ATRIAL NATRIURETIC PEPTIDE AND BRAIN NATRIURETIC PEPTIDE CHANGES AFTER EPICARDIAL PERCUTANEOUS LEFT ATRIAL APPENDAGE SUTURE LIGATION USING LARIAT DEVICE
INTRODUCTION
Over 90% of all thrombosis in patients with non-valvular atrial fibrillation (AF) is formed in the left atrial appendage (LAA) (1). Oral anticoagulation (OAC) therapy is the most effective prophylactic approach to reduce thromboembolic risk in patients with AF. Current guidelines recommend use of oral anticoagulation in patients with CHA2DS2-VAS-score Ł 2 (2, 3).
However, there is a large number of patients in whom oral anticoagulation is ineffective or contraindicated or refusal of anticoagulation therapy for various reasons (cost of novel oral anticoagulants, inconvenience of warfarin monitoring, high risk of bleeding and bleeding complications, liver disorders) (4, 5). For those patients, percutaneous left atrial appendage occlusion or exclusion system are an alternative treatment for stroke and systemic thromboembolism risk reduction in non-valvular AF.
Endothelial cells of the LAA are specialized in the production and release of natriuretic peptides: atrial natriuretic peptide and brain natriuretic peptide. The LAA is the main producer of ANP in the human heart (6-9). The ANP densely granulated cells concentration is 40 times higher in the LAA walls than in the rest of the atrial free wall and in the ventricles (8). BNP, in addition released of the left ventricle, is also produced and released from left atrium and LAA in AF patients (10). Also elevated level of ANP and BNP correlate with presence of AF (11, 12).
It has been shown that in patients with AF after Maze procedure and bilateral appendectomy, ANP levels was decreased, which is consistent with reduction in ANP release after removal of the LAA (13, 14). Study that evaluated the acute effect of percutaneous endocardial occlusion of the LAA using WATCHMAN device showed significant attenuation of ANP and secretion in early post-procedural period (15). However, in contrast to percutaneous endocaridial occluders like WATCHMAN, LARIAT device is epicardial system based on LAA exclusion using suture. This two approach causes different location and type of leaks with clinical implications (16).
To date, there are no data evaluate any clinically significant effects due to alteration of ANP and BNP after percutaneous epicardial LAA exclusion with the LARIAT device.
The aim of this study was to evaluate the amount of ANP and BNP in patients with atrial fibrillation after percutaneous epicardial LAA suture ligation using LARIAT device.
MATERIALS AND METHODS
Patients
A prospective, single-center study was performed in 66 patients with atrial fibrillation. The mean age of was 63.4 ± 10.4 years, and 45.4% were women. The mean CHADS2 was 1.8 ± 0.9, CHA2DS2-VASc was 3.0 ± 1.7 and HAS-BLED was 3.3 ± 1 respectively. AF persistent was presents 53% in paroxysmal AF was presents in 47%. Further baseline characteristics are presented in Table 1.
All patients were fully informed about the procedure and gave written informed consent. The protocol was conducted with the approval of the Polish Ministry of Health and the ethics committee at John Paul II Hospital, Cracow, Poland.
Blood sampling
Venous blood samples were taken before the procedure and during 3 months follow-up visit at different times of day. The blood was drawn into plastic tubes containing aprotinin and ethylenediaminetetraacetic acid disodium and was promptly centrifuged. The plasma obtained was stored at -20 °C until assayed. The plasma BNP (Blue Gene, Shanghai, China) and ANP (Blue Gene, Shanghai, China) concentrations were determined with commercially available enzyme immunoassay kits. All measurements were performed in the same laboratory, at Central Laboratory of the John Paul II Hospital.
Epicardial left atrial appendage suture ligation using LARIAT device
Closure of left atrial appendage (LAA) performed by percutaneous suture ligation using LARIAT device has been previously described (17-19). Briefly, procedure involves 4 basic steps: 1) pericardial and transseptal access; 2) placement of the endocardial magnet-tipped guidewire in the apex of the LAA with balloon identification of the LAA os; 3) connection of the epicardial and endocardial magnet-tipped guidewires for stabilization of the LAA; and 4) snare capture of the LAA with closure confirmation and release of the pre-tied suture for LAA ligation (Fig. 1 and Fig. 2) (18).
