CORTICOTROPIN RELEASING FACTOR RECEPTOR 1 ANTAGONIST DIFFERENTIALLY INHIBITS FREEZING BEHAVIOR AND CHANGES
GAMMA-AMINOBUTYRIC ACID-ERGIC ACTIVITY IN THE AMYGDALA
IN LOW- AND HIGH-ANXIETY RATS
2Department of Experimental and Clinical Pharmacology, Medical University, Warsaw, Poland
INTRODUCTION
The amygdala and medial prefrontal cortex (mPFC) are structures that are engaged in the modulation of anxiety and fear learning (1-4). Evidence strongly supports a role for the basolateral amygdala (BLA) as a critical structure for the formation and storage of fear memory (3-5). The central nucleus of the amygdala (CeA) is required for the acquisition, consolidation and expression of fear memories in parallel with the BLA (1-3, 6). Corticotropin-releasing factor (CRF) has been shown to play an important role within the amygdala in fear learning processes by acting at the CRF1 and CRF2 receptors. Stimulation of the CRF1 receptors induces hormonal and behavioral stress-like responses (7-9). Mice lacking CRF1 receptors display reduced anxiety and selective CRF1 receptor antagonists inhibit the anxiogenic action of CRF (10). The BLA contains a high density of CRF1 receptors. In contrast, the CeA contains many CRF-expressing neurons but lacks strong CRF receptor expression (3, 11). The infusion of a CRF1 receptor antagonist (DMP696) into the BLA disrupts contextual fear conditioning (12). Other studies have found that intra-CeA infusion of a non-selective CRF antagonist (alpha-helical CRF9–41) prior to contextual fear conditioning or administration of the CRF antisense oligonucleotide at different time points after contextual fear conditioning is effective at attenuating the acquisition and expression of the fear response (3, 13-15).
The mPFC is recognized as important in mediating learning, attention and emotional behavior (16-18). Rats given mPFC lesions prior to training express a stronger fear reaction than control rats (19). Immunocytochemical studies revealed that CRF was present in the mPFC and was expressed in glutamate decarboxylase-positive interneurons in the cerebral cortex (20-21). In situ hybridization studies showed that CRF1 receptors were found in large densities in the mPFC. Moreover, CRF injection into the mPFC increased anxiety-like behavior in the elevated plus maze in both acutely and repeatedly stressed animals compared to vehicle (16).
GABA is the main inhibitory neurotransmitter in the central nervous system. Its transmission in the amygdala is particularly important for controlling fear and anxiety levels (22). Clinical evidence suggests that alterations in normal GABA transmission might contribute to the pathophysiology of anxiety disorders in humans. For instance, various studies using nuclear imaging techniques have revealed diminished central GABA and GABA-A receptor activity in patients suffering from anxiety (panic disorder) and trauma/stress-related disorders (posttraumatic stress disorder) (22). GABA is synthesized from glutamate by the enzyme glutamate decarboxylase (GAD), which exists in two isoforms: GAD67 (thought be involved in GABA synthesis) and GAD65 (controls the synaptic release of GABA) (23-25). Deletion of the GAD67 gene in mice resulted in a > 90% reduction in the basal GABA levels in the brain, whereas GAD65-deleted homozygous mice expressed normal GABA levels (22, 26-27). The preclinical study indicated that alterations in the GAD67 levels might participate in the development and/or expression of symptoms associated with fear and anxiety (22).
The aim of this study was to examine the interaction between the CRF system and the inhibitory neurotransmitter (GABA) in the modulation of amygdala and prefrontal cortex activity in low- and high-anxiety rats. Recently, we studied the central mechanisms that are responsible for individual vulnerability to stressors by employing a model that divided rats into high-anxiety (HR) and low-anxiety (LR) groups (28-31). Our model is based on differences in the expression of a conditioned fear to the context. We did this on purpose, considering that in the clinic there occur different types of anxiety, e.g. panic fear, phobias or trauma (PTSD). Thus our model refers mainly to the post-traumatic stress disorder. Different types of anxiety and trauma/stress-related disorders are treated in a different way using pharmacological or psychotherapeutic methods. This indicates that the neurobiological mechanisms of various types of anxiety are different at the level of cortical and limbic structures, and only at the final point of execution of emotional reactions in the structures like the brainstem and the hypothalamus, they show a similar expression of behavioral and hormonal symptoms of fear. Consequently, both populations of animals should be defined as groups of HR and LR rats, in the model of a conditioned fear. The division of HR and LR rats is validated and justified by the results of many already published reports indicating a different reactivity of HR and LR rats in different models of chronic stress (immobilization stress, chronic corticosterone) (28-31), and their different reactivity to the ligands changing the activity of brain neurotransmitter systems which regulate emotional reactions (GABA, 5-HT, CRF, glutamate, glucocorticoid receptors) (32-34). Moreover, we demonstrated in a recent study that LR rats were more sensitive to re-exposure to fear stimuli and that midazolam pretreatment was associated with the attenuation of brain activity in the amygdala and prefrontal cortex (c-Fos and CRF immunocytochemistry) in this group of animals (35). Based on these data, in the present study, we tested the hypotheses that animals with different vulnerabilities to fear stimuli would be differentially sensitive to the effects of the non-peptide CRF1 antagonist (antalarmin) on amygdala-prefrontal cortex activity and fear expression and that these effects would be accompanied by changes in the local activity of the GABAergic system.
MATERIALS AND METHODS
Animals
The experiments were performed on 70 male Wistar rats (200 – 220 g body weight), purchased from a licensed breeder (The Center for Experimental Medicine of the Medical University, 24A Sklodowskiej-Curie Str., Bialystok, Poland) and housed under standard laboratory conditions with a 12 h light/dark cycle (lights on at 7 a.m.) at a constant temperature (21 ± 2°C). The rats were group-housed, 4 per cage in the polycarbonate cages (556 × 324 × 195 mm, floor area, 1875 cm2) containing bedding (Lignocel; Hygienic Animal Bedding; JRS GmbH + Co KG, Germany). Cages were cleaned and the bedding replaced twice a week.
The experiments were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609 EEC). The Local Committee for Animal Care and Use at the Warsaw Medical University, Poland approved all experimental procedures using animals.
