EXPRESSION OF NEUROTROPHINS AND THEIR RECEPTORS
IN HUMAN CD34+ BONE MARROW CELLS
2Department of General Surgery and Transplantation, Pomeranian Medical University, Szczecin, Poland
INTRODUCTION
Neurological disorders affect people in all countries irrespective of age, sex, education or income, and stroke remains the second-most common cause of death worldwide. Thrombolytic therapy is able to restore cerebral blood flow in some patients with acute ischemic stroke and can lead to the improvement or resolution of neurologic deficits. However, only a limited number of patients can be treated in this way, because the treatment must occur within 3 hours of the onset of stroke (1). Neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and others affect millions of people worldwide. These diseases result in the gradual and progressive loss of neural cells, leading to nervous system dysfunction. Spinal cord injury and optic nerve injury results in loss of neurons, degeneration of axons, formation of glial scar, and severe functional impairment. Due to the lack of effective therapies for neurodegenerative diseases, the development of stem cell-based therapeutic strategies aiming to exert trophic neuroprotective activity or replace the lost neurons have begun and show promise for treating these diseases.
In recent decades, a number of studies have been performed to assess the feasibility and benefits of stem cell-based experimental approaches to regeneration and trophic support for neural tissue. Stem/progenitor cells (SPCs) have the potential to self-renew and to differentiate into mature cells. These cells have also been demonstrated to exert trophic activity by producing important growth factors (2). The cell membrane protein CD34 is an important marker of early SPCs, and the CD34+ cell population is thought to comprise early hematopoietic SPCs and endothelial progenitor cells. Since it has been reported that the infusion of large numbers of CD34+ cells has had a positive impact on the outcome in patients undergoing bone marrow (BM) transplantation in terms of faster engraftment and fewer infectious episodes (3), the estimation of the number of CD34+ cells has been widely used to monitor hematopoietic stem cell transplantation. The efficacy and safety of intravenous administration of immunoselected CD34+ cells in hematopoietic reconstitution following high-dose chemotherapy in patients with cancer has also been demonstrated (4). CD34+ cells isolated by magnetic sorting have also been used in clinical trials studying SPC transplantation for the purpose of tissue regeneration (5). In addition to their hematopoietic potential, CD34+ cells are of potential use in stem cell-based therapies for neural diseases, including spinal cord injury (6) and stroke (7). Human CD34+ cells have been administered intravenously to immunocompromised mice 48 hours prior to experimentally induced ischemia and have been shown to induce long-term neovascularization in the ischemic zone (8). Similarly, a recent study demonstrated that human CD34+ cells injected intravenously to immunocompromised mice 48 hours after neonatal stroke modestly reduced ischemic brain damage with a transient augmentation of cerebral blood flow in the peri-infarct area (9).
We and others have previously demonstrated that endogenous BM CD34+ cells are mobilized into the peripheral blood in response to central nervous system (CNS) injury following ischemic or hemorrhagic stroke and acute spinal cord injury (10-12). This finding suggests an intrinsic mechanism for ameliorating tissue damage that involves circulating SPCs. Moreover, it has been shown that, in patients with intracerebral hemorrhage, the concentration of circulating CD34+ progenitor cells at day 7 is independently associated with good functional outcome at 3 months (13). This finding suggests that CD34+ progenitor cells might participate in the functional recovery of these patients. These observations have provided the basis for two experimental approaches. The first was the intra-arterial transplantation of BM mononuclear cells (MNCs) in an experimental model of CNS injury and in clinical trials. This procedure has been shown to be feasible and safe in patients; however, there were no significant differences in neurological function in stroke patients at 180 days after transplantation (14). The second study used granulocyte-colony stimulating factor (G-CSF) as a SPC-mobilizing cytokine and a neuroprotective factor initially in an experimental stroke model (15). Recent clinical trials have suggested that G-CSF is safe when administered subacutely in stroke patients; however, it is too early to know whether G-CSF improves functional outcomes (16, 17).
The characterization of the SPC population and optimal choice of SPCs in terms of their ability to exert paracrine activity following their transplantation into the neural microenvironment are important prerequisites for the successful application of stem cells in future experimental studies. BM CD34+ cells are heterogeneous and easily available from whole BM aspirates, which can be enriched by immunomagnetic separation. Therefore, in the present study, we sought to better characterize this population specifically by investigating whether human BM CD34+ cells express NTs and their relevant receptors along with a comparison of their expression levels with those of unseparated BM nucleated cells (NCs).
MATERIALS AND METHODS
Cells
Bone marrow cells were collected from ten adult heparinized deceased organ donors (HDODs). The mean age of the HDODs was 46 ± 15 years (5 men, 5 woman). The donors’ families gave their consent in each case. All procedures were approved by the Local Ethics Committee and in accordance with the Helsinki Declaration.