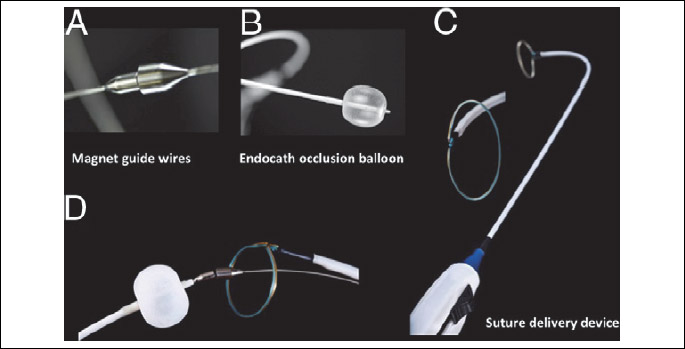
Adapted from: Bartus K., et al., Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62: 108-118 (19).
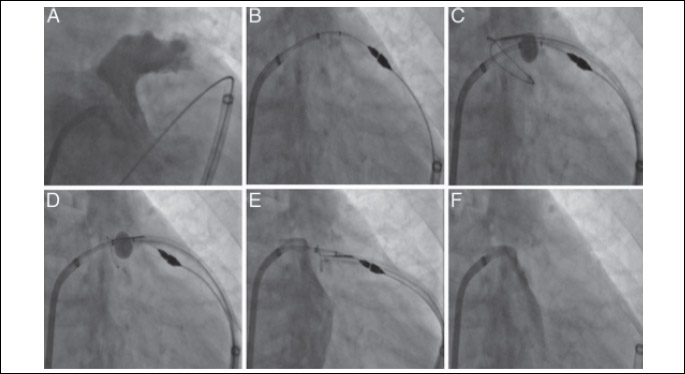
All images are in the right anterior oblique projection. Left atrial (LA) angiography identifies the ostium and body of the left atrial appendage (LAA) (A). Attachment of the magnet-tipped endocardial and epicardial guidewires (B) allows for the LARIAT suture delivery device to be guided over the LAA by the magnet-tipped epicardial guidewire using an over-the-wire approach (C). After verification of the correct position of the snare with the balloon catheter (D), an LA angiogram is performed prior to the release of the pre-tied suture to exclude the existence of a remnant trabeculated LAA lobe (E). A final LA angiogram is performed to verify LAA exclusion (F).
Adapted from: Bartus K., et al., Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62: 108-118 (19).
Statistical analysis
Descriptive statistics were made, and all data were expressed as mean ± standard deviation. Continuous variables were checked for normal distribution with the Shapiro-Wilk test. Data are expressed as mean ± standard deviation or median (interquartile range) unless otherwise stated. To assess the differences between two continuous variables, Student’s t-test (for normally distributed values), or the Mann-Whitney U-test (for non-normally distributed values) were applied. Statistical analysis was performed with STATISTICA 10.0 (StatSoft, Tulsa, OK, USA). A two-sided P-value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristic of patients
Patient characteristics at baseline are summarized in Table 1. LAA CT angiography examination revealed a mean ostial diameter of 28.2 ± 6.7 mm and a mean length of 31.5 ± 7.9 mm. The LAA was single-lobed in 44%. Most of the patients before procedure receive warfarin (n = 59; 85%).
Peri-procedural results
Device implantation was successful in all 66 patients. During procedure the most common hear rhythm was normal sinus rhythm (NSR) observed in 54.5%, then AF in 42.5% and atrial flutter and NSR with AF marked, both observed in 1.5%. Mean non-invasive blood pressure was 117/71 mmHg. Post-procedural transesophageal echocardiogram (TEE) showed complete LAA closure in all 66 patients, no leak was observed. Also TTE examination 3 months after procedure showed no leak in all patients.
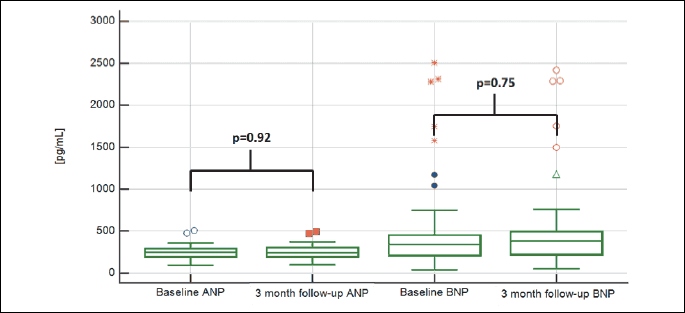
Before procedure in all 66 patients, mean ANP level was 249 ± 77 pg/mL (range from 95 pg/mL to 503 pg/mL) and mean BNP level was 481 ± 517 pg/mL (range from 34 pg/mL to 2508 pg/mL). Three months after procedure natriuretic peptide level was measured in 59 patients, 7 patients refused further follow-up visit. Three months after procedure mean ANP level was 249 ± 79 pg/mL (range from 98 pg/mL to 492 pg/mL) and mean BNP level was 495 ± 526 pg/mL (range from 52 pg/mL to 2420 pg/mL) (Fig. 1). The highest change in ANP level was 271 pg/mL, the lowest was 110 pg/mL, mean differences was 22 pg/mL. The highest change in BNP level was 327 pg/mL, the lowest was 200 pg/mL, mean differences was 24 pg/mL. There was no significant statistical difference in level of ANP (P = 0.92) and BNP (P = 0.75) (Fig. 3). Left ventricle mean EF before procedure was estimated at 44.39% ± 10.6 (range from 20 to 60). Three months after procedure EF was 45.25% ± 10.68 (range from 20 to 60) (Table 2). There was no significant statistical difference in EF before procedure and 3 month after procedure (P = 0.68).