Experimental protocol
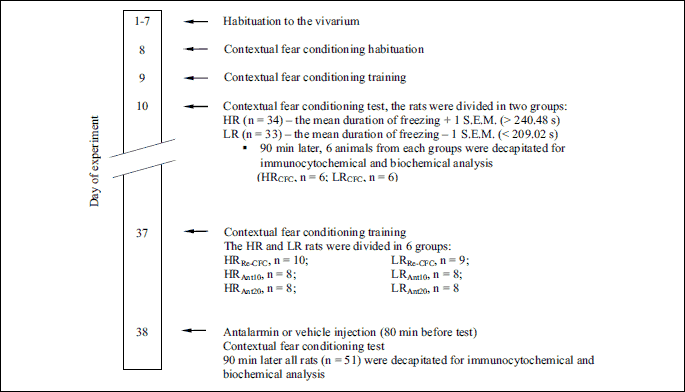
After seven days of acclimatization to the vivarium, the animals (n = 70) were subjected to a contextual fear-conditioning test to assess individual responses to conditioned aversive stimuli (28, 34). The rats were divided into low-anxiety (LR, n = 33) and high-anxiety (HR, n = 34) groups based on the duration of their conditioned freezing in a contextual fear test. Three rats did not meet either criterion. 90 min after exposure to the first aversive context, 6 animals from each group were decapitated (LRCFC - low anxiety animals, n = 6; HRCFC - high-anxiety animals, n = 6) for immunocytochemistry and biochemical analyses. The remaining animals (HR, n = 28 and LR, n = 27) were housed in their home cages for 28 days. Two rats from HR group and two rats from LR group were excluded from the study, because of their bad physical condition. Next, the HR (n = 26) and LR (n = 25) rats were randomly divided into six experimental groups as follows: LRRe-CFC - low anxiety animals pretreated with vehicle solution and conditioned for a second time to the aversive context (n = 9); LRAnt10 - low-anxiety rats administered antalarmin at a dose of 10 mg/kg and conditioned for a second time to the aversive context (n = 8); LRAnt20 - low-anxiety rats administered antalarmin at a dose of 20 mg/kg and conditioned for a second time to the aversive context (n = 8); HRRe-CFC - high-anxiety animals pretreated with vehicle solution and conditioned for a second time to the aversive context (n = 10); HRAnt10 - high-anxiety rats administered antalarmin at a dose of 10 mg/kg and conditioned for a second time to the aversive context (n = 8); and HRAnt20 - high-anxiety rats administered antalarmin at a dose of 20 mg/kg and conditioned for a second time to the aversive context (n = 8). Next, the animals were subjected to the contextual fear training and retested. The rats received antalarmin or vehicle injections 80 min before the second contextual fear test. The animals were decapitated ninety minutes after the second exposure to the aversive context (180 min after drug administration) (Fig. 1). Their brains were removed, frozen, and stored at –70°C for the immunocytochemistry (GAD67 expression) and biochemistry (GABA concentration) analyses (Fig. 2).
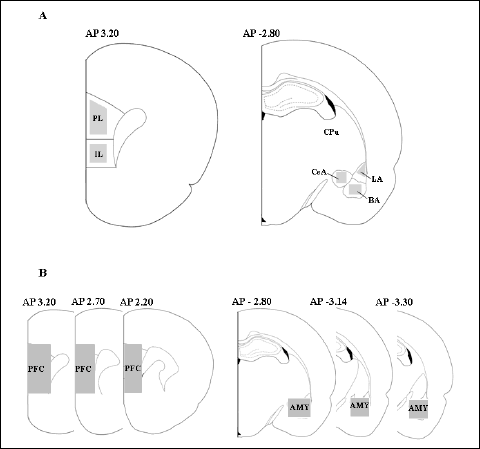 |
Fig. 2. (A) Schematic view of brain regions analyzed for immunocytochemistry. (B) Schematic view of brain regions used for biochemical study. The number indicates the distance from bregma (mm). AMY – amygdala complex, BA – basal nucleus of the amygdala, CeA – central nucleus of the amygdala, CPU – striatum, IL – infralimbic cortex, LA – lateral nucleus of the amygdala, PFC – prefrontal cortex, PL – prelimbic cortex. The marked areas indicated regions of tissue collections. |
Contextual fear-conditioning test
The fear-conditioning experiment was performed using a computerized fear-conditioning system (TSE, Bad Homburg, Germany; FCS 04.11) in a Plexiglas cage (36 × 21 × 20 cm, w × l × h) with a steel foot-shock grid (the 38 floor bars were 0.4 cm in diameter and spaced 0.5 cm apart) under constant white noise (65 dB) and constant illumination (12 V, 10 W halogen lamp, ~150 l×). Freezing behavior was recorded using an infrared photobeam system (10 Hz detection rate) controlled by the fear-conditioning system. The photobeams were spaced 1.3 cm in the direction of the x-axis and 2.5 cm in the direction of the y-axis. This method and equipment have been used in our and other laboratories for years and have been validated pharmacologically using many clinically effective and experimental anxiolytic and anxiogenic agents (36-37).
The total duration of inactivity was calculated by the fear-conditioning system. The total duration was defined as no interruption of any photobeam over a 5-s period; these periods were summarized for the entire experimental session to yield the total freezing time. The box was cleaned with 95% ethanol after each trial. The testing was performed from 8.30 to 12.00. The animals were transported from the vivarium to the experimental room in pairs, and 3 hours after the end of the experiment the rats returned to the vivarium. The experiment was performed on three consecutive days in the same testing box and experimental chamber. On the first day, the animals were placed separately for 2 min in a training box without aversive stimulation to adapt to the experimental conditions. On the second day (a training day), the animals were placed for 10 min in the training box. After 5 min of sitting undisturbed in the box, the rat received 4 footshocks (0.7 mA) delivered through the stainless steel floor grid, lasting for 1 s each, with 59 s breaks between stimuli, for final 5 min. The animals were removed from the testing boxes 1 min after the last shock was delivered. On the third experimental day, the freezing behavior of rats was observed for 10 min in the same box. The conditioned response (freezing reaction) was analyzed and recorded by the fear-conditioning system. The absolute duration of inactivity was calculated from the activity plots and expressed as the total time during which the animals were inactive. The computerized method is based on the latency between the photobeam interruption measures obtained during the contextual fear-conditioning tests, which is highly correlated with hand-scored freezing (r values ranged from 0.87 to 0.94) (38-39). The rats were divided into two experimental groups according to the duration of the context-induced freezing responses. The LR group had a total duration of freezing responses at least one S.E.M or more below the mean value (224.75 – 15.73, i.e., < 209.02 s). The HR group had a total duration of freezing responses at least one S.E.M or more above the mean value (224.75 + 15.73, i.e., > 240.48 s). The mean value of freezing for the LR group = 98.18 s and for HR group = 345.88 s. Three rats did not meet either criterion.
Drug treatment
Antalarmin hydrochloride (N-butyl-N-ethyl-2,5,6-trimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine hydrochloride, Tocris Bioscience, Bristol, United Kingdom) was suspended in a vehicle composed of 10% Tween 80 and distilled water and administered intraperitoneally (i.p.) in a 1 ml/kg injection volume. The injection doses and timing were selected based on previous studies (40-41). For vehicle injection, 10% Tween 80 alone was administered in the same volume.