BM cells were aspirated from pelvic bones and subsequently resuspended in collecting medium phosphate-buffered saline (PBS) and heparin (20 U/mL; Life Technologies, Paisley, UK). Before organ harvest, every HDOD was infused with heparin (25,000 U/donor, Teva, Kutno, Poland) and subsequently BM was aspirated from iliac crest before disconnection from the respirator. Whole human BM samples were lysed in BD PharmLyse Lysing Solution (BD Biosciences, San Jose, CA, USA) for 15 min at room temperature in the dark and washed twice in PBS. A part of the obtained suspension of BM NCs was subjected to immunomagnetic separation procedure. CD34+ cell population enriched in SPCs were isolated from NCs using commercially available CD34 MicroBead Kit (Miltenyi Biotec, Auburn, CA, USA). Isolation procedure was performed according to the manufacturer’s instructions, as described (18).
qRT-PCR
Total mRNA was isolated from NCs or BM CD34+ cells using the RNeasy Mini Kit (Qiagen Inc., CA, USA). Subsequently, the mRNA was reverse-transcribed using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, MA, USA). A quantitative assessment of gene expression was performed using real time qRT-PCR carried out on a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Inc., CA, USA). The 15 µL reaction mixture contained 7.5 µL of SYBR Green PCR Master Mix, 10 ng of cDNA template, and one pair of forward and reverse primers. The primers used for these reactions are listed in Table 1. The threshold cycle (Ct), i.e. the cycle number at which the amount of the amplified gene of interest reached a fixed threshold, was subsequently determined. The relative target gene mRNA expression was quantified using the comparative Ct method. The relative quantification value of the target was normalized to the endogenous control BMG gene and expressed as 2ΔCt, where ΔCt = [Ct of endogenous control gene (BMG)] – [Ct of target genes].

Immunofluorescence
Freshly isolated BM CD34+ cells and NCs were subjected to immunofluorescence (IF) staining of neurotrophic factors. First, the cells were fixed with 3.7% paraformaldehyde and then smeared on polylysine-coated slides. After permeabilization in 0.5% Tween 20 (Bio-Rad Inc.) and blocking with 10% normal goat serum, the smears were incubated at 4°C overnight with one of the following primary antibodies: anti-BDNF, anti-NGF, anti-NT3, anti-NT4, anti-TrkA, anti-TrkB, anti-TrkC, or anti- p75NTR. Subsequently, the cells were incubated in the dark with the relevant secondary antibodies. The antibodies used for immunofluorescence studies are listed in Table 2. Upon termination, all of the sections were counterstained with DAPI solution (Thermo Fisher Scientific, Waltham, MA, USA), mounted, and examined using an LSM700 confocal system (Carl Zeiss, Jena, Germany). For quantification of the percentage of cells expressing a specific marker in any given experiments the number of positive cells was determined in relation to the total number of DAPI labeled nuclei. Counts of immunoreactive cells were made in 10 random fields in two slides for each with a 20 objective.
Western blot
Western blot analysis was performed to evaluate the expression of the BDNF, NGF, NT-3, and NT-4 as well as TrkA, TrkB, TrkC, p75NTR. The BM CD34+ cells and NCs (2 × 106) were lysed for 10 min on ice in M-Per lysing buffer (Pierce, Rockford, IL) containing protease and phosphatase inhibitors (Sigma-Aldrich, MO, USA) (10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 µg/ml pepstatin A, 1 mM sodium fluoride, and 2 mM Na3VO4). The cell lysates were clarified via centrifugation at 14,000 rpm for 10 min at 4°C, and the protein concentrations were determined using the Bradford protein assay (Sigma-Aldrich). Three cell lysates were pooled and equal amounts of protein (20 µg/well) were loaded and separated on a 4 – 0% sodium dodecyl sulfate polyacrylamide gel via electrophoresis (SDS-PAGE, mini-PROTEAN II electrophoresis system, Bio-Rad Inc.) and then transferred to a 0.2 µm polyvinylidene fluoride (PVDF) membrane (Bio-Rad Inc.). Kaleidoscope polypeptide standard wide range (10 – 250 kD) protein markers (Bio-Rad Inc.) were used to determine the molecular weights of the analyzed proteins. After blocking non-specific binding for 2 hours at room temperature with a 3% BSA, Tris-HCl and NaCl solution with 0.05% Tween 20, the membrane was probed with a specific monoclonal/polyclonal IgG antibody directed against amino acid sequences of the selected proteins (BDNF, NGF, NT-3, NT-4, TrkA, TrkB, TrkC, p75NTR) and incubated overnight at 4°C. The antibodies used for Western blotting studies are listed in Table 2. Immunoreactive bands were detected using horseradish peroxidase-conjugated secondary antibody specific to the primary antibody used in the previous step. Chemiluminescence detection was performed using the ECL Select Detection Kit (Amersham Life Sciences, Buckinghamshire, UK), and the bands were subsequently visualized with a UVP camera (Gel DOC-It Imaging system, Bio-Rad).
Statistical analysis
Because the distribution of most variables significantly deviated from normal distribution, non-parametric tests were used. The significance of differences between the CD34+ cell and NC populations was assessed using the Kruskal-Wallis test followed by the Mann-Whitney test. P values < 0.05 were considered statistically significant.
RESULTS
Human BM is a rich source of heterogeneous SPCs. In this study, we isolated BM CD34+ cells from NCs obtained from HDODs and subsequently used these cells to better characterize this SPC-enriched population. Non-separated NCs obtained after the lysis of erythrocytes were used as a control. The average total number of NCs recovered from a single collection was 544 ± 262 × 106 cells. We successfully isolated the CD34+ cells from NCs using immunomagnetic separation. The number of CD34+ cells recovered from 100 × 106 NCs was 1.13 ± 0.63 × 106. Thus, in our studies, the NC fraction contained 1.13 ± 0.63% BM CD34+ cells.
Human bone marrow CD34+ cells spontaneously express neurotrophins
Accumulating data indicate that CD34+ have the neuroprotective properties. By employing qRT PCR, we evaluated the expression of various NTs (BDNF, NGF, NT-3, and NT-4) at the mRNA level in BM CD34+ cells and compared to those observed in unsorted NCs. We found that NT mRNAs are robustly expressed in human BM CD34+ cells; the expression data are shown in Fig. 1A. Univariate statistical analysis revealed that BM CD34+ cells expressed significantly higher levels of each analyzed neurotrophic factor compared to NCs. Furthermore, we observed a tendency towards an increase in the expression of NTs in BM CD34+ cells at the protein level, as determined by Western blot analysis (Fig. 1B).
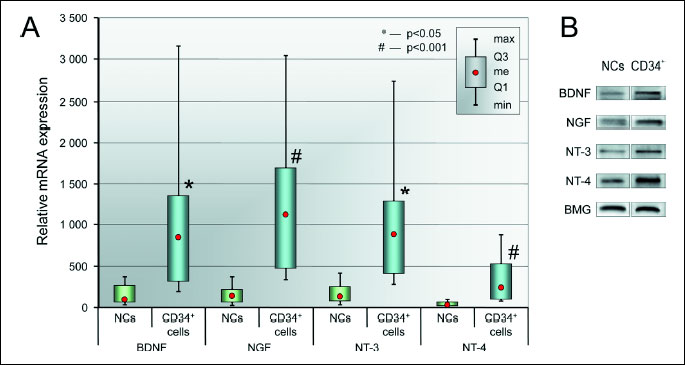
(A) NT mRNA expression levels in BM CD34+ cells and NCs. Relative of mRNA expression values are normalized against BMG expression values. Data are presented as median, quartiles, interquartile range, minimum, and maximum (N = 6 for CD34+ cells, N = 10 for NCs). *P < 0.05 and #P < 0.001 versus NCs.
(B) Representative Western blot analysis of NT protein levels in BM CD34+ and NC cell lysates.
Finally, we confirmed the expression of NTs in CD34+ cells at the protein level by visualizing their intracellular expression through immunofluorescence analyses. We determined that the isolated CD34+ population expressed select NTs (BDNF, NGF, NT-3, and NT-4), as shown by immunofluorescent staining (Fig. 2A). Freshly isolated BM CD34+ cells were immunostained for specific neurotrophins including BDNF, NGF, NT-3, and NT-4, which showed that 3.41 ± 7.53% of CD34+ cells strongly expressed BDNF; 2.13 ± 4.0% expressed NGF; approximately 3.32 ± 6.34% of CD34+ cells were positive for NT-3; and a few cells (2.62 ± 5.15%) expressed NT-4. These data indicate that human BM CD34+ cells express neurotrophic and neuroprotective factors. Similarly, NCs were immunostained for specific neurotrophins and we could observe very few cells expressing NTs, i.e. 0.49 ± 1.27% of NCs expressed BDNF; 0.26 ± 0.72% expressed NGF; approximately 0.22 ± 0.68% of NCs were positive for NT-3; and 0.37 ± 0.92% expressed NT-4 (Fig. 2B). We observed a tendency towards higher number of cells expressing NTs in BM CD34+ cells compared to NCs.
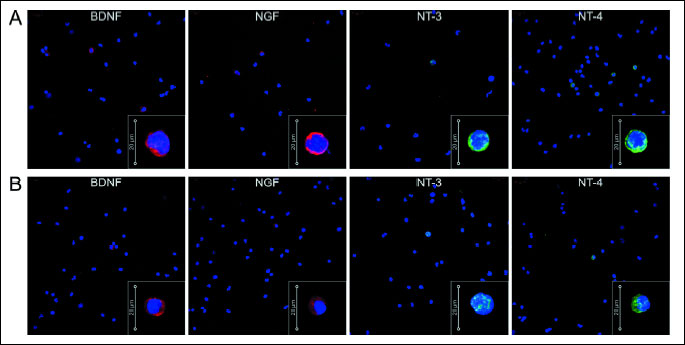
(B) Immunofluorescent analysis of intracellular NT expression in NCs. Nuclei were visualized by DAPI staining. Pseudo-coloring was assigned to stain as follows: anti-BDNF, anti-NGF are red, anti-NT-3, and anti-NT-4 are green, and nuclei are blue. All images were captured using an LSM700 confocal system (Carl Zeiss, Jena, Germany). Representative data are shown from two independent experiments.
Collectively, we found that human BM CD34+ cells express all of the examined NTs at higher levels than NCs. These results strongly support the hypothesis that BM CD34+ cells might exert a neurotrophic and neuroprotective effect on neural cells.
The expression of neurotrophin receptors is more pronounced in human bone marrow CD34+ cells compared to nucleated cells
In recent years, numerous studies have shown that BM cell-induced neuroprotection involves neurotrophic factors acting through paracrine and/or autocrine interactions between the neural microenvironment and transplanted cells (19, 20). Using qRT-PCR, we evaluated the expression levels of the neurotrophin receptors TrkA, TrkB, TrkC, and p75NTR in BM CD34+ cells compared to NCs.
All neurotrophin receptors were expressed at detectable levels in each cell type, as shown in Fig. 3A. Univariate statistical analysis revealed that BM CD34+ cells expressed significantly higher levels of each neurotrophin receptor compared to unsorted NCs. Moreover, we observed a tendency towards an increase in the expression of NT receptors in BM CD34+ cells at the protein level, as determined by Western blot analysis (Fig. 3B). Furthermore, we assessed the percentage of BM CD34+ cells that expressed NT-specific receptors by visualizing the surface protein on freshly isolated BM CD34+ cells using immunofluorescent analyses. We determined that the isolated BM CD34+ cell population expressed the specific NT receptors (TrkA, TrkB, TrkC), as shown in Fig. 4A. Freshly isolated BM CD34+ cells were also immunostained for the nonspecific NT receptor p75NTR. Immunostaining showed that 3.81 ± 5.48% of CD34+ cells expressed TrkA (Fig. 4A); 1.70 ± 2.97% expressed TrkB; approximately 1.5 ± 0.83% of CD34+ cells were positive for TrkC; and a few cells (1.0 ± 0.53%) expressed p75NTR. These data indicate that human BM CD34+ cells express neurotrophin receptors on their cell membranes. Similarly, we performed analysis of NCs using immunofluorescence staining. We observed few cells expressing NT receptors among NCs (0.45 ± 1.46% TrkA positive cells, 0.27 ± 0.66% TrkB positive cells, 0.25 ± 0.62% TrkC positive cells, and 0.26 ± 0.88% positive for p75NTR) (Fig. 4B).
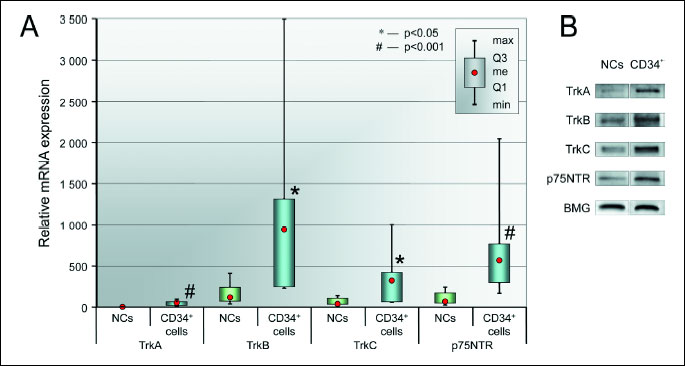
(A) NT receptor mRNA expression levels in BM CD34+ cells and NCs. Relative mRNA expression values are normalized against BMG levels. Data are shown as median, quartiles, interquartile range, minimum, and maximum (N = 6 for CD34+ cells, N = 10 for NCs). *P < 0.05 and #P < 0.001 versus NCs.
(B) Representative Western blot analysis of NT receptor protein levels in BM CD34+ and NC cell lysates.
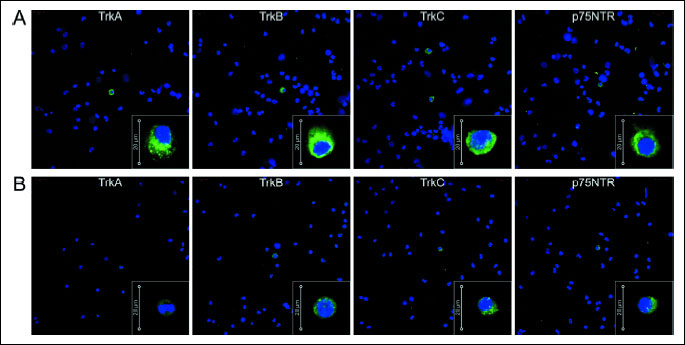
(B) Immunofluorescent analysis of intracellular NT receptor expression in NCs. Nuclei were visualized by DAPI staining. Pseudo-coloring was assigned to each stain, consequently anti-TrkA, anti-TrkB, anti-TrkC, and anti-p75NGR are green, and nuclei are blue. All images were captured using an LSM700 confocal system (Carl Zeiss, Jena, Germany). Representative data are shown from two independent experiments.
Taken together, these results demonstrate that human BM CD34+ cells express specific NT receptors at the mRNA and protein levels, suggesting that these cells could respond to neurotrophic factors in neural tissue.
DISCUSSION
The involvement of trophic support in addition to the replacement of damaged neurons has been proposed as an explanation for the beneficial effects observed following the infusion/transplantation of SPCs in experimental animal models of neural tissue injury (20, 21). We and others have shown that human SPCs could be a source for growth factors (2, 18, 22). In this study, we report the expression of neurotrophins in human BM CD34+ cells at the mRNA and protein levels. Moreover, these cells expressed mRNAs encoding BDNF, NGF, NT-3, and NT-4 on a higher level than nucleated cells. Taken together, these findings support the hypothesis that human BM SPCs exert paracrine effects following their transplantation into injured neural tissue. These cells could be a source for neuroprotective factors that inhibit apoptosis and promote neurogenesis and neovascularization in neural tissue. Because BM cells have been demonstrated to exert positive effects in an experimental model of nervous tissue injury, it could be speculated that neurotrophins produced by these cells interact with other effector cells in the injured neural tissue. A thorough characterization of the SPC population is an important factor in determining the optimal choice of stem cells for experimental studies of specific tissue injury models. In our study, we used BM harvested from HCOD, because our previous studies have demonstrated that HCOD BM cells have similar characteristics to BM cells from healthy subjects (23). We have not observed statistically significant differences in the transplantation potential of HSPCs harvested from the BM of HDODs compared to healthy donors.
CD34+ cells appear to have a number of significant advantages when considering their use in stem cell-based therapies. The CD34+ cell population has been demonstrated to secrete various growth factors (kit ligand, fibroblast growth factor-2, vascular endothelial growth factor, hepatocyte growth factor, insulin-like growth factor-1, and thrombopoietin), cytokines (tumor necrosis factor-α, Fas ligand, interferon α, interleukin 1, and interleukin 16), and chemokines (macrophage inflammatory protein-1α, and 1β, monocyte chemotactic protein-3, interleukin 8, and others) (2). Consequently, in this study, we show that human BM CD34+ cells express crucial neurotrophins that are required for the repair of neural tissues. Neurotrophins are a family of proteins that are well characterized by their influence on the survival, differentiation and apoptosis of cells in the nervous system (24, 25). The classical group of neurotrophins includes NGF, BDNF, NT-3, and NT-4. The effects of neurotrophins are mediated via high-affinity tropomyosin receptor kinase (Trk) receptors TrkA, TrkB, and TrkC and the low-affinity receptor p75NTR, which is a member of the tumor necrosis factor receptor family (26). It has been hypothesized that under some pathological conditions, neural cells fail to be exposed to a necessary level of neurotrophic factors and consequently undergo apoptosis (27, 28). Here we demonstrated that freshly isolated BM CD34+ cells produce BDNF, NGF, NT-3, NT-4 on protein level. We did not culture BM CD34+ cells and conduct secretion analysis by ELISA or Western blot on the cell culture supernatants. However, the secretion of NTs by BM cells and neuroprotection of conditioned media has been demonstrated in numerous studies (29, 30). One study demonstrated that BM mesenchymal stem cells produce a number of neuroprotective proteins including BDNF and suggested that secretion of these factors may play an important role in observed mesenchymal stem cell-mediated retinal ganglion cell neuroprotection. Authors confirmed the secretion of BDNF by BM cells using ELISA (29). The quantification of secreted NTs by ELISA analysis of cell culture supernatants, the defining cell culture conditions as well as strategies involving pre-treatment of BM CD34+ before using in experimental studies could be a subject of further research.
