might interfere with leptin action in hypovolaemia. Experimental studies demonstrate the influence of anaesthesia on cardiovascular regulation in hypovolaemia i.e. the shortening of the sympathoexcitatory phase (24). In the present studies, all injections of leptin were performed in the second, sympathoinhibitory phase, however, we cannot exclude a possible influence of anaesthesia on leptin-mediated cardiovascular effects, although both in conscious (13) and anaesthetized normotensive rats (14) centrally acting leptin induces a pressor effect.
According to the model used, the animals were monitored for 2 hours and in that time leptin evoked an improvement in measured cardiovascular parameters and survival rate. However, since the main mechanism of action is the activation of the sympathetic nervous system (15) associated with an increase in energy expenditure, further studies are needed to fully assess a long-lasting metabolic effects of leptin action.
Central neuronal pathways involved in leptin-mediated resuscitating action in haemorrhagic shock are not clear. According to the published literature, we suggest indirect mechanisms since leptin is able to stimulate neurons located in the arcuate nucleus and projecting to the paraventricular nucleus (PVN) of the hypothalamus, where they release a-melanocyte stimulating hormone (α-MSH) (25). On the other hand, in vivo electrophysiological studies demonstrate that leptin activates the sympathetic nervous system by stimulating α-MSH release in PVN (19). Since α-MSH and other POMC-derived peptides have protective effects due to the activation of the cholinergic anti-inflammatory pathway in haemorrhagic shock, multiple organ dysfunction syndrome and other critical conditions (26-28), we hypothesize that the leptin-induced resuscitating effect may be mediated via POMC-derived peptides; further, the central melanocortinergic system favourably influences the sympathetic tone and, this way, MAP values, as results of in vivo (29) and in vitro (30) studies demonstrate. In addition, findings by Fekete and Liposits (31) show that the α-MSH-containing neurons of the arcuate nucleus directly innervate histaminergic neurons in the tuberomammillary nucleus and therefore, may be involved in the mediation of leptin action on this neuronal system. Indeed, in vivo microdialysis studies demonstrate an increase in histamine release at the anterior hypothalamus after intraperitoneal administration of leptin in normotensive anaesthetised rats (32) and mice (16). In the present study, we demonstrate for the first time leptin-induced increase in hypothalamic histamine release in haemorrhage-shocked animals.
To further determine the role of the histaminergic system in the central leptin-induced resuscitating action, we used histamine receptor ligands. Haemodynamic results show that H1 receptors are involved in leptin-mediated resuscitating effect in haemorrhagic shock. These data are in line with the previous studies in which we demonstrated the role of H1 receptor in the resuscitating effect of centrally acting endogenous and exogenous histamine (9). H1 receptors are involved also in the mediation of the pressor effect of centrally acting cytidine 5’-diphosphocholine (17) and the agonist of 5-HT1A receptor 8-OH-DPAT (33). Moreover, pre-treatment with chlorpheniramine decreases leptin-induced increase in MAP and iliac vasoconstriction (15), and the H1 receptor antagonist diphenhydramine blocks leptin-evoked increases in MAP and the renal sympathetic nerve activity in normotensive rats (33).
Similarly to our previous studies concerning the role of the histaminergic system in the central cardiovascular regulation in shock (18), here we did not find any influence of central H2, H3 and H4 receptor blockade on leptin-mediated effects. Presynaptic H3 receptors mediate autoinhibition of histamine release from the histaminergic neurons as well as regulation of the synthesis/release of other neurotransmitters (7). Tanida et al. (34) demonstrated that H3/4 receptor antagonist/inverse agonist thioperamide inhibits changes in the renal sympathetic nerve activity and MAP evoked by low doses of histamine in normotensive rats. In contrast, VUF 5681, a silent/neutral H3 receptor antagonist did not affect measured cardiovascular parameters in shock. We hypothesize that the difference can be explained by the pharmacological properties of the used H3 ligands.
Despite a single report on the functional expression of histamine H4 receptors in the mammalian brain (35), we showed no influence of the H4 receptor antagonist JNJ 10191584 on the central cardiovascular regulation in leptin-injected and saline-treated animals. These results are in agreement with our previous studies in which thioperamide did not affect the action of cholinergic (17) and serotonergic ligands (18) in haemorrhagic shock.