Immunocytochemistry of GAD67
The immunocytochemical reaction was performed on slide-mounted frozen brain sections. Based on the atlas of Paxinos and Watson (42), coronal 15 µm cryostat slices were cut, mounted on silane-coated slides and fixed in cold methanol for 10 min. In the study, three slices from each section per animal were taken for immunostaining (Fig. 2A), and the rest of the tissue samples were used for biochemical analysis of the GABA concentration (see below) (Fig. 2B). The specimens were washed twice (2 × 15 min) in 0.01 M PBS solution (pH 7.4), incubated in a 3% hydrogen peroxide (H2O2) solution for 30 min to block the activity of endogenous peroxidase, washed twice again (2 × 15 min) in 0.01 M PBS and incubated in a 3% normal horse serum blocking solution. Subsequently, the slide-mounted brain sections were incubated with a rabbit polyclonal antibody directed against GAD67 (1:200, Santa Cruz) at 4 – 8°C for 72 h. Following incubation, the slides were washed in 0.01 M PBS three times (3 × 15 min) and detected with peroxidase-conjugated anti-rabbit IgG (1:1000, ImmunoJackson Research). The peroxidase reaction was developed with DAB (0.2 mg/ml) and hydrogen peroxide (0.003%) in Tris buffer. The sections were dehydrated by serial alcohol rinsing, cleared in xylene, and coverslipped in a histofluid mounting medium. Western blotting analysis confirmed the specific binding of the antibodies.
Cells counts were assessed by light microscopy (Olympus BX-51 light microscope, DP-70 digital camera) at a total magnification of ×100. The number of positive cells was counted with a computerized image analysis system (Olympus CellSens software, Munster, Germany) in the following subregions: AP 3.20: infralimbic cortex (IL) and prelimbic cortex (PL) and AP(–) 2.80: basal nucleus of the amygdala (BA), central nucleus of the amygdala (CeA), and lateral nucleus of the amygdala (LA) (42). The total number of positive cells was manually counted for each region of each rat brain as shown as Fig. 2A and expressed as the number of positive nuclei per 1 mm2. An independent researcher blinded to the groups to which the rats had been assigned performed the analysis.
Biochemical analysis of the GABA concentration
After serial sections were cut for the immunocytochemistry analysis, the anatomical structures of the cortex (bregma 3.20 – 2.20) and the amygdala (bregma –2.80 – –3.30) were micropunched under a dissecting microscope as shown as Fig. 2B. Each tissue was weighed (the average weight was 11 mg), placed in a dry ice-cooled polypropylene vial, and homogenized with a polytron-type homogenizer (30 s, 4°C) in a solution containing perchloric acid (0.2 M). The homogenates were centrifuged (26,880 × g at 4°C for 8 min) and the supernatants were filtered through Syringe Driven Filter Units (Millipore) prior to the analysis.
HPLC analysis of GABA was performed using a Luna 5 µm C18(2) 100A (250 × 4.6 mm) reverse phase column according to the previously described procedure (43). The compounds were eluted isocratically with the mobile phase delivered at 0.70 ml/min using a Shimadzu Clas VP LC 10AD pump. An electrochemical detector with a flow-through cell (Intro-AntecLeyden) linked to the Shimadzu Class VP Integrator SCL-10 Avp was used. A high-density glass carbon-working electrode (Antec) was operated at +0.85 V. A rheodyne injection valve with a 20-µl sample loop was used to manually inject the samples. The preparation of the mobile phase and the derivatizing agents were based on the method of Rowley et al. (44) with some modifications. The mobile phase consisted of 45 mM disodium phosphate and 0.15 mM ethylenediaminetetraacetic acid (EDTA) with 24% methanol (v/v) in water adjusted to pH 3.9 with 0.2 M citric acid. Then, the mobile phase was filtered through a 0.45 µm filter and degassed for 15 min. A stock solution (0.01 M) of the GABA standard was prepared in double-deionized water and kept at 4°C for five days. The standard was prepared in polyethylene vials to prevent adhesion to the glass. Working solutions were prepared daily by diluting the stock solution. To obtain agents for derivatization, o-phthaldialdehyde (OPA, 22 mg, Fluka) was dissolved in 0.5 ml of 1 M sodium sulfite, 0.5 ml of methanol, and 0.9 ml of sodium tetraborate buffer (0.1 M) adjusted to pH 10.4 with 5 M sodium hydroxide. The derivatization reaction was performed at room temperature. The derivatizing agent (20 µl) was reacted with 1 ml of the GABA standard for 15 min in a polyethylene vial prior to injection onto the column. The GABA concentration was calculated in µM.
Statistical analysis
The data are shown as the means and standard errors of the mean (S.E.M). To verify the differences between the HR and LR groups in the contextual fear-conditioning test, we used Student’s t test. In the first part of the study, we performed the analysis for the first and second contextual fear conditioning test to determine how fear re-conditioning affected the behavior and local GABA activity in the HR and LR rats. In the second part of the study, we performed the analysis to determine how antalarmin administration modulated the behavioral and biochemical activity after the second contextual fear test in the HR and LR rats. In the analysis of the different brain structures, the number of the analyzed animals may be different from the number of animals in the test group, because for technical reasons some brain sections slices were lost. The data were analyzed by two-way ANOVA followed by the most conservative Tukey’s post hoc test. A probability value of P < 0.05 was considered significant in this study. The statistical analyses were performed using Stat-Soft Statistica 12.0 for Windows (StatSoft Inc., USA).
RESULTS
Contextual fear-conditioning test
Student’s t-test did not revealed a significantly differences between LR and HR groups in the 5 min pre-shock period (t = 0.04, df = 65, P > 0.1) (Fig. 3A). Student’s t-test revealed a significantly weaker freezing response in the LR group compared to the HR group (t = 15.70, df = 65, P < 0.01) (Fig. 3B).
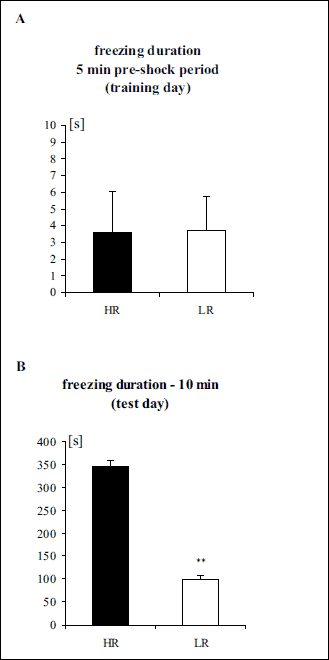 |
Fig. 3. (A) Freezing duration in the 5 min pre-shock period in the contextual fear test (training day). (B) Freezing duration in the contextual fear test (10 min – test day). The data are shown as the means + S.E.M. HR – high-anxiety rats (n = 34), LR – low-anxiety rats (n = 33). **P < 0.01, differs from HR. For more details, see the experimental procedure. |
The effects of fear re-conditioning
1. Contextual fear-conditioning test
Two-way ANOVA revealed significant differences in the freezing durations between the experimental groups (LRCFC, LRRe-CFC, HRCFC, HRRe-CFC): group effect [F(1,27) = 35.95 (P < 0.01)], fear effect [F(1,27) = 14.48 (P < 0.01)], and group x fear interaction effect [F(1,27) = 4.51 (P < 0.05)]. Tukey’s post hoc test revealed a lower freezing duration in the LRCFC group compared with the HRCFC group (P < 0.01) and in the LRRe-CFC group compared with the HRRe–CFC group (P < 0.05). The post hoc test also indicated a much higher freezing duration in the LRRe–CFC group compared with the LRCFC group (P<0.01) (Fig. 4A).
