APPLICATION OF STEM CELLS IN DENTISTRY FOR BONE REGENERATION
2Institute of Human Genetics Polish Academy of Sciences, Poznan, Poland
INTRODUCTION
Crestal bone, in which dental restorations are grafted, constitutes the major element of the bone surface. A tooth loss or extraction trigger the irreversible process of bone resorption. This process may continue over a lifetime, however, it proceeds most intensively over the first six months following the extraction. Ultimately, it results in the complete loss of bone structure.
As the resorption progresses, implant-prosthodontic reconstruction becomes increasingly challenging. Both horizontal and vertical deficits in bone hinder the adequate choice of the size and shape of the endoosseous implant. Grafting and retaining the implant in reduced bone is impeded as well. Moreover, deficits in bone also hinder effective reconstruction of the masticatory organ in the adequate range to conditions in the patient’s oral cavity, as well as the patient’s general well-being.
The appropriate amount of bone tissue enables direct structural and functional amalgamation of the surface of the implant with the bone, as well as it supports the soft tissues surrounding the graft. The shape of the bone in three-dimensional space also conditions the appropriate shape of the marginal gingiva and gingival papilla, which provides the desired aesthetic result, and influences the overall level of the patient’s satisfaction with the treatment.
BONE REGENERATION
Bone regeneration is a complex physiological biological process in which new bone tissue is produced. It is a part of the healing process of bones, and it is subjected to changes throughout the whole adult life. The goal of surgical treatments in the area of the facial skeleton which aims at retaining or rebuilding the lost bone mass is to provide appropriate conditions for implanting endosseous grafts. Recently, there has been a considerable progress in techniques enabling rebuilding and regenerating the lost bone tissue, which in turn enables attaining satisfactory results of dental treatment, both aesthetic and functional (1-5).
Biological principles
Bone augmentation is a form of treatment, which allows to increase the spatial dimensions of resorbed bone by placing substitutive bone material or bone substitute in the cavity. The appropriate conditions for the ingrowth of osteogenic elements (osteoconduction), stimulation of undifferentiated cells to transform into osteoblasts (osteoinduction), and the presence of osteoblasts able to synthesise a new bone (osteogenic properties) are required for the correct and efficient augmentation process (6).
Osteoconduction is defined as creating foundations for osteoplastic elements and blood vessels to penetrate into. Autologous and synthethic bone materials (resorbable, non-resorbable, gels) have osteoconductive properties.
Signalling molecules of the healing process demonstrate osteoinductive properties, which are indispensable at stimulating osteoblasts to bone production. Examples of such molecules include bone morphogenetic proteins (BMPs), adhesins, hormones, vