DYNAMICS OF THE MELATONIN MT1 RECEPTOR IN THE RAT PAROTID GLAND UPON MELATONIN ADMINISTRATION
INTRODUCTION
Melatonin is regarded as a natural component of saliva. In the oral cavity, a number of potential activities related to its anti-inflammatory and anti-oxidant properties, either receptor dependent or receptor independent, are discussed (1). Immunoblotting shows that the rat parotid gland expresses receptors of melatonin of types MT1 and MT2 (2). On the administration of melatonin, the gland responds with the acinar secretion of amylase and the synthesis of secretory proteins, both phenomena preferentially associated with the MT2 type and the intracellular activity of NO synthase of the neuronal type (2, 3). Moreover, using the rat parotid gland as an experimental model, the lipopolysaccharide-induced inflammatory response of the gland is markedly reduced by the administration of melatonin (4). In vitro, fragments of the human parotid gland exposed to melatonin display ultrastructural changes associated with secretory activity and analytical pharmacology shows that the MT2 receptors are preferentially involved (5). In human salivary glands, light microscopy demonstrates the diffuse cellular distribution of MT1 and MT2 receptors (6-8). By transmission electron microscopy (TEM), the subcellular localisation of MT1 and MT2 receptors in human parotid glands in both the cell membrane and the cytosolic organelles emerges, which led us to suggest the presence of an active uptake/transport system for melatonin, linked to its receptors (8).
It could be expected that exposure to increased blood levels of melatonin would influence the amount and (or) the distribution pattern of the melatonin receptors in the gland cells. After having initially confirmed the subcellular distribution of the two types of melatonin receptor in the rat parotid gland, similar to that previously described for the human parotid gland (8), we put our hypothesis to the test by exposing the anaesthetised rat to a continuous intravenous infusion of melatonin over a period of two hours. Gland specimens were processed for TEM, using the gold post-embedding technique, and for immunoblotting. The secretory granules were the particular focal point of the microscopic investigation. Our previous study of the human parotid gland showed that reactivity for the two types of melatonin receptor was preferentially localised in the granules. Moreover, the granules are the most representative organelle in the serous cells and, further, they have a distinct membrane border. Our present study showed that the two types of melatonin receptor differ in their response to melatonin administration, implying that they perform different functional roles.
MATERIAL AND METHODS
The in-vivo experiments were approved by the Ethics Committee for Animal Experiments (CESA), University of Cagliari, Italy. A total of seven adult, female Sprague-Dawley rats, weighing between 260 g and 310 g, were used. The rats were housed at a constant temperature with light-dark cycles of 12 hours, lights on at 6 am and off at 6 pm. The rats were maintained on a pelleted standard diet and tap water ad libitum. The experiments were performed between 9 am and 1 pm.
In-vivo experiments
The animals were anaesthetised with 0.5 ml/100 g of equitesin intraperitoneally (9). The body temperature of the anaesthetised animal, measured with a rectal probe, was maintained at 38°C by means of a thermostatically controlled blanket. The animals were fitted with a femoral venous polyethylene catheter, which served as a conduit for the infusion of melatonin, and a tracheal cannula. Prior to the start of the infusion, the left parotid gland was removed to serve as (untreated) control tissue; bleeding, if any, was stopped by Spongostan™. The wound was then sutured. Melatonin (3 mg/kg per hour) was intravenously infused continuously over a period of two hours. Thirty minutes after the end of the infusion period, the corresponding right gland was removed. From each parotid gland, specimens were taken for electron microscopy and immunoblotting. Whereas the specimens for microscopy were prepared immediately following the removal of the gland (see below), those for immunoblotting were frozen and kept at –70°C until homogenised (see below). The animals were killed by an overdose of equitesin.
Transmission electron microscopy
Samples were cut into small pieces and fixed for two hours with a mixture of 3% paraformaldehyde and 1% glutaraldehyde in 0.1 M cacodylate buffer (10). They were then rinsed in cacodylate buffer supplemented with 3.5% sucrose, dehydrated and embedded in Epon resin (glycide ether 100). In order to preserve the antigenicity of the tissue, osmium tetroxide was omitted in the preparation of immunocytochemical samples. Ultrathin sections (80 nm) were collected on nickel grids and processed for the immunohistochemical analysis. For MT1 immunolocalisation, the grids were treated with 1% bovine serum albumin (BSA) and 5% normal goat serum (NGS) in phosphate-buffered saline (PBS) solution to block non-specific binding. They were then incubated over night at 4°C with a rabbit polyclonal antibody anti-melatonin receptor 1, diluted 1:50 in 1% BSA and 5% NGS. The grids were then incubated for one hour at room temperature with the secondary antiserum, a goat anti-rabbit IgG conjugated to 15 nm gold particles, diluted 1:50 in 1% BSA-PBS. For MT2, the grids were treated with 1% bovine serum albumin (BSA) and 5% normal rabbit serum (NRS) in phosphate-buffered saline (PBS) solution to block non-specific binding. They were then incubated over night at 4°C with anti-MT2 goat polyclonal antibody diluted 1:50 in 1% BSA and 5% NRS. The grids were then incubated for one hour at room temperature with the secondary antiserum, a rabbit anti-goat IgG conjugated to 10 nm gold particles, diluted 1:50 in 1% BSA-PBS. After washing, they were stained with uranyl acetate and bismuth subnitrate, after which they were finally observed and photographed in a JEOL 100S transmission electron microscope. Micrographs were acquired by TEM in different parts of the grid that were randomly chosen. Fragments were also incubated with a non-immune serum or omitting the primary antibody.
Immunoblotting
Parotid glands were homogenised, following a standard procedure for membrane protein isolation (11). Briefly, tissues were homogenised in the homogenising buffer (10 mM Tris pH 7.4, 0.32 M sucrose, 5 mM EDTA), with the addition of 1:100 protein inhibitor cocktail, centrifuged at 1000 g for 20 min (in order to eliminate nuclei and large-scale cellular debris), after which the supernatant was centrifuged at 12,000g for 20 min. The resulting pellet was washed three more times in the same buffer. It mainly represents plasma membranes, membranes of granules and endocytic vesicles and similar sized organelles that precipitate in the range of 2500–12,000 g (12, 13). Protein determination was obtained using the Lowry method. Samples (50 µg) were loaded into 4 – 15% Mini-PROTEAN® TGX™ precast polyacrylamide gels and allowed to run at 80V for 1.5 h. Gels were blotted onto PVDF membranes and blotted membranes were blocked with 5% milk in Tris buffered saline with 0.1% Tween 20 (TBS-T) for two hours. The incubation of primary antibodies against MT1 or MT2 receptors (dilution 1:300 in TBS-T with 5% milk) followed over night at 4°C. Secondary antibodies (goat anti-rabbit peroxidase conjugate 1:5000, for MT1, or rabbit anti-goat peroxidase conjugate 1:3000, for MT2, both from Sigma-Aldrich, St. Louis, MO, USA) were incubated for two hours at room temperature. Immunoreactive bands were detected using the ECL Prime chemiluminescence kit and images were acquired using a Fujifilm Luminescent Image Analyzer LAS4000 System (Fujifilm, Tokyo, Japan). The densitometry of immunoreactive bands was analysed by Image Studio Lite Software (LI-COR, Nebraska, USA). Receptor quantification was expressed as the ratio between the relative intensity of receptor signals and the expression of the housekeeping gene for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In fact, our protein extracts enriched with membrane proteins also contained GAPDH, as revealed by incubation with 1:1,000 rabbit anti-GAPDH antibody (Sigma-Aldrich, St. Louis, MO, USA), followed by 1:5,000 goat anti-rabbit peroxidase conjugate.
Chemicals
The Epon resin was purchased from Merck (Darmstadt, Germany), the rabbit polyclonal antibody anti-melatonin receptor 1 from Biorbyt (Cambridge, Cambridgeshire, UK) and the goat anti-rabbit IgG from GE Healthcare (Milan, Italy). The goat antibody anti-melatonin receptor 2 and the rabbit anti-goat IgG were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA), the Mini-PROTEAN® TGX™ precast polyacrylamide gels from Biorad (CA, USA) and the PVDF membranes and ECL Prime chemiluminescence kit from GE Healthcare (NJ, USA). The other chemical substances and drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Statistical analysis
The quantitative analysis of labelling density was performed with Image Tool for Windows (University of Texas Health Science Center in San Antonio) and with Image Pro Plus (Media Cybernetics, Silver Spring, MD, USA). Image analysis was performed on a total of 36 micrographs, randomly acquired by TEM, 18 images for untreated specimens and 18 for melatonin-treated specimens. MT1-receptor immunoreactivity was searched for in 382 granules of untreated specimens and in 499 granules of melatonin-treated specimens, while the corresponding figures were 491 and 342 with respect to MT2-receptor immunoreactivity. The data are expressed as the mean labelling density (i.e. number of gold particles of granules per µm2). Moreover, the number of immunolabelled MT1 receptors associated with the membrane part of the granules and the content of the granules respectively were determined. The gold particles directly localised on the membrane and those within a range of about ± 25 nm (related to the membrane) were considered to be associated with the membrane. The gold particles localised further away, and inside the granule, were considered to be associated with granule content.
The immunoblotting data for MT1 and MT2 receptors of both untreated and melatonin-treated glands were normalised to the mean value of the untreated glands for the respective receptor type, set at 100 percent.
The Shapiro-Wilk test was used to determine the distribution pattern of the samples. For non-normal distribution, the statistical significance of differences was calculated using the Mann-Whitney U-test for two groups and the Kruskal-Wallis one-way of analysis and Dunn's post-hoc test were used for multiple comparisons. For normal distribution, the unpaired Student's two-tailed t-test was used. Statistical significances were calculated on raw data. Probabilities of < 5% were considered significant.
RESULTS
MT1- and MT2-receptor labelling in untreated glands
The parotid glands expressed immunoreactivity for both MT1 (Fig. 1a) and MT2 receptors (Fig. 1b). The intracellular localisation of MT1 reflected that of MT2, although the MT1 staining was weaker. The preferential site for the reactivity of the two types of receptor was the acinar secretory granules, where they appeared in both the membranous and the cytosolic parts of the granule. Small positive vesicles were observed throughout the cytoplasm and, in particular, surrounding the granules (Fig. 2a). Moreover, the basolateral cell membranes were also marked for both MT1 (Fig. 2b) and MT2 receptors.
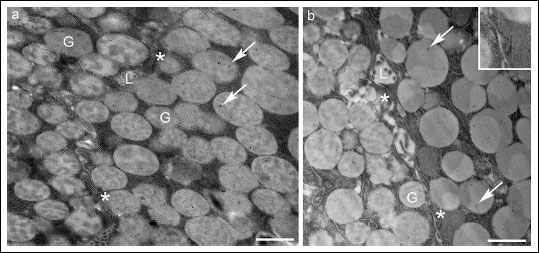 |
Fig. 1. Secretory granules of acinar serous cells in untreated parotid gland. (a) The gold particles marking the MT1 receptor were retrieved in a restricted number of secretory granules (G). (b) The gold particles indicating the MT2 receptor, on the other hand, occurred more frequently. The asterisks and inset show the MT2-receptor reactivity on the lateral membrane. L, lumen; Bars, 1 µm. |
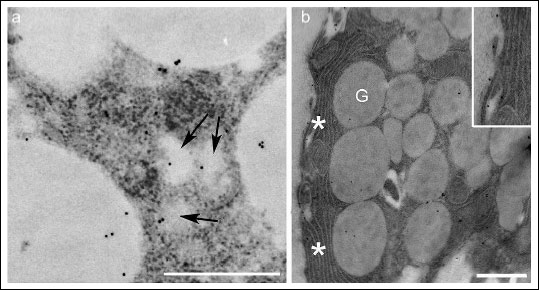 |
Fig. 2. Portions of acinar serous cells in untreated parotid gland staining for the MT1 receptor. The MT1 receptor was observed in small vesicles (a, arrows) among the secretory granules and in the basal membrane (b, asterisks). The vesicles limiting membranes are not so contrasted due to the omission of osmium tetroxide in order to preserve the antigenicity of the tissue. Inset: a detail of basal membrane labelled for MT1 receptors. G, granules; Bars, 1 µm. |
In the duct cells of the intercalated segments, immunoreactivity was occasionally detected for both MT1 and MT2 receptors (Fig. 3a). Few positive MTl vesicles were found, principally near the apical area. In the striated duct cells, few marked vesicles were observed next to the apical compartment, but most of the MT1-positive vesicles were located close to the basal compartment, or in the basolateral folds, which are the most representative feature of this ductal portion. In all these parts of the ductal system, the granules were always unstained. The MT2 receptor showed the same distribution pattern in the striated ducts as the MT1 receptor, although the presence of gold particles for MT2 was more frequent (Fig. 3b). Occasionally, the nuclei and mitochondria of acinar and duct cells were decorated with gold particles showing reactivity for the two receptor types. None of the samples, where the primary antibody was omitted, exhibited positivity (Fig. 4a, 4b); a lack of positivity was also seen when non-immune serum was used.
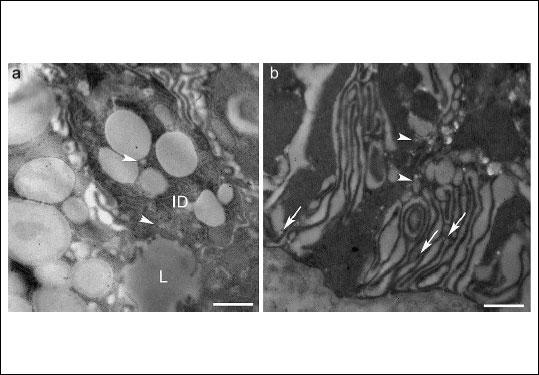 |
Fig. 3.Untreated parotid gland. (a) Intercalated duct cells (ID). A few positive vesicles showing MT1 receptors (arrowheads) were found, principally near the apical area, while the granules of this ductal portion were unstained for MT1. Similarly, a few gold particles indicating MT2 receptors were sporadically observed in these cells (photo not shown). (b) Striated duct cells. The gold particles indicating the MT2 receptor decorated the basolateral membrane (arrows) and often appeared in association with small vesicles (arrowheads). A similar distribution was observed for MT1 receptors (photo not shown). L, lumen; Bars, 1 µm. |
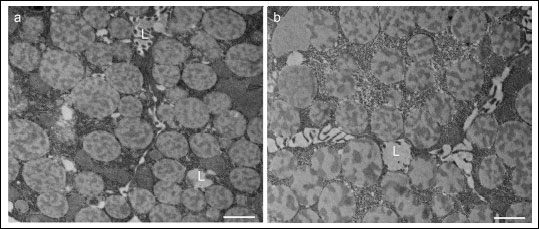 |
Fig. 4. Specimens incubated without primary antiserum. The acinar serous cells completely lacked staining for MT1 (a) and MT2 (b) receptors. L, lumen; Bars, 1 µm. |
MT1- and MT2-receptor labelling in melatonin-treated glands
The pattern of MT1- and MT2-receptor staining appeared unchanged in melatonin-treated parotids in relation to untreated ones. As in the untreated glands, the preferential site for gold particle deposition was the secretory granules of the acinar cells in the melatonin-treated glands. However, a quantitative assessment revealed changes in response to melatonin not only in the number of gold particles deposited on the granule membrane and inside the granule but also in the number of stained granules.
Morphometric evaluation of untreated and melatonin-treated glands
In the glands exposed to melatonin, the density of the secretory granules did not differ (unpaired two-tailed t-test) from that of the secretory granules of the contralateral glands removed before the infusion of melatonin, where the total number of granules was 45.2 ± 3.5 and 47.7 ± 4.2 per 100 µm2 of cell area in MT1-receptor-labelled samples and 45.4 ± 2.5 and 43.4 ± 3.5 per 100 µm2 of cell area in MT2-receptor-labelled samples respectively.
1. The MT1 receptor
The granule population stained for the MT1 receptor was 45% (223 of 499) in the melatonin-treated gland and 25% (94 of 382) in the untreated gland, i.e. almost twice as many granules showed reactivity for the MT1 receptor after the infusion of melatonin (Fig. 7). The labelling density of gold particles showing immunoreactivity for the MT1 receptor associated with the granules (Fig. 5) was higher (P < 0.001, Kruskal-Wallis test, Fig. 6a) in the treated glands (1.21 ± 0.1 per µm2, total number of granules examined: 499) than in the untreated glands (0.48 ± 0.05 per µm2, total number of granules examined: 382). In the melatonin-treated glands, the mean number of MT1 receptors located on the membrane part of the granule (Fig. 6b) was about three times higher than that of MT1 receptors located on the membrane part of the granule in the untreated glands (0.24 ± 0.03 versus 0.07 ± 0.01 respectively; P < 0.001, Mann-Whitney U-test). Similarly, in the treated glands, the mean amount of MT1-receptor reactivity located on the inner side of the granules (Fig. 6b) was about twice that in the corresponding part of the untreated glands (0.79 ± 0.08 versus 0.35 ± 0.05 respectively; P < 0.001, Mann-Whitney U-test).
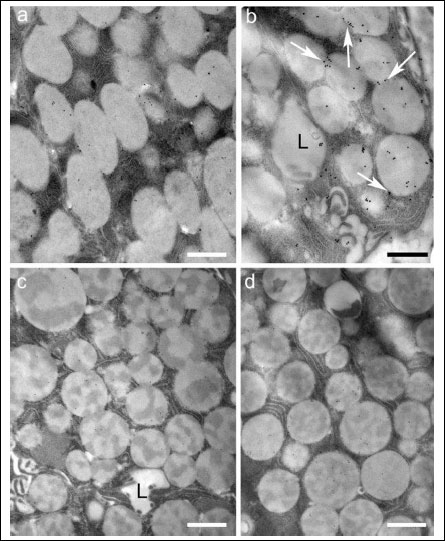 |
Fig. 5. Portions of serous cell stained for MT1 receptors in untreated (a) and (b) melatonin-treated glands and for MT2 receptors in untreated (c) and (d) melatonin-treated glands. The number of MT1-stained secretory granules appeared more numerous in the treated glands. Moreover, the granule membranes (arrows) appeared more decorated. With respect to the MT2 receptor, the number of gold particles in treated glands appeared not to differ from that of untreated samples. L, lumen; Bar, 1 µm. |
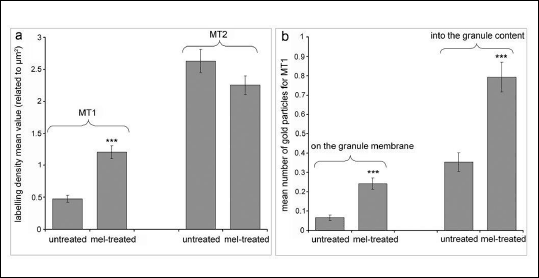 |
Fig. 6. Morphometric evaluation of the MT1- and MT2-receptor labelling density in untreated glands and in melatonin (mel)-treated glands; (a) number of gold particles per µm2 of the granules expressed as mean labelling density ± S.E.M, (b) mean number of gold particles for MT1 receptors related to all observed granules, in untreated and mel-treated glands, associated with the membrane part and with the content of the granules. *** P < 0.001. |
2. The MT2 receptor
The granule population stained for the MT2 receptor in the melatonin-treated glands (68%: 233 of 342) did not differ from that in the untreated ones (67%: 327 of 491) (Fig. 7). In the untreated glands, the labelling density of gold particles associated with the secretory granules and showing reactivity for the MT2 receptor was about five times higher than that for the MT1 receptor (P < 0.001, Kruskal-Wallis test). In treated glands, the labelling density of gold particles associated with the secretory granules and showing reactivity for the MT2 receptor was about two times higher than that for the MT1 receptor (P < 0.001, Kruskal-Wallis test). In contrast to the MT1 receptor, the density of the MT2 receptor was not affected in a statistically significant way (Kruskal-Wallis test) by melatonin exposure and was 2.3 ± 0.1 per µm2 (n = 342) versus a mean value for untreated glands of 2.6 ± 0.2 per µm2 (n = 491).
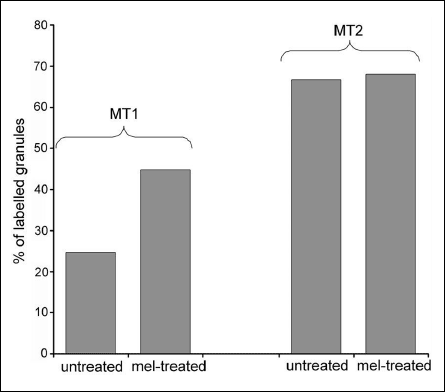 |
Fig. 7. Percentage of the MT1- and MT2-labelled granules in untreated and melatonin (mel)-treated glands related to all observed granules. The morphometric evaluation revealed that melatonin stimulation increased the number of granules labelled for MT1 but not for MT2. |
Immunoblotting analysis
The immunoblotting of membrane-enriched samples from the glands revealed the presence of both MT1 and MT2 receptors. In samples of untreated glands, optical densitometry data, normalised for GAPDH expression, were 0.09 ± 0.03 for MT1 and 0.20 ± 0.09 for MT2 (arbitrary units). After melatonin treatment, the expression of MT1 receptors was about three times that of the untreated gland, i.e. 0.27 ± 0.06 (P < 0.05, unpaired two-tailed t-test). The immunoblotting for MT2 receptors revealed a fairly constant signal in the membranous fraction before and after (0.22 ± 0.046) melatonin infusion (Fig. 8).
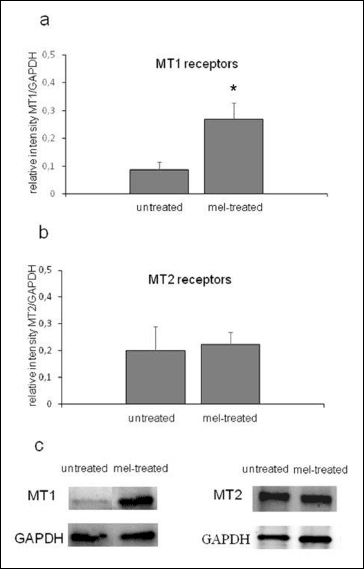 |
Fig. 8. Immunoblotting of membrane-enriched samples from untreated and melatonin (mel)-treated parotid glands. Both samples showed the presence of MT1 (a, c) and MT2 (b, c) receptors, but melatonin infusion caused a marked increase in MT1-receptor expression, whereas MT2-receptor expression was unchanged. The housekeeping protein, GADPH, served as a reference against which the target proteins were normalised in order accurately to quantify fluorescent western blots. Mean values ± S.E.M. * P < 0.05. |
DISCUSSION
As judged by the distribution of gold particles, the two types of melatonin receptor in the rat parotid gland displayed a pattern similar to that previously reported for the human parotid gland (8). Although present at the level of the cell membrane, their predominant localisation was the acinar secretory granules and, in addition, the small vesicles among these granules. Moreover, they were also associated with vesicles of the ductal cells. However, in contrast to the human parotid gland, the number of gold particles in the rat parotid gland showing immunoreactivity for the MT1 receptor was smaller than those showing immunoreactivity for the MT2 receptor.
Melatonin has previously been found to cause the in-vivo secretion of amylase/protein from the rat parotid gland, a phenomenon probably associated with granule exocytosis (2). This assumption is supported by the recent ultrastructural findings of exocytotic events in human parotid tissue exposed to melatonin in vitro (5). In the present study, however, no support for acinar degranulation was found. The density of granules was the same in melatonin-treated glands and untreated glands, a fact that simplifies comparisons between the two glands. The current dose exposure per unit of time was probably not high enough to reach the threshold for evoking granule secretion, although the blood level of melatonin achieved by the current intravenous infusion was much higher than can be expected to cause blood levels of melatonin in rats near physiological amounts (14). The protocol used did, however, produce reproducible data demonstrating dynamic effects on the melatonin-receptor population of potential physiological and pharmacological importance.
In the acinar cells of parotid glands, an increased load of circulating melatonin was found to be accompanied by an increase in the density of immunogold particles signalling the MT1-receptor type but not of those signalling the MT2-receptor type. The dissection of the MT1-receptor response showed not only more granules stained with immunogold particles but also an increased number of immunogold particles per granule. Furthermore, this increase involved particles located both in the membrane of the granule, probably reflecting the membrane affinity of the G-protein receptor, and its content, which was three and two times that of untreated glands respectively. Additional support for the increased localisation of the MT1 receptor to membranes in response to melatonin was obtained from the immunoblotting study, showing a threefold increase in immunoreactivity compared with the value for untreated glands; here, too, the immunoreactivity of the MT2 receptor remained unchanged. It should, however, be pointed out that the membrane-enriched extract of the gland prepared for the immunoblotting technique represents membranes from various sources, including plasma membranes and membranes of intracellular organelles, such as granules, vesicles and mitochondria.
The two types of melatonin receptor belong to the family of G-protein-coupled, seven transmembrane receptors (15). Although the cell-membrane location of this receptor family was initially the focal point, there are now numerous reports showing intracellular locations of G-proteins and, further, that these proteins translocate between various sites upon stimulation. Agonist exposure of the G-protein receptors over a period of minutes or hours induces receptor internalisation, as a part of acute receptor desensitisation, and trafficking (16, 17). With respect to the two types of melatonin receptor, they do not respond uniformly and, as a result, one receptor type may be internalised while the other is not; factors of importance to differences are the melatonin concentration, the time of exposure and cell types under study (18). Although the possibility cannot be excluded that the present increase in the density of the MT1 receptor associated with the secretory granules is part of a desensitising process in response to elevated blood levels, it is possible that the receptor is involved in the uptake and transport of melatonin that is to be stored in the secretory granules.
Acinar small vesicles, distributed throughout the granules and nearby basolateral membranes, displayed reactivity for the melatonin receptors. In the present study, neither the number of vesicles nor the number of the two types of melatonin receptor associated with the vesicles was calculated. Although the vesicles may perform a number of functions in the cells (19), it is tempting, in line with our hypothesis, to suggest that the presence of reactivity for the melatonin receptors associated with the small vesicles reflects the trafficking of the melatonin-receptor complex from the plasma membrane either towards the secretory granules or directly towards the glandular lumen; the latter pathway also perhaps serves as an explanation of the presence of reactive vesicles in the ductal cells.
In previous work by Bubenik (20), melatonin has been demonstrated in the palate salivary glands of the rat and, further, in the stomach, duodenum and colon of this species (21). However, as pointed out by Bubenik (22), the immunofluorescence method used does not permit a distinct description of the distribution of the melatonin-positive cells or their cellular localisation. In the human acinar cells of the parotid gland, support for the granular storage of melatonin has recently been obtained (23).
Hypothetically, inside the granule, the anti-oxidant effect of melatonin may contribute to the protection of the various constituents of the granule, including the various enzymes. Upon granule exocytosis, released melatonin may exert a number of beneficial effects in the oral cavity in the event of caries, ulcers, periodontal diseases and candidiasis (24). The presence of melatonin in the saliva is usually attributed to the passive passage of the non-protein bound fraction, from the blood to the oral cavity, thus reflecting the blood levels of melatonin (1, 25-28). The intestinal tract may both synthesise melatonin and act as a sink for pineal gland-derived melatonin (14, 29). As a result, particularly during the night, when the blood level of melatonin is high, the salivary glands may take up and store melatonin in the secretory granules via the MT1 receptor. Subsequently, upon the reflex stimulation of the glands, melatonin may be released by the regulated secretory pathway (30). In this connection it may be mentioned that a melatonin synthesis has been suggested to occur in salivary duct cells and possibly melatonin may exert paracrine effects in the acinar cells via its receptors (31). Likewise, in the rat thyroid gland a paracrine secretion of melatonin was suggested. C-cell secreted melatonin seems to influence the activity of follicular cells within the gland (32).