Here, we report that mRNAs for neurotrophin receptors are highly expressed in human BM CD34+ cells. A comparison of receptor mRNA expression showed a higher level of expression of these surface proteins in BM CD34+ cells compared to NCs. It has been previously shown that TrkA is expressed by and mediates functional responses to NGF in immature hematopoietic cell lines and mouse precursor BM cells (31). Another study by Laurenzi et al. has demonstrated the expression of neurotrophin mRNA in human granulocytes and BM cells (32). The presence of NGF receptors, which creates the physiological microenvironment for hematopoietic stem cells, on BM stromal cells has been reported previously (33). Bracci-Laudiero and co-workers have provided the first evidence that human umbilical cord blood (UCB) CD34+ stem cells and progenitors are able to respond to NGF through the expression of TrkA receptors and that they are able to synthesize NGF themselves (34). These findings are in agreement with our previous studies showing that UCB CD34+ cells express NGF and its relevant receptor at the mRNA and protein levels (18). NGF expression together with the expression of its relevant receptor, TrkA, has been observed in whole BM cells (35) and in BM stromal cells (36); however, the expression of NGF in CD34+ cells from BM had not been investigated previously. Here, we clearly demonstrate that human BM CD34+ cells express NGF and its relevant receptor, TrkA, at a significantly higher level than NCs, suggesting that the cells are more responsive to NGF stimulation than mononuclear cells.
In a previous study, it was demonstrated that B lymphocytes and their precursors express the BDNF receptors p75NTR and TrkB, while no BDNF expression was found in these cells (37). In the BM, BDNF has been found to be expressed in megakaryocytes, endothelial cells, and osteoblasts (35). The presence of BDNF receptors on lymphocyte precursors together with strong BDNF expression in BM stromal cells suggests a role for BDNF in normal B lymphocyte development (36). TrkB expression has also been shown in BM stromal cells in culture (36). Here, we observed the differences in the spontaneous expression levels of NTRs between selected BM CD34+ cells with immature characteristics and BM nucleated cells, suggesting that NTs may play different functional roles depending on the level of differentiation and maturity of the cells. In mice with experimental stroke injury, it has been observed that following a systemic injection of CD34+-enriched human cord blood cells, there was a significant increase in subgranular zone proliferation compared to control mice (38). Neurotrophin receptors could be involved in the recruitment of SPCs to specific sites. In an interesting finding by Kermani et al, the induction of neoangiogenesis in an ischemic limb model was observed following treatment with BDNF (39). The mechanism by which BDNF enhances capillary formation was mediated in part through the local activation of the TrkB receptor and by the recruitment of specific subsets of BM-derived hematopoietic cells that co-express TrkB, which provide peri-endothelial support for the newly formed vessels.
It is interesting that in a clinical study involving intra-arterial BM MNC transplantation in stroke patients, there was a negative correlation between the total number of CD34+ cells injected and MMP-2 levels 4 days post-transplantation, and lower plasma levels of MMP-2 at this time point was associated with better functional outcomes (40). In addition, Moniche et al. observed changes in the serum levels of GM-CSF and PDGF-BB even 3 months post-transplantation, which could be associated with better functional outcomes. These results suggest that the effects of BM MNC transplantation on functional outcomes in stroke patients are achieved by multifocal mechanisms that depend on changes in growth factors, enzymes and possibly the expression of other genes.
The availability of SPCs and their ease of isolation are important factors in considering their use in clinical applications. Autologous BM CD34+ cells can be obtained by aspiration of BM under local anesthesia and separated in a closed system, which is in contrast to endothelial progenitor cells and mesenchymal stem cells that require ex vivo culture. CD34+ cells isolated by magnetic sorting have been used in SPC transplantation clinical trials for the purposes of tissue regeneration (5, 41). In a previous clinical study, the possibility of delivering autologous BM-derived CD34+ cells into the spinal cord of patients with spinal cord injury via the lumbar puncture technique has been demonstrated. The results of studies using magnetic resonance imaging to monitor the fate of cells labeled with magnetic nanoparticles suggest that BM CD34+ cells migrate into the injured site in patients with chronic spinal cord injury (42). The neuroprotective effects of CD34+ cells have been observed by Boltze et al., who reported that in vivo, intravenously transplanted human UCB MNCs and CD34+ and CD34– cells reduced neurofunctional deficits and the lesion volume in rats following middle cerebral artery occlusion. This study also showed that human CD34+ cells within MNCs were preferably attracted to damaged hippocampal tissue in vitro and that the depletion of CD34-expressing cells abolished a reduction in neural damage, indicating that these cells were particularly involved in the protective action of MNCs (43). Separation to remove mature cells, e.g., granulocytes, prior to transplantation could decrease the cellular and cytokine interactions that can lead to inflammation and increased capillary permeability at the site of transplantation.
Conclusions
We found evidence that BM CD34+ cells have the potential to respond to NTs by expressing their relevant receptors TrkA, TrkB, TrkC, p75NTR and that they are also able to synthesize NTs (BDNF, NGF, NT-3, and NT-4) themselves. These cells show higher levels of NT expression compared to NCs. The spontaneous expression of neurotrophins and neurotrophin receptors at the mRNA and protein levels demonstrates the potential for the neurotrophic activity of BM CD34+ cells. However, a better understanding of the trophic influence of CD34+ cells on neural tissue following transplantation requires additional functional secretome studies using proteomic techniques and in vivo experiments. The simplicity and safety of immunomagnetic isolation, which has been confirmed in clinical settings, and the potential to respond to neural stimuli and to produce neurotrophic factors lays the foundation for stem cell-based regenerative therapy for neurological diseases using CD34+ isolated from autologous BM as an alternative source of cells for transplantation purposes.
Acknowledgments: This work was supported by The National Centre for Research and Development grant STRATEGMED1/234261/2NCBR/2014 (to BM).
E. Paczkowska and K. Piecyk equally contributed to this work.
Conflict of interests: None declared.
REFERENCES
- Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929-1935.
- Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001; 97: 3075-3085.
- Bittencourt H, Rocha V, Chevret S, et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood 2002; 99: 2726-2733.
- Chou T, Sano M, Ogura M, Morishima Y, Itagaki H, Tokuda Y. Isolation and transplantation of highly purified autologous peripheral CD34+ progenitor cells: purging efficacy, hematopoietic reconstitution following high dose chemotherapy in patients with breast cancer: results of a feasibility study in Japan. Breast Cancer 2005; 12: 178-188.
- Fujita Y, Kinoshita M, Furukawa Y, et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J 2014; 78: 490-501.
- Walczak P, Chen N, Hudson JE, et al. Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors? J Neurosci Res 2004; 76: 244-254.
- Willing AE, Vendrame M, Mallery J, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant 2003; 12: 449-454.
- Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004; 114: 330-338.
- Tsuji M, Taguchi A, Ohshima M, et al. Effects of intravenous administration of umbilical cord blood CD34(+) cells in a mouse model of neonatal stroke. Neuroscience 2014; 263: 148-158.
- Paczkowska E, Golab-Janowska M, Bajer-Czajkowska A, et al. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of endothelin-1. J Neurol Sci 2013; 325: 90-99.
- Paczkowska E, Roginska D, Pius-Sadowska E, et al. Evidence for proangiogenic cellular and humoral systemic response in patients with acute onset of spinal cord injury. J Spinal Cord Med 2015; 38: 729-744.
- Taguchi A, Nakagomi N, Matsuyama T, et al. Circulating CD34-positive cells have prognostic value for neurologic function in patients with past cerebral infarction. J Cereb Blood Flow Metab 2009; 29: 34-38.
- Sobrino T, Arias S, Perez-Mato M, et al. CD34+ progenitor cells likely are involved in the good functional recovery after intracerebral hemorrhage in humans. J Neurosci Res 2011; 89: 979-985.
- Moniche F, Gonzalez A, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke 2012; 43: 2242-2244.
- Piao CS, Gonzalez-Toledo ME, Gu X, Zhao LR. The combination of stem cell factor and granulocyte-colony stimulating factor for chronic stroke treatment in aged animals. Exp Transl Stroke Med 2012; 4: 25. doi: 10.1186/2040-7378-4-25
- England TJ, Abaei M, Auer DP, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke 2012; 43: 405-411.
- Bath PM, Sprigg N, England T. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev 2013; 6: CD005207.
- Paczkowska E, Kaczynska K, Pius-Sadowska E, et al. Humoral activity of cord blood-derived stem/progenitor cells: implications for stem cell-based adjuvant therapy of neurodegenerative disorders. PLoS One 2013; 8: e83833.
- Machalinska A, Roginska D, Pius-Sadowska E, et al. Neuroprotective and antiapoptotic activity of lineage-negative bone marrow cells after intravitreal injection in a mouse model of acute retinal injury. Stem Cells Int 2015; 2015: 620364. doi: 10.1155/2015/620364.