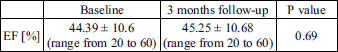
DISCUSSION
Percutaneous epicardial LAA exclusion using LARIAT suture delivery system is an alternative and safety method for stroke prevention in AF patients intolerant to oral anticoagulation therapy (17, 18, 20). The target LARIAT suture delivery system is to eliminate from cardiovascular system LAA, the source of more than 90% of thrombus in AF (1).
Oxidative stress and systemic inflammation promote cardiac structural and electrophysiologic remodeling and induce development of AF. The adhesive and gap junctions is suggested to be implicated in pathogenesis of arrhythmias. Modulation of adhesive proteins, N-cadherin and catenin may develop some new pharmacological treatment methods aimed on synchronization of heart function (21). Although, some new insights are published recently in the literature many questions still remain unanswered and therefore LAA ligation is considered to be a promissing treatment option for those patients.
Among patients with AF thrombus formation plays a crucial role in the development of stroke. As known from the aortic aneurysm studies thrombotic/fibrinolysis imbalance may favour faster thrombus formation and possibly the aneurysm progression (22).
BNP level is an important factor in patients with heart failure (HF). In animal studies the association with noradrenaline was significant when adjusted to left ventricular ejection fraction (LVEF) and plasma BNP level. Reduced left ventricle ejection fraction and elevated plasma BNP level are reflecting HF severity (23). The association between ANP and BNP levels in patients with LAA suture ligation remained unknown. Herein, we present the first study that evaluate the amount of ANP and BNP in patients with atrial fibrillation after percutaneous epicardial LAA suture ligation using LARIAT device. There were no significant differences in ANP and BNP levels in all patients before and 3 month after percutaneous LAA suture ligation. Moreover, the mean natriuretic peptide levels was similar before and after procedure, in ANP was 249 ± 77 pg/mL versus 249 ± 79 pg/mL and in BNP was 481 ± 517 pg/mL versus 495 ± 526 pg/mL, respectively.
Endothelial cells of the LAA are specialized in the production and release of natriuretic peptides. The LAA is the main producer of ANP in the human heart (6-8). The ANP densely granulated cells concentration is 40 times higher in the LAA walls than in the rest of the atrial free wall and in the ventricles (8). ANP plasma levels are directly proportional to the maximal LAA area. The most important factor increasing the ANP plasma concentration levels is distension of the LAA wall rather than elevation in right or left atrial pressure or distension of the body of the LA in patients with left-sided cardiac dysfunction (24). LAA is also a source of BNP (10).
ANP is secreted in response to atrial stretch by increased pressure and/or volume (25). The overall effect of ANP on the body is to counter increases in blood pressure, fluids volume as well as of salt and water (25, 26). The main ANP targets are kidney, vessels and adrenal glands. ANP, by different mechanisms, produces potent diuretic, natriuretic and vasorelaxant effects. In kidney, ANP increase the glomerular filtration rate (GFR) by the stimulation of afferent arterioles vasodilation together with vasoconstriction of efferent arterioles and also increases sodium excretion (27).
In experiments performed in rats, monkeys, and dogs (28-32), the removal of both LAA attenuated the release of ANP during volume expansion, and reduced the natriuretic and diuretic responses. The LAA role in mediating thirst was demonstrate in an in vivo study in sheep. The LAA was eliminated by mechanical compression after thoracotomy. Sheep with crushed LAA did not increase their water intake after volume depletion, whereas control sheep with intact LAA did. These findings suggest that stretch receptors of the LAA play a role in mediating thirst in hypovolemia (30). In contrast, Garcia et al. (32) showed that in rats in whom atrial appendectomy was performed, there was no inducible increase in plasma ANP levels.
Only a small amount of data is available about the consequences of LAA removal on ANP in humans (7). In patients with AF after Maze procedure and appendectomy, ANP levels was decreased, which is consistent with reduction in ANP release after removal of the LAA (13, 14). Moreover, ANP secretion by exercise 2 years after Maze procedure was still lower compare with patients who had not undergone the maze procedure (13). However, performed studies of LAA removal on ANP in humans have various restrictions. First of all, number of analyzed patients was very small, 12 patients in Yoshihara et al. study (13) and 15 patients in Nakamura et al. (14). Moreover, in both study, there were heterogeneity groups of patients with separately AF and AF with comorbid valve disease, in whom not only the Maze procedure had been performed, but also valve surgery or surgery was performed in patients with chronic heart failure (14). In that case, improvement of heart failure cause decrease of BNP level (11, 12). It should be also noted, that BNP and ANP may be and diagnostic and predictive markers in heart failure with left ventricular systolic dysfunction (9).
In our study conduct only non-valvular AF patients and CHF was present only in 15%.
Majunke et al. (15) showed that after endocardial percutaneous LAA closure, ANP and BNP values increased significantly compared with baseline measurements, which is most likely due to injection of contrast dye into the LAA and stretching of the LAA by the closure device. Twenty-four hours after the procedure, ANP and BNP values decreased significantly compared with baseline values. In contrast, to Majunke et al. (15) we present result of long term follow-up using epicardial system for LAA exclusion. Endocardial and epicardial approach causes different location and type of leaks with different clinical implications (16). The LARIAT device is associated with a lower rate of leaks at 1 year as compared with the Watchman device. In our study there were no leaks in post-procedural period and 3 months after procedure. Unfortunately, there is no information on the number of leaks in study of Majunke et al. (15).
What seems to be important, our study correlate with study Maybrook et al. (33) were electrolytes, BP, and heart rate (HR) were monitored before LARIAT and post-LARIAT (24 and 72 hours and 6 months). There were significant early decline in sodium and HR and diastolic BP (24 h and 72 h), which normalizes at long-term follow-up. They hypothesized that LARIAT ligation due to ischemic necrosis resulting in degranulation and release ANP what cause massive natriuresis and hyponatremia. As the acute LAA necrosis progresses to fibrosis, the ANP secretion from the LAA goes down and subsequently the right atrium probably takes over the ANP secretory function. These events are then likely followed by a homeostatic response that raises serum sodium levels over time.
This hypothesis seems to be consistent with our previous study confirm, where authors demonstrated that percutaneous suture ligation of LAA resulted in extensive inflammation of LAA leading to fibrosis, scarring, and permanent closure of LAA (34).
In endocardial LAA occlusion strategy, occluder is implanted inside LAA therefore rapid necrosis may be not present. However device implantation in the early phase inside LAA may lead to the observed reduction in circulating ANP and BNP levels (15).
Study limitations
There are several important limitations of the study. This is a single center study with all the inherent limitations. First and foremost, the influence of percutaneous LAA suture ligation on secretion of natriuretic peptides has been analyzed only in the 3 months after procedure. The impact of this procedure on the amount of circulating ANP and BNP post-procedural remains unclear. It is possible that ANP and BNP values initially may increase according to hypothesis due to ischemic necrosis resulting in degranulation and ANP release. As the acute LAA necrosis progresses to fibrosis, the ANP secretion from the LAA goes down and subsequently the other myocytes takes over the ANP secretory function. Second, in the study there are no information about LA hemodynamics parameters and measurements. In addition, we didn’t evaluate the clinical impact of our study. Therefore, further studies are necessary to reassess these findings to evaluate potential clinical effects.
In summary, there were no significant differences in ANP and BNP levels after percutaneous epicardial LAA suture ligation using LARIAT device 3 months after procedure. However, due to relatively short follow-up period and lack of such studies in the literature, post-procedural and long-term observations should be followed in future.
K. Bartus and J. Podolec contributed equally to the content of this paper.
Acknowledgements: This study was supported by the research grant No. UMO-2015/17/B/NZ5/00125 funded by the National Science Centre.
Conflict of interests: None declared.
REFERENCES
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61: 755-759.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016; 50: e1-e88.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1-76.
- Ansell J, Hirsh J, Hylek E, et al., Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008; 133: 160S-198S.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285: 2370-2375.
- Genest J, Cantin M. Atrial natriuretic factor. Circulation 1987; 75: 118-124.
- Stollberger C, Schneider B, Finsterer J. Elimination of the left atrial appendage to prevent stroke or embolism?: anatomic, physiologic, and pathophysiologic considerations. Chest 2003; 124: 2356-2362.
- Rodeheffer RJ, Naruse M, Atkinson JB, et al. Molecular forms of atrial natriuretic factor in normal and failing human myocardium. Circulation 1993; 88: 364-371.
- Falcao LM, Pinto F, Ravara L, van Zwieten PA. BNP and ANP as diagnostic and predictive markers in heart failure with left ventricular systolic dysfunction. J Renin-Angiotensin-Aldosterone Syst 2004; 5: 121-129.
- Inoue SI, Murakami Y, Sano K, Katoh H, Shimada T. Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J Card Fail 2000; 6: 92-96.
- Rossi A, Enriquez-Sarano M, Burnett JC, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol, 2000; 35: 1256-1262.
- Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol 2005; 45: 82-86.
- Yoshihara F, Nishikimi T, Kosakai Y, et al. Atrial natriuretic peptide secretion and body fluid balance after bilateral atrial appendectomy by the maze procedure. J Thorac Cardiovasc Surg 1998; 116: 213-219.
- Nakamura M, Niinuma H, Chiba M, et al. Effect of the maze procedure for atrial fibrillation on atrial and brain natriuretic peptide. Am J Cardiol, 1997; 79: 966-970.
- Majunke N, Sandri M, Adams V, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the Watchman device. J Invasive Cardiol 2015; 27: 448-452.
- Pillarisetti J, Reddy YM, Gunda S, et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm 2015; 12: 1501-1507.
- Bartus K, Bednarek J, Myc J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm 2011; 8: 188-193.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62: 108-118.
- Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA clinical perspective. Circ Cardiovasc Interv 2010; 3: 224-229.
- Stone D, Byrne T, Pershad A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation. Catheter Cardiovasc Interv 2015; 86: 121-127.
- Tribulova N, Egan Benova T, Szeiffova Bacova B, Viczenczova C, Barancik M. New aspects of pathogenesis of atrial fibrillation: remodelling of intercalated discs. J Physiol Pharmacol 2015; 66: 625-634.
- Siennicka A, Drozdzynska M, Chelstowski K, Cnotliwy M, Jastrzebska M. Hemostatic factors and intraluminal thrombus thickness in abdominal aortic aneurysm. Is secondary fibrinolysis relevant? J Physiol Pharmacol 2013; 64: 321-330.
- Tomaszek A, Kiczak L, Bania J, et al. Increased gene expression of catecholamine-synthesizing enzymes in adrenal glands contributes to high circulating catecholamines in pigs with tachycardia-induced cardiomyopathy. J Physiol Pharmacol 2015; 66: 227-231.
- Tabata T, Oki T, Yamada H, Abe M, Onose Y, Thomas JD. Relationship between left atrial appendage function and plasma concentration of atrial natriuretic peptide. Eur J Echocardiogr 2000; 1: 130-137.
- Zhang YH, Youm JB, Earm YE. Stretch-activated non-selective cation channel: a causal link between mechanical stretch and atrial natriuretic peptide secretion. Prog Biophys Mol Biol 2008; 98: 1-9.
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev 1990; 70: 665-699.
- De Vito P. Atrial natriuretic peptide: an old hormone or a new cytokine? Peptides 2014; 58: 108-116.
- Nishimura K, Saito Y, Hidaka T, et al. Does atrial appendectomy aggravate secretory function of atrial natriuretic polypeptide? J Thorac Cardiovasc Surg 1991; 101: 502-508.
- Villarreal D, Freeman RH, Davis JO, Verburg KM, Vari RC. Effects of atrial appendectomy on circulating atrial natriuretic factor during volume expansion in the rat. Proc Soc Exp Biol Med 1986; 183: 54-58.
- Stewart J, Dean R, Brown M, et al. Bilateral atrial appendectomy abolishes increased plasma atrial natriuretic peptide release and blunts sodium and water excretion during volume loading in conscious dogs. Circ Res 1992; 70: 724-732.
- Benjamin BA, Metzler CH, Peterson TV. Chronic atrial appendectomy alters sodium excretion in conscious monkeys. Am J Physiol 1988; 254: R699-R705.
- Garcia R, Cantin M, Thibault G. Role of right and left atria in natriuresis and atrial natriuretic factor release during blood volume changes in the conscious rat. Circ Res 1987; 61: 99-106.
- Maybrook R, Pillarisetti J, Yarlagadda V, et al. Electrolyte and hemodynamic changes following percutaneous left atrial appendage ligation with the LARIAT device. J Interv Card Electrophysiol 2015; 43: 245-251.
- Bartus K, Morelli RL, Szczepanski W, Kapelak B, Sadowski J, Lee RJ. Anatomic analysis of the left atrial appendage after closure with the LARIAT device. Circ Arrhythm Electrophysiol 2014; 7: 764-767.
A c c e p t e d : February 24, 2017