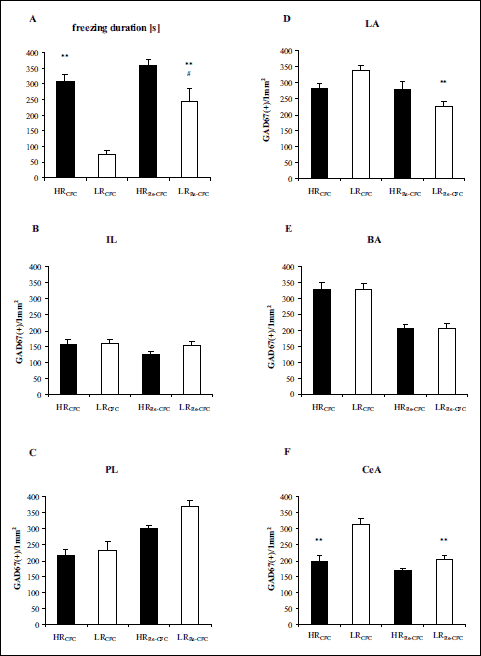 |
Fig. 4. (A) Freezing duration in the contextual fear test after the first and second contextual fear test. (B-F) GAD67 expression 90 min after the first and second contextual fear test. The data show the number of immunoreactive neurons per 1 mm2. BA – basal nucleus of the amygdala, CeA – central nucleus of the amygdala, IL – infralimbic cortex, LA – lateral nucleus of the amygdala, PL – prelimbic cortex. HRCFC – high-anxiety animals after the first contextual fear test, n = 6 (A-F); LRCFC – low anxiety animals after the first contextual fear test, n = 6 (A-F); HRRe-CFC – high-anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context, n = 8 (F), n = 9 (C, E), n = 10 (A, B, D); LRRe-CFC – low anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context, n = 9 (A-F). The data are shown as the means + S.E.M. **P < 0.01, differs from LRCFC; #P < 0.05, differs from HRRe-CFC. For more details, see the experimental procedure. |
2. GAD67 expression
In the IL, two-way ANOVA did not reveal significant differences between the experimental groups (LRCFC, LRRe-CFC, HRCFC, HRRe-CFC): no group effect [F(1,27) = 1.08 (P > 0.1)], no fear effect [F(1,27) = 2.04 (P > 0.1)], and no group × fear interaction effect [F(1,27) = 0.75 (P > 0.1)] (Fig. 4B).
In the PL, two-way ANOVA revealed significant differences between groups (LRCFC, LRRe-CFC, HRCFC, HRRe-CFC): group effect [F(1,26) = 4.76 (P < 0.05)], fear effect [F(1,26) = 31.81 (P < 0.01)], but no group × fear interaction effect [F(1,26) = 1.72 (P > 0.1)] (Fig. 4C).
In the LA, two-way ANOVA revealed significant differences between groups (LRCFC, LRRe-CFC, HRCFC, HRRe-CFC): fear effect [F(1,27) = 8.17 (P < 0.01)], group × fear interaction effect [F(1,27) = 7.57 (P < 0.05)], and no group effect [F(1,27) = 0.01 (P > 0.1)]. Tukey’s post hoc test revealed a decrease in the number of GAD67-positive nuclei in the LRRe-CFC group compared with the LRCFC group (P < 0.01) (Fig. 4D).
In the BA, two-way ANOVA revealed significant differences between groups (LRCFC, LRRe-CFC, HRCFC, HRRe-CFC): fear effect [F(1,26) = 52.38 (P < 0.01)] but no group effect [F(1,26) = 0.06 (P > 0.1)] and no group × fear interaction effect [F(1,26) = 0.03 (P > 0.1)] (Fig. 4E).
In the CeA, two-way ANOVA revealed significant differences between groups (LRCFC, LRRe-CFC, HRCFC, HRRe-CFC): group effect [F(1,25) = 30.11 (P < 0.01)], fear effect [F(1,25) = 25.12 (P < 0.01)], and group × fear interaction effect [F(1,25) = 7.97 (P < 0.01)]. Tukey’s post hoc test revealed lower GAD67 expression in the HRCFC group than in the LRCFC group (P < 0.01) and in the LRRe-CFC group than in the LRCFC group (P < 0.01) (Fig. 4F).
The effects of antalarmin pretreatment
1. Contextual fear-conditioning test
Two-way ANOVA revealed significant differences in the freezing duration between the experimental groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,45) = 11.28 (P < 0.01)], group × drug interaction effect [F(1,45) = 7.64 (P < 0.01)], but no group effect [F(1,45) = 1.30 (P > 0.1)]. Tukey’s post hoc test revealed a lower freezing duration in the HRAnt10 group compared with the HRRe-CFC group (P < 0.01), and in the HRAnt20 group compared with the HRRe-CFC group (P < 0.05) (Fig. 5A).
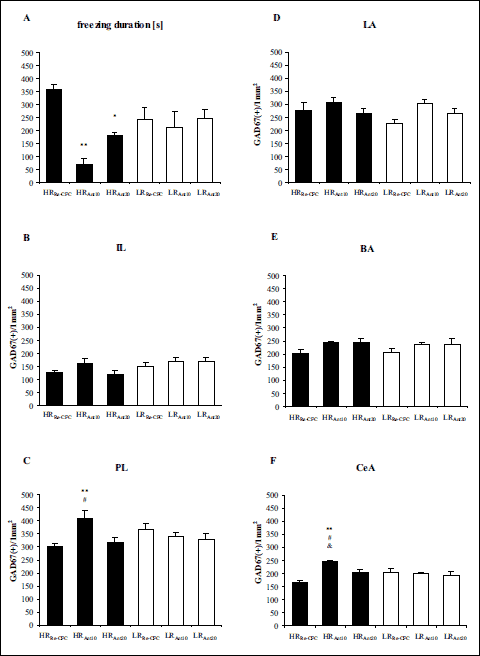 |
Fig. 5. (A) The influence of antalarmin pretreatment on rat behavioral in the second conditioned fear test. (B-F) GAD67 expression 180 min after antalarmin administration and 90 min after exposure to the conditioning boxes. The data show the number of immunoreactive neurons per 1 mm2. BA – basal nucleus of the amygdala, CeA – central nucleus of the amygdala, IL – infralimbic cortex, LA – lateral nucleus of the amygdala, PL – prelimbic cortex. HRRe-CFC – high-anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context, n = 8 (F), n = 9 (C, E), n = 10 (A, B, D); HRAnt10 – high-anxiety rats administered with antalarmin at a dose of 10 mg/kg, and conditioned for a second time to the aversive context, n = 8 (A-F); HRAnt20 – high-anxiety rats administered with antalarmin at a dose of 20 mg/kg, and conditioned for a second time to the aversive context, n = 8 (A-F); LRRe-CFC – low anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context, n = 9 (A-F); LRAnt10 – low-anxiety rats administered with antalarmin at a dose of 10 mg/kg, and conditioned for a second time to the aversive context, n = 7 (F), n = 8 (A-E); LRAnt20 – low-anxiety rats administered with antalarmin at a dose of 20 mg/kg, and conditioned for a second time to the aversive context, n = 7 (B-F), n = 8 (A). The data are shown as the means + S.E.M. *P < 0.05, **P < 0.01, differs from HRRe-CFC; #P < 0.05, differs from HRAnt20; &P < 0.05, differs from LRAnt10. For more details, see the experimental procedure. |
2. GAD67 expression
In the IL, two-way ANOVA revealed significant differences between groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): group effect [F(1,44) = 4.99 (P < 0.05)], but no drug effect [F(1,44) = 1.61 (P > 0.1)], and no group × drug interaction effect [F(1,44) = 0.96 (P > 0.1)] (Fig. 5B).