- Uemura M, Kasahara Y, Nagatsuka K, Taguchi A. Cell-based therapy to promote angiogenesis in the brain following ischemic damage. Curr Vasc Pharmacol 2012; 10: 285-288.
- Chen SH, Wang JJ, Chen CH, et al. Umbilical cord blood-derived CD34+ cells improve outcomes of traumatic brain injury in rats by stimulating angiogenesis and neurogenesis. Cell Transplant 2014; 23: 959-979.
- Racz GZ, Kadar K, Foldes A, et al. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol 2014; 65: 327-339.
- Baumert B, Kawa MP, Kotowski M, et al. Bone marrow of multiorgan donors underutilized: implications for improvement of accessibility of hematopoietic cells for transplantations. Transplantation 2012; 93: 165-171.
- Sakr HF, Abbas AM, El Samanoudy AZ. Effect of vitamin E on cerebral cortical oxidative stress and brain-derived neurotrophic factor gene expression induced by hypoxia and exercise in rats. J Physiol Pharmacol 2015; 66: 191-202.
- Murawska-Cialowicz E, Wojna J, Zuwala-Jagiello J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J Physiol Pharmacol 2015; 66: 811-821.
- Machalinski B, Lazewski-Banaszak P, Dabkowska E, Paczkowska E, Golab-Janowska M, Nowacki P. The role of neurotrophic factors in regeneration of the nervous system. [article in Polish] Neurol Neurochir Pol 2012; 46: 579-590.
- Rashedi I, Panigrahi S, Ezzati P, Ghavami S, Los M. Autoimmunity and apoptosis - therapeutic implications. Curr Med Chem 2007; 14: 3139-3151.
- Sakr HF, Khalil KI, Hussein AM, Zaki MS, Eid RA, Alkhateeb M. Effect of dehydroepiandrosterone (DHEA) on memory and brain derived neurotrophic factor (BDNF) in a rat model of vascular dementia. J Physiol Pharmacol 2014; 65: 41-53.
- Johnson TV, DeKorver NW, Levasseur VA, et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014; 137: 503-519.
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 2014; 9: e109305. doi: 10.1371/journal.pone.0109305.
- Chevalier S, Praloran V, Smith C, et al. Expression and functionality of the trkA proto-oncogene product/NGF receptor in undifferentiated hematopoietic cells. Blood 1994; 83: 1479-1485.
- Laurenzi MA, Beccari T, Stenke L, Sjolinder M, Stinchi S, Lindgren JA. Expression of mRNA encoding neurotrophins and neurotrophin receptors in human granulocytes and bone marrow cells-enhanced neurotrophin-4 expression induced by LTB4. J Leukoc Biol 1998; 64: 228-234.
- Cattoretti G, Schiro R, Orazi A, Soligo D, Colombo MP. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood 1993; 81: 1726-1738.
- Bracci-Laudiero L, Celestino D, Starace G, et al. CD34-positive cells in human umbilical cord blood express nerve growth factor and its specific receptor TrkA. J Neuroimmunol 2003; 136: 130-139.
- Labouyrie E, Dubus P, Groppi A, et al. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol 1999; 154: 405-415.
- Li N, Yang H, Lu L, Duan C, Zhao C, Zhao H. Spontaneous expression of neural phenotype and NGF, TrkA, TrkB genes in marrow stromal cells. Biochem Biophys Res Commun 2007; 356: 561-568.
- Schuhmann B, Dietrich A, Sel S, et al. A role for brain-derived neurotrophic factor in B cell development. J Neuroimmunol 2005; 163: 15-23.
- Kadam SD, Chen H, Markowitz GJ, et al. Systemic injection of CD34(+)-enriched human cord blood cells modulates poststroke neural and glial response in a sex-dependent manner in CD1 mice. Stem Cells Dev 2015; 24: 51-66.
- Kermani P, Rafii D, Jin DK, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest 2005; 115: 653-663.
- Moniche F, Montaner J, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cell transplantation correlates with GM-CSF, PDGF-BB, and MMP-2 serum levels in stroke patients: results from a clinical trial. Cell Transplant 2014; 23 (Suppl. 1): 57-64.
- Cordes AL, Jahn K, Hass R, et al. Intramedullary spinal cord implantation of human CD34+ umbilical cord-derived cells in ALS. Amyotroph Lateral Scler 2011; 12: 325-330.
- Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev 2007; 16: 461-466.
- Boltze J, Reich DM, Hau S, et al. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell Transplant 2012; 21: 723-737.
A c c e p t e d : January 20, 2016