Although we demonstrated the involvement of the histaminergic system in leptin-mediated cardiovascular effects in shock, there are limitations of our study. In addition to the melanocortinergic and histaminergic systems, centrally acting leptin is able to influence the activity of other neuronal pathways, and their role in the observed resuscitating effect cannot be excluded. The study by Garcia et al. (36) showed a leptin-mediated increase in the central thyrotropin-releasing hormone (TRH) synthesis and release, and TRH evokes an increase in the sympathetic tone in normotensive animals (37) and induces a resuscitating effect in experimental haemorrhagic shock (38). Moreover, leptin is involved in AVP synthesis and secretion (32), and AVP belongs to essential compensatory pathways in haemorrhagic shock (39). Finally, we did not study particular neuronal pathways activated by leptin. We injected it icv., similarly as it was performed in the studies concerning the function of other poorly penetrating the blood-brain barrier neurotransmitters/neuromodulators (40-42).
In conclusion, the results of our studies directly demonstrate for the first time the involvement of the histaminergic system in centrally-acting leptin-induced resuscitating effect in haemorrhagic shock in rats.
Acknowledgements: The study was supported by a grant of the Medical University of Silesia, Katowice, Poland (KNW-1-065/P/2/0) and by COST Action BM0806 “Recent advances in histamine receptor H4R research”. We thank Dr M. Ozgur Ozyigit for the technical assistance at histological procedures.
Conflict of interests: None declared.
REFERENCES
- Secher NH, Jacobsen J, Friedman DB, Matzen S. Bradycardia during reversible hypovolaemic shock: associated neural reflex mechanisms and clinical implications. Clin Exp Pharmacol Physiol 1992; 19: 733-743.
- Henderson LA, Keay KA, Bandler R. Delta- and kappa-opioid receptors in the caudal midline medulla mediate haemorrhage-evoked hypotension. Neuroreport 2002; 13: 729-733.
- Guarini S, Bini A, Bazzani C, et al. Adrenocorticotropin normalizes the blood levels of nitric oxide in hemorrhage-shocked rats. Eur J Pharmacol 1997; 336: 15-21.
- Sowa P, Adamczyk-Sowa M, Zwirska-Korczala K, et al. Proopiomelanocortin but not vasopressin or renin-angiotensin system induces resuscitative effects of central 5-HT1A activation in haemorrhagic shock in rats. J Physiol Pharmacol 2014; 65: 659-671.
- Yalcin M, Aydin C, Savci V. Cardiovascular effect of peripheral injected melittin in normotensive conscious rats: mediation of the central cholinergic system. Prostaglandins Leukot Essent Fatty Acids 2009; 81: 341-347.
- Jochem J. Cardiovascular effects of histamine administered intracerebroventricularly in critical haemorrhagic hypotension in rats. J Physiol Pharmacol 2000; 51: 229-239.
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol 2001; 63: 637-672.
- Bealer SL. Central neuronal histamine contributes to cardiovascular regulation. News Physiol Sci 1999; 14: 100-105.
- Jochem J, Kasperska-Zajac A. The role of the histaminergic system in the central cardiovascular regulation in haemorrhagic hypotension. Folia Med Cracov 2012; 52: 31-41.
- Jochem J. Haematological, blood gas and acid-base effects of central histamine-induced reversal of critical haemorrhagic hypotension in rats. J Physiol Pharmacol 2001; 52: 447-458.
- Jochem J. Central histamine-induced reversal of critical haemorrhagic hypotension in rats – haemodynamic studies. J Physiol Pharmacol 2002; 53: 75-84.
- Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol 2010; 31: 377-393.
- Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension 2001; 37: 936-942.
- Rao SP, Dunbar JC. A role for the central histaminergic system in the leptin-mediated increase in cardiovascular dynamics. Brain Res Bull 2005; 64: 425-432.
- Jochem J, Kalarus Z, Spaccapelo L, et al. Centrally acting leptin induces a resuscitating effect in haemorrhagic shock in rats. Regul Pept 2012; 176: 45-50.
- Ishizuka T, Hatano K, Murotani T, Yamatodani A. Comparison of the effect of an H(3)-inverse agonist on energy intake and hypothalamic histamine release in normal mice and leptin resistant mice with high fat diet-induced obesity. Behav Brain Res 2008; 188: 250-254.
- Jochem J, Savci V, Filiz N, Rybus-Kalinowska B, Fogel WA, Yalcin M. Involvement of the histaminergic system in cytidine 5’-diphosphocholine-induced reversal of critical haemorrhagic hypotension in rats. J Physiol Pharmacol 2010; 61: 37-43.
- Jochem J, Rybczyk R, Irman-Florjanc T, Zwirska-Korczala K, Niwecka A. Central serotonin-induced pressor effect in rats is mediated in part via the histaminergic system. Inflamm Res 2008; 57 (Suppl. 1): S35-S36.