In the PL, two-way ANOVA revealed significant differences between groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,43) = 3.25 (P < 0.05)], group × drug interaction effect [F(1,43) = 6.09 (P < 0.01)], but no group effect [F(1,43) = 0.02 (P > 0.1)]. Tukey’s post hoc revealed higher GAD67 expression in the HRAnt10 group compared with the HRRe-CFC and HRAnt20 groups (P < 0.01 and P < 0.05, respectively) (Fig. 5C).
In the LA, two-way ANOVA revealed significant differences between groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,44) = 3.74 (P < 0.05)], but no group effect [F(1,44) = 1.17 (P > 0.1)] and no group × drug interaction effect [F(1,44) = 1.12 (P > 0.1)] (Fig. 5D).
In the BA, two-way ANOVA revealed significant differences between groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,43) = 3.94 (P < 0.05)], but no group effect [F(1,43) = 0.14 (P > 0.1)] and no group × drug interaction effect [F(1,43) = 0.06 (P > 0.1)] (Fig. 5E).
In the CeA, two-way ANOVA revealed significant differences between groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,41) = 7.31 (P < 0.01)], group × drug interaction effect [F(1,41) = 9.93 (P < 0.01)], but no group effect [F(1,41) = 0.83 (P > 0.1)]. Tukey’s post hoc test revealed higher GAD67 expression in the HRAnt10 group compared with the HRRe-CFC (P < 0.01), LRAnt10 (P < 0.05), and HRAnt20 (P < 0.05) groups (Fig. 5F and 6).
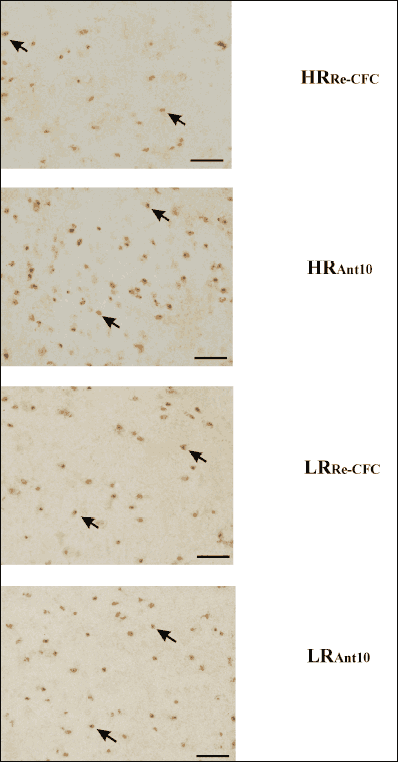 |
Fig. 6. Photomicrographs showing representative expression of GAD67 in the central nucleus of the amygdala. Slices were photographed with an objective lens at 20 × magnification (total magnification ×200). Scale bar indicates 75 µm. The arrow heads show representative immunopositive cells. HRRe-CFC – high-anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context (n = 8); HRAnt10 – high-anxiety rats administered with antalarmin at a dose of 10 mg/kg, and conditioned for a second time to the aversive context (n = 8); LRRe-CFC – low anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context (n = 9); LRAnt10 – low-anxiety rats administered with antalarmin at a dose of 10 mg/kg, and conditioned for a second time to the aversive context (n = 7). |
3. GABA concentration
In the cortex, two-way ANOVA revealed significant differences between groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,37) = 15.87 (P < 0.01)] but no group effect [F(1,37) = 0.01 (P > 0.1)] and no group × drug interaction effect [F(1,37) = 2.40 (P > 0.1)] (Fig. 7A).
In the amygdala, two-way ANOVA revealed significant differences between the experimental groups (LRRe-CFC, LRAnt10, LRAnt20, HRRe-CFC, HRAnt10, HRAnt20): drug effect [F(1,39) = 6.22 (P < 0.01)], group × drug interaction effect [F(1,39) = 3.25 (P < 0.05)], but no group effect [F(1,39) = 2.17 (P > 0.1)]. Tukey’s post hoc test revealed a higher GABA concentration in the HRAnt10 group compared with the HRRe-CFC group (P < 0.01) (Fig. 7B).
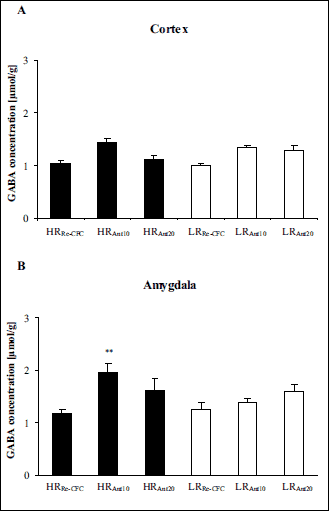 |
Fig. 7. GABA concentration in the cortex (A) and the amygdala (B) 180 min after antalarmin administration and 90 min after exposure to the conditioning boxes. The data are shown as the means + S.E.M. HRRe-CFC – high-anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context, n = 8 (A-B); HRAnt10 – high-anxiety rats administered with antalarmin at a dose of 10 mg/kg, and conditioned for a second time to the aversive context, n = 7 (A), n = 8 (B); HRAnt20 – high-anxiety rats administered with antalarmin at a dose of 20 mg/kg, and conditioned for a second time to the aversive context, n = 7 (A-B); LRRe-CFC – low anxiety animals pretreated with vehicle solution, and conditioned for a second time to the aversive context, n = 7 (A), n = 8 (B); LRAnt10 – low-anxiety rats administered with antalarmin at a dose of 10 mg/kg, and conditioned for a second time to the aversive context, n = 7 (A-B); LRAnt20 – low-anxiety rats administered with antalarmin at a dose of 20 mg/kg, and conditioned for a second time to the aversive context, n = 7 (A-B). **P < 0.01, differs from HRRe-CFC. For more details, see the experimental procedure. |
DISCUSSION
In the present study, we found that fear re-conditioning increased the freezing duration compared with the first conditioned fear response in the LR group, similar to our previous report (Fig. 4) (35). However, the behavioral response of these animals remained lower compared with the HR group. The behavioral changes in the LRRe-CFC rats were accompanied by decreased GAD67 expression in the LA and CeA compared with the LRCFC group. Pretreatment of the rats with antalarmin (10 mg/kg or 20 mg/kg), prior to the second exposure to the aversive context, decreased the conditioned fear response only in the HR group (Fig. 5). The behavioral effect of the lower dose (10 mg/kg) of antalarmin was accompanied by increased the GAD67 expression in the PL and CeA (Fig. 5) and GABA concentration in the amygdala, in the HR group (Fig. 7).
The effect of fear re-conditioning on rat behavior and GABAergic system activity in the amygdala of the high- and low-anxiety rats
Fear re-conditioning significantly increased the freezing duration in the LR rats compared with the first conditioned fear response. In the HR rats, the second exposure to the aversive context also increased the freezing duration, albeit not significantly (the mean value of the freezing duration for the HRCFC group = 310.00 s, and for the HRRe-CFC group = 359.10 s). This effect confirms the result of our previous study that the LR rats appeared to be more sensitive to re-exposure to the contextual fear stimuli. In this group of animals, the fear re-conditioning increased neuronal activity in the amygdala and decreased neuronal activity in the prefrontal cortex (c-Fos and CRF immunocytochemistry) (35). In the current study, we showed that a second exposure to the aversive context also decreased GABAergic neurotransmission (measured by GAD67 expression) in the LA and CeA in the LR rats.
Our findings are consistent with other data indicating the important role of amygdala GABA innervation in fear memory processing. It was found that, during acquisition and consolidation of fear memory, fear conditioning decreases the extracellular GABA levels in the BLA and reduces the mRNA level of the GABA-synthesizing enzyme GAD67 in the amygdala (2, 45-46). Reduced GAD67 expression during acquisition may be associated with a loss of inhibitory control of the amygdala, thereby contributing to the hyperactivity or prolonged activation of this limbic nucleus (45, 47). Accordingly, in the current study a stronger fear reaction after the second exposure to the aversive context in the LR rats could be due to a decrease in the GABAergic activity (represented by a decrease GAD67 expression) in the amygdala, leading to the disinhibition of amygdala-related processes and enhancement of the fear reaction. This hypothesis is substantiated by our earlier findings that fear re-conditioning induces an increase in the freezing duration that is accompanied by an increase in amygdala activity (measured by c-Fos and CRF expression) and weaker prefrontal cortex activity (measured by CRF expression) in both the HR and LR groups. However, these effects were significantly stronger in the LR group (35).
The effect of antalarmin on the fear response and GABAergic system activity in the amygdala of the high- and low-anxiety rats
The CRF plays an important role in modulating the activity of the brain structures involved in fear learning and fear expression. Exposure to footshock increases CRF expression in the amygdala of male rats (48). This increase may be critical for fear conditioning because reducing the effects of CRF in both the BLA (BA and LA) and the CeA disrupts the consolidation or stabilization of fear memories in male rats (12, 14). Compelling evidence indicates that CRF activation of the CRF1 receptor is sufficient and in many cases necessary to initiate an anxiety-like response (7-9). The anxiogenic character of the CRF1 receptor ligands was verified by the consistent anxiolytic effects of peptide or non-peptide CRF1 receptor antagonists (49). For example, pharmacological blockade at the CRF1 receptor by the nonpeptide corticotropin-releasing factor antagonist antalarmin produced anxiolytic-like effects in animal models of anxiety, including a blockade of the anxiogenic-like effects of CRF in the elevated plus maze test (10 and 20 mg/kg), impaired induction and expression of conditioned fear (20 mg/kg), and reduced burying behavior in rats (10 and 20 mg/kg) (11, 41, 50, 51). In agreement with these findings, in the present study the pretreatment of rats with antalarmin (10 mg/kg and 20 mg/kg) prior to the second exposure to the aversive context inhibited the conditioned fear response in the HR group. We did not observe any significant inhibitory effects of antalarmin in the LR rats. Similar results were presented by Keck et al. (52). In this report, the anxiolytic-like effects of a different non-peptide CRF1 receptor antagonist (R121919) were found to depend on the level of innate emotionality in the rats. The authors found that R121919 displayed anxiolytic effects in the elevated plus maze only in rats selectively bred for high anxiety-like behavior (HAB rats) and had no anxiolytic effects in rats selectively bred for low anxiety-like behavior (LAB). Similarly, Rotzinger et al. (49) suggested that the effects of a CRF1 receptor antagonist in animal models of anxiety were dependent upon the baseline anxiety state of the animal and the test parameters (49, 52).
In the present study, the behavioral effect of a lower dose of antalarmin was accompanied by increased GAD67 expression in the PL and CeA and increased the GABA concentration in the amygdala in the HR rats. The PL, which is a subregion of the medial prefrontal cortex, seems to be critical for the expression of fear-related behavior (18, 53-54). PL activity increases during and following fear conditioning (17, 55). Additionally, PL has a reciprocal connection with the amygdala, especially with the BA. Subsequently, augmented BA activity mediated through the PL is a necessary condition to activate CeA output neurons, which results in fear responses (53-54). Thus, the antalarmin treatment-related increase in GAD67 activity in the PL of HR rats via the enhancement of local GABA synthesis might diminish the activity of this important neuronal loop for the expression of fear.
Pretreatment of the HR rats with antalarmin enhanced GABAergic neurotransmission (shown by the increased GAD67 expression) in the CeA. Similar results were observed in our previous study, where the non-selective CRF receptor antagonist a-helical CRF(9-41) significantly decreased the rat freezing responses and increased the GABA concentration in the CeA during the first 30 min of observation (microdialysis) (56). The CeA is largely GABAergic, receives glutamatergic projections from the LA and expresses a wide variety of neuropeptides (CRF, vasopressin, neuropeptide Y, and oxytocin). The role of all these peptides in regulating of anxiety-related behavior has been suggested (2, 57-58). We can not exclude that antalarmin could disinhibit the activity of other neuropeptide systems found in the CeA by blocking the action of CRF, thereby contributing to the anxiolytic effect. For example, Huber et al. (58) demonstrated that oxytocin, which is a neuropeptide with a strong anxiolytic potency, excited a subpopulation of GABAergic neurons in the CeA. When activated by oxytocin, these neurons exerted tonic inhibition by reducing the excitability of CeA neurons (2, 58).
Some data from the literature indicate that CRF has a stimulating effect on GABAergic activity in the amygdala (10, 59). However, these data are limited and significantly differ methodologically from our study. For example, Nie et al. (10) based their conclusions solely on the analysis of electrophysiological changes in IPSC (inhibitory postsynaptic current) amplitudes in CRF1 and CRF2 receptor knock-out mice. Roberto et al. (59) analyzed the effect of a different CRF1 antagonist (R121919) on the ethanol-induced release of GABA in the CeA in vivo. However, these authors did not observe any effect of this CRF1 receptor antagonist on the basal release of GABA. The mechanisms underlying the effect of CRF antagonists on the GABA system require further analysis. The possibility that this effect is indirect and secondary to the influence of the CRF1 receptor antagonist on the equilibrium between the other neurotransmitter systems present in the prefrontal cortex and amygdala also cannot be excluded. As mentioned earlier, CRF is expressed in GAD-positive interneurons in the cerebral cortex (20-21). Another point is that fear conditioned context may stimulate corticosterone secretion, which has actions in the amygdala not only inhibiting GABA release, but also facilitating glutamate release (32, 60-62). The effects of CRF1 antagonist could be linked to these activities, as shown by a number of other publications (51, 63-65), however, more accurate discussion of this topic is beyond the scope of our work.
The effects of a lower dose (10 mg/kg) of antalarmin were more potent than the effects of the higher dose (20 mg/kg) of the antagonist. Accordingly, Heinrichs et al. (66) found that only the lowest dose (1 µg, i.c.v.) of α-helical CRF9-41 tested was effective at blocking the stress-induced decrease in exploration on the elevated plus maze test, whereas higher doses (5 and 25 µg) were ineffective (49, 66). Although there are some important differences between the experimental protocols of Heinrichs et al. (66) report and our study (e.g., administration of a different CRF1 receptor antagonist and a different type of aversive stimulation), these results indicate a non-linear dose response curve of the CRF receptor antagonists effects. It is widely accepted in the pharmacological sciences that a lower dose of a drug is more selective than a higher dose. Therefore, the higher doses of antalarmin could activate other receptors (e.g., alpha-2 and beta-2 adrenergic, kappa opioid, cholecystokinin B, and D2 receptor) and impair the selectivity of the drug action through these interactions (67-71).
It is noteworthy, that the CRF1 receptors are expressed in numerous extrahypothalamic brain regions including the ventral tegmental area (VTA) a structure that appears important for aversive learning (72-73). The recent study showed that intra-VTA injection of a lentivirus against CRF1 mRNA did not affect tone-elicited freezing during conditioning but increased freezing duration to the tone even after extinction and reinstatement (72). This study demonstrated that CRF1 receptors located in the VTA also play an important role in the conditioned fear. Another structures of the brain, which could contribute to the effects of CRF antagonist, may involve the central serotonin system (74), as well as CRF receptors within the periaqueductal gray matter, regulating pain responses (75).
In summary, this study shows that the LR rats appear to be more sensitive to the second exposure to contextual fear stimuli. This phenomenon was accompanied by increased neuronal activity in the amygdala. Furthermore, this study demonstrates that HR rats are more sensitive to the anxiolytic effects of acute antalarmin administration, which are accompanied by changes in CRF-GABA system activity. In this group of animals, antalarmin administration enhanced GABA synthesis and the GABA concentration in the medial prefrontal cortex and amygdala. These results indicate that the fear responses in the HR rats may be regulated by innate and individually variable changes in the activity of the local CRF and GABAergic systems. The current data may help increase our understanding of the neurobiological mechanism controlling the CRF-GABA interaction within the prefrontal cortex-amygdala circuitry, which is responsible for individual differences in reactivity to stressors. This knowledge can be applied to elucidate the pathophysiology of the predisposition to anxiety and trauma/stress-related disorders.
Acknowledgments: The study was supported by Grant No. 501-003-17017 from the Institute of Psychiatry and Neurology in Warsaw and Grant No. 2014/15/B/NZ4/05305 from the National Science Centre, Poland. The authors thank Mrs. Ala Biegaj for the excellent technical assistance.
Conflict of interests: None declared.
REFERENCES
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron 2014; 82: 966-880.
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009; 62: 757-771.
- Gafford GM, Ressler KJ. GABA and NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Horm Behav 2015; 76: 136-142.
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155-184.
- Johansen JP, Hamanaka H, Monfils MH, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA 2010; 107: 12692-12697.
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 1995; 15: 2301-2311.
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann NY Acad Sci 2009; 1179: 120-143.
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 1999: 848: 141-152.
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2002: 2: 23-33.
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 2004; 303: 1512-1514.
- Chen X, Li S, Kirouac GJ. Blocking of corticotrophin releasing factor receptor-1 during footshock attenuates context fear but not the upregulation of prepro-orexin mRNA in rats. Pharmacol Biochem Behav 2014; 120: 1-6.
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience 2007; 150: 818-828.
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci 2009; 29: 7379-7388.
- Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Mem 2011; 95: 86-91.
- Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res 1993; 623: 229-234.
- Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res 2007; 1186: 212-223.
- Likhtik E, Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci 2015; 38: 158-166.
- Maroun M. Medial prefrontal cortex: multiple roles in fear and extinction. Neuroscientist 2013; 19: 370-383.
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res 2003; 146: 121-130.
- Ohata H, Shibasaki T. Microinjection of different doses of corticotropin-releasing factor into the medial prefrontal cortex produces effects opposing anxiety-related behavior in rats. J Nippon Med Sch 2011; 78: 286-292.
- Yan XX, Baram TZ, Gerth A, Schultz L, Ribak CE. Co-localization of corticotropin-releasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons. Exp Brain Res 1998; 123: 334-340.
- Heldt SA, Mou L, Ressler KJ. in vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry 2012; 2: e181. doi: 10.1038/tp.2012.101
- Lussier AL, Romay-Tallon R, Caruncho HJ, Kalynchuk LE. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience 2013; 231: 38-48.
- Namchuk M, Lindsay L, Turck CW, Kanaani J, Baekkeskov S. Phosphorylation of serine residues 3, 6, 10, and 13 distinguishes membrane anchored from soluble glutamic acid decarboxylase 65 and is restricted to glutamic acid decarboxylase 65alpha. J Biol Chem 1997; 272: 1548-1557.
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci USA 1999; 96: 12911-12916.
- Asada H, Kawamura Y, Maruyama K, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun 1996; 229: 891-895.
- Condie BG, Bain G, Gottlieb DI, Capecchi MR. Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proc Natl Acad Sci USA 1997; 94: 11451-11455.
- Skorzewska A, Lehner M, Wislowska-Stanek A, Krzascik P, Ziemba A, Plaznik A. The effect of chronic administration of corticosterone on anxiety- and depression-like behavior and the expression of GABA-A receptor alpha-2 subunits in brain structures of low- and high-anxiety rats. Horm Behav 2014; 65: 6-13.
- Skorzewska A, Lehner M, Wislowska-Stanek A, et al. GABAergic control of the activity of the central nucleus of the amygdala in low- and high-anxiety rats. Neuropharmacology 2015; 99: 566-576.
- Wislowska-Stanek A, Lehner M, Skorzewska A, et al. Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in low- and high-anxiety rats. Behav Brain Res 2013; 253: 337-345.
- Wislowska-Stanek A, Lehner M, Skorzewska A, Krzascik P, Plaznik A. Behavioral effects and CRF expression in brain structures of high- and low-anxiety rats after chronic restraint stress. Behav Brain Res 2016; 310: 26-35.
- Lehner M, Taracha E, Skorzewska A, et al. Expression of c-Fos and CRF in the brains of rats differing in the strength of a fear response. Behav Brain Res 2008; 188: 154-167.
- Lehner M, Taracha E, Maciejak P, et al. Colocalisation of c-Fos and glucocorticoid receptor as well as of 5-HT(1A) and glucocorticoid receptor immunoreactivity-expressing cells in the brain structures of low and high anxiety rats. Behav Brain Res 2009; 200: 150-159.
- Lehner M, Wislowska-Stanek A, Skorzewska A, et al. Differences in the density of GABA-A receptor alpha-2 subunits and gephyrin in brain structures of rats selected for low and high anxiety in basal and fear-stimulated conditions, in a model of contextual fear conditioning. Neurobiol Learn Mem 2010; 94: 499-508.
- Skorzewska A, Lehner M, Wislowska-Stanek A, et al. Midazolam treatment before re-exposure to contextual fear reduces freezing behavior and amygdala activity differentially in high- and low-anxiety rats. Pharmacol Biochem Behav 2015; 129: 34-44.
- Maciejak P, Lehner M, Turzynska D, et al. The opposite role of hippocampal mGluR1 in fear conditioning in kindled and non-kindled rats. Brain Res 2008; 1187: 184-193.
- Stiedl O, Birkenfeld K, Palve M, Spiess J. Impairment of conditioned contextual fear of C57BL/6J mice by intracerebral injections of the NMDA receptor antagonist APV. Behav Brain Res 2000; 116: 157-168.
- Takahashi H. Automated measurement of freezing time to contextual and auditory cues in fear conditioning as a simple screening method to assess learning and memory abilities in rats. J Toxicol Sci 2004; 29: 53-61.
- Valentinuzzi VS, Kolker DE, Vitaterna MH, et al. Automated measurement of mouse freezing behavior and its use for quantitative trait locus analysis of contextual fear conditioning in (BALB/cJ x C57BL/6J)F2 mice. Learn Mem 1998; 5: 391-403.
- Cippitelli A, Damadzic R, Singley E, et al. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav 2012; 100: 522-529.
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res 2002; 952: 188-199.
- Paxinos G, Watson CH. The Rat Brain in Stereotaxic Coordinates. Academic Press Inc., San Diego CA. 1998.
- Szyndler J, Piechal A, Blecharz-Klin K, et al. Effect of kindler seizures on rat behavior in water Morris maze test and amino acid concentrations in brain structures. Pharmacol Rep 2006; 58: 75-82.
- Rowley HL, Martin KF, Marsden CA. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods 1995; 57: 93-99.
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci 2007; 26: 3631-3644.
- Stork O, Ji FY, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci Lett 2002; 327: 138-142.
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 1998; 19: 500-505.
- Yamano Y, Yoshioka M, Toda Y, et al. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci 2004; 66: 1323-1327.
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 2010; 31: 736-756.
- Cippitelli A, Ayanwuyi LO, Barbier E, et al. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology (Berl) 2015; 232: 1083-1093.
- Deak T, Nguyen KT, Ehrlich AL, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology 1999; 140: 79-86.
- Keck ME, Welt T, Wigger A, et al. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001; 13: 373-380.
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci 2015; 9: 298.
- Lee S, Kim SJ, Kwon OB, Lee JH, Kim JH. Inhibitory networks of the amygdala for emotional memory. Front Neural Circuits 2013; 7: 129.
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 2007; 27: 840-844.
- Skorzewska A, Bidzinski A, Hamed A, et al. The effect of CRF and alpha-helical CRF(9-41) on rat fear responses and amino acids release in the central nucleus of the amygdala. Neuropharmacology 2009; 57: 148-156.
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol 2012; 46: 329-337.
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005; 308: 245-248.
- Roberto M, Cruz MT, Gilpin NW, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 2010; 67: 831-839.
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2011; 13: 22-37.
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci 1997; 111: 503-511.
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci 2007; 25: 3109-3114.
- Asok A, Schulkin J, Rosen JB. Corticotropin releasing factor type-1 receptor antagonism in the dorsolateral bed nucleus of the stria terminalis disrupts contextually conditioned fear, but not unconditioned fear to a predator odor. Psychoneuroendocrinology 2016; 70: 17-24.
- Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp Neurol 2013; 239: 120-132.
- Cratty MS, Birkle DL. N-methyl-D-aspartate (NMDA)-mediated corticotropin-releasing factor (CRF) release in cultured rat amygdala neurons. Peptides 1999; 20: 93-100.
- Heinrichs SC, Menzaghi F, Pich EM, et al. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology 1994; 11: 179-186.
- Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur J Neurosci 2006; 23: 3319-3327.
- Funk D, Coen K, Le AD. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav 2014; 4: 356-367.
- Marinelli PW, Funk D, Juzytsch W, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007; 195: 345-355.
- Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J Neurosci 2014; 34: 12504-12514.
- Wang H, Spiess J, Wong PT, Zhu YZ. Blockade of CRF1 and CCK2 receptors attenuated the elevated anxiety-like behavior induced by immobilization stress. Pharmacol Biochem Behav 2011; 98: 362-368.
- Chen NA, Ganella DE, Bathgate RA, Chen A, Lawrence AJ, Kim JH. Knockdown of corticotropin-releasing factor 1 receptors in the ventral tegmental area enhances conditioned fear. Eur Neuropsychopharmacol 2016; 26: 1533-1540.
- Van Pett K, viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 2000; 428: 191-212.
- Kaminska K, Rogoz Z. The antidepressant- and anxiolytic-like effects following co-treatment with escitalopram and risperidone in rats. J Physiol Pharmacol 2016; 67: 471-480.
- Yarushkina NI, Bagaeva TR, Filaretova LP. Involvement of corticotropin-releasing factor receptors type 2, located in periaquaductal gray matter, in central and peripheral CRF-induced analgesic effect on somatic pain sensitivity in rats. J Physiol Pharmacol 2016; 67: 595-603.
A c c e p t e d : February 13, 2017