Interestingly, in vitro studies of prostate cancer cells suggest that melatonin crosses the cell membrane and reaches cytoplasmic and nuclear compartments by active transport or facilitated diffusion rather than by simple passive diffusion due to the amphiphilic nature of melatonin (33); in this case, glucose transporters, in particular GLUT1, were suggested as a carrier of melatonin (34). Transcytotic receptor-mediated passages over acinar cells have, for example, been demonstrated with respect to immunoglobulin A and transferrin (35, 36). As is the case for immunoglobulin A and transferrin, once in the saliva, melatonin is supposed to separate from its transporter.
The infusion time for melatonin in the present study was two hours, followed by a 30-minute post-infusion period. There is thus a possibility that a minor fraction of the MT1-receptor population represents newly synthesised MT1 receptors (37). The present study, combined with our previous observations (2, 3, 5), may, at first sight, suggest that the two types of receptor are potentially involved in different functions in the gland, the MT1 receptor in the transport and granular storage of melatonin and the MT2 receptor in the secretion of amylase/protein and the synthesis of secretory proteins. Nevertheless, it should be remembered that, in this study, M2 receptors also occurred in association with the secretory granules and the vesicles; in fact, this type of receptor was in the majority, at least when its association with the secretory granules was counted. Although the MT2 receptors were not influenced by the melatonin infusion in the current experimental conditions, a role for this type of receptor in melatonin transport cannot be excluded. Perhaps the MT2 receptor, in contrast to the MT1 receptor, represents a slowly adapting transport system or is only mobilised in physiological conditions. It is interesting to question whether, in physiological conditions, increased glandular activity evoked by autonomic innervation stimulates intracellular, melatonin-receptor-linked melatonin transport and, further, whether pharmacological doses of melatonin, by the MT1-receptor-mediated translocation of the hormone, may be of importance in situations in which salivary gland functions fail.
To conclude, an increased load of circulating melatonin selectively induced an increase in the number of MT1 receptors (but not of MT2 receptors) associated with the acinar secretory granules, suggesting a functional role for MT1 receptors in the salivary gland transport, storage and secretion of melatonin. In order to obtain further support for this concept, future studies, in our rat model, have to show a parotid gland output of melatonin into the saliva that gradually decreases upon prolonged autonomic receptor stimulation (during constant blood levels of melatonin) concomitantly with the depletion of the gland content of melatonin. Further, pre-treatment with melatonin receptor antagonists and, as judged from the present study, particularly those involving the MT1 receptor, should prevent melatonin from accumulating in the gland. When exploring the effect of melatonin antagonists it should be noted that currently there is no selective MT1 receptor on the market; comparisons have to be made between the effects of the non-selective antagonist luzindole and the selective MT2 receptor antagonist 4-P-PDOT (38).
Acknowledgements: The authors thank Mr A. Cadau for his excellent technical assistance. J. Ekstrom acknowledges support from the University of Cagliari for periods as visiting professor. M. Isola and M.A. Lilliu gratefully acknowledge the Sardinia Regional Government for financial support (P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007-2013).
R. Isola and F. Loy equally contributed to this work.
Conflict of interests: None declared.
REFERENCES
- Reiter RJ, Rosales-Corral SA, Liu XY, Acuna-Castroviejo D, Escames G, Tan DX. Melatonin in the oral cavity: physiological and pathological implications. J Periodontal Res 2015; 50: 9-17.
- Cevik-Aras H, Ekstrom J. Melatonin-evoked in vivo secretion of protein and amylase from the parotid gland of the anaesthetised rat. J Pineal Res 2008; 45: 413-421.
- Cevik-Aras H, Godoy T, Ekstrom J. Melatonin-induced protein synthesis in the rat parotid gland. J Physiol Pharmacol 2011; 62: 95-99.
- Cevik-Aras H, Ekstrom J. Anti-inflammatory action of cholecystokinin and melatonin in the rat parotid gland. Oral Dis 2010; 16: 661-667.
- Loy F, Isola M, Isola R, et al. Ultrastructural evidence of a secretory role for melatonin in the human parotid gland. J Physiol Pharmacol 2015; 66: 847-853.
- Arias-Santiago S, Aneiros-Fernandez J, Arias-Santiago B, et al. MTNR1A receptor expression in normal and pathological human salivary glands. Anticancer Res 2012; 32: 4765-4771.
- Aneiros-Fernandez J, Arias-Santiago S, Arias-Santiago B, et al. MT1 melatonin receptor expression in Warthin's tumor. Pathol Oncol Res 2013; 19: 247-250.
- Isola M, Ekstrom J, Diana M, et al. Subcellular distribution of melatonin receptors in human parotid glands. J Anat 2013; 223: 519-524.
- Loy F, Isola M, Isola R, et al. The antipsychotic amisulpride: ultrastructural evidence of its secretory activity in salivary glands. Oral Dis 2014; 20: 796-802.
- Isola M, Solinas P, Proto E, Cossu M, Lantini MS. Reduced statherin reactivity of human submandibular gland in diabetes. Oral Dis 2011; 17: 217-220.
- Follesa P, Porcu P, Sogliano C, et al. Changes in GABA-A receptor gamma 2 subunit gene expression induced by long-term administration of oral contraceptives in rats. Neuropharmacology 2002; 42: 325-336.
- Hoppel C, DiMarco JP, Tandler B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem 1979; 25: 4164-4170.
- Fanali C, Inzitari R, Cabras T, et al. Mass spectrometry strategies applied to the characterization of proline-rich peptides from secretory parotid granules of pig (Sus scrofa). J Sep Sci 2008; 31: 516-522.
- Huether G, Messner M, Rodenbeck A, Hardeland R. Effect of continuous melatonin infusions on steady-state plasma melatonin levels in rats under near physiological conditions. J Pineal Res 1998; 24: 146-151.
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 2012; 351: 152-166.
- Willard FS, Crouch MF. Nuclear and cytoskeletal translocation and localization of heterotrimeric G-proteins. Immunol Cell Biol 2000; 78: 387-394.
- Kallal L, Benovic JL. Using green fluorescent proteins to study G-protein-coupled receptor localization and trafficking. Trends Pharmacol Sci 2000; 21: 175-180.
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 2010; 62: 343-380.
- Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res 2005; 84: 500-509.
- Bubenik GA. Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Horm Res 1980; 12: 313-323.
- Bubenik GA. Gastrointestinal melatonin, localization, function and clinical relevance. Dig Dis Sci 2002; 47: 2336-2348.
- Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol 2008; 59 (Suppl 2): 33-51.
- Isola M, Lilliu MA. Melatonin localization in human salivary glands. J Oral Pathol Med 2015; Dec 23. doi: 10.1111/jop.12409
- Gomez-Moreno G, Guardia J, Ferrera MJ, Cutando A, Reiter RJ. Melatonin in diseases of the oral cavity. Oral Dis 2010; 16: 242-247.
- Kennaway DJ, Voultsios A. Circadian rhythm of free melatonin in human plasma. J Clin Endocrinol Metab 1998; 83: 1013-1015.
- Czesnikiewicz-Guzik M, Konturek SJ, Loster B, Wisniewska G, Majewski S. Melatonin and its role in oxidative stress related diseases of oral cavity. J Physiol Pharmacol 2007; 58 (Suppl. 3): 5-19.
- Le Bars D, Thivolle P, Vitte PA, et al. PET and plasma pharmacokinetic studies after bolus intravenous administration of [11C] melatonin in humans. Int J Rad Appl Instrum B 1991; 18: 357-362.
- Costa EJ, Shida CS, Biaggi MH, Ito AS, Lamy-Freund MT. How melatonin interacts with lipid bilayers: a study by fluorescence and ESR spectroscopies. FEBS Lett 1997; 416: 103-106.
- Messner M, Hardeland R, Rodenbeck A, Huether G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J Pineal Res 1998; 25: 251-259.
- Castle JD, Castle AM. Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci 1996; 109: 2591-2599.
- Shimozuma M, Tokuyama R, Tatehara S, et al. Expression and cellular localizaion of melatonin-synthesizing enzymes in rat and human salivary glands. Histochem Cell Biol 2011; 135: 389-396.
- Garcia-Marin R, Fernandez-Santos JM, Morillo-Bernal J, et al. Melatonin in the thyroid gland: regulation by thyroid-stimulating hormone and role in thyroglobulin gene expression. J Physiol Pharmacol 2015; 66: 643-552.
- Hevia D, Sainz RM, Blanco D, et al. Melatonin uptake in prostate cancer cells: intracellular transport versus simple passive diffusion. J Pineal Res 2008; 45: 247-257.
- Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, et al. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res 2015; 58: 234-250.
- Nashida T, Yoshie S, Imai A, Shimomura H. Transferrin secretory pathways in rat parotid cells. Arch Biochem Biophys 2009; 487: 131-138.
- Xu S, Ma L, Evans E, Okamoto CT, Hamm-Alvarez SF. Polymeric immunoglobulin receptor traffics through two distinct apically targeted pathways in primary lacrimal gland acinar cells. J Cell Sci 2013; 126: 2704-2717.
- Koenig JA, Edwardson JM. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol Sci 1997; 18: 276-287.
- Juszczak M, Roszczyk M, Kowalczyk E, Stempniak B. The influence of melatonin receptors antagonists, luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT), on melatonin-dependent vasopressin and adrenocorticotropic hormone (ACTH) release from the rat hypothalamo-hypophysial system. in vitro and in vivo studies. J Physiol Pharmacol 2014; 65: 777-784.
A c c e p t e d : December 31, 2015