- Dunbar JC, Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) products. Brain Res Bull 1999; 50: 215-221.
- Wu J, Zhao D, Wu S, Wang D. Ang-(1-7) exerts protective role in blood-brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol 2015; 748: 30-36.
- Lochhead JJ, McCaffrey G, Quigley CE, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 2011; 30: 1625-1636.
- Zhang JY, Yan GT, Liao J, et al. Leptin attenuates cerebral ischemia/reperfusion injury partially by CGRP expression. Eur J Pharmacol 2011; 671: 61-69.
- Voelckel WG, Raedler C, Wenzel V, et al. Arginine vasopressin, but not epinephrine, improves survival in uncontrolled hemorrhagic shock after liver trauma in pigs. Crit Care Med 2003; 31: 1160-1165.
- Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol 1991; 260: H305-H318.
- Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 2007; 49: 647-652.
- Giuliani D, Mioni C, Bazzani C, et al. Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. Br J Pharmacol 2007; 150: 595-603.
- Bitto A, Polito F, Altavilla D, et al. Melanocortins protect against multiple organ dysfunction syndrome in mice. Br J Pharmacol 2011; 162: 917-928.
- Ottani A, Galantucci M, Ardimento E, et al. Modulation of the JAK/ERK/STAT signaling in melanocortin-induced inhibition of local and systemic responses to myocardial ischemia/reperfusion. Pharmacol Res 2013; 72: 1-8.
- Da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 2008; 51: 884-890.
- Ye ZY, Li DP. Activation of the melanocortin-4 receptor causes enhanced excitation in presympathetic paraventricular neurons in obese Zucker rats. Regul Pept 2011; 166: 112-120.
- Fekete C, Liposits Z. Histamine-immunoreactive neurons of the tuberomammillary nucleus are innervated by alpha-melanocyte stimulating hormone-containing axons. Generation of a new histamine antiserum for ultrastructural studies. Brain Res 2003; 969: 70-77.
- Morimoto T, Yamamoto Y, Yamatodani A. Leptin facilitates histamine release from the hypothalamus in rats. Brain Res 2000; 868: 367-369.
- Jochem J, Zak A, Rybczyk R, Irman-Florjanc T. Interactions between the serotonergic and histaminergic systems in the central cardiovascular regulation in haemorrhage-shocked rats: involvement of 5-HT1A receptors. Inflamm Res 2009; 58 (Suppl. 1): 38-40.
- Tanida M, Kaneko H, Shen J, Nagai K. Involvement of the histaminergic system in renal sympathetic and cardiovascular responses to leptin and ghrelin. Neurosci Lett 2007; 413: 88-92.
- Connelly WM, Shenton FC, Lethbridge N, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol 2009; 157: 55-63.
- Garcia SI, Landa MS, Porto PI, et al. Thyrotropin-releasing hormone decreases leptin and mediates the leptin-induced pressor effect. Hypertension 2002; 39: 491-495.
- Siren AL, Paakkari I. Cardiovascular effects of TRH icv. in conscious rats. Clin Exp Hypertens 1984; 6: 2073-2077.
- Vergoni AV, Marrama D, Guarini S, et al. Afferent vagal fibres and central cholinergic mechanisms are involved in the TRH-induced reversal of haemorrhagic shock. Pharmacol Res 1991; 23: 271-278.
- Cossu AP, Mura P, De Giudici LM, et al. Vasopressin in hemorrhagic shock: a systematic review and meta-analysis of randomized animal trials. Biomed Res Int 2014; 2014: 421291. doi: 10.1155/2014/421291.
- Juszczak M, Roszczyk M, Kowalczyk E, Stempniak B. The influence od melatonin receptors antagonists, luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT), on melatonin-dependent vasopressin and adrenocorticotropic hormone (ACTH) release from the rat hypothalamo-hypophysial system. in vitro and in vivo studies. J Physiol Pharmacol 2014; 65: 777-784.
- Ozturk CC, Oktay S, Yuksel M, Akakin D, Yarat A, Kasimay Cakir O. Anti-inflammatory effects of nesfatin-1 in rats with acetic acid-induced colitis and underlying mechanisms. J Physiol Pharmacol 2015; 66: 741-750.
- Merroun I, El Mlili N, Martinez R, et al. Interaction between orexin A and cannabinoid system in the lateral hypothalamus of rats and effects of subchronic intraperitoneal administration of cannabinoid receptor inverse agonist on food intake and the nutritive utilization of protein. J Physiol Pharmacol 2015; 66: 181-190.
A c c e p t e d : January 23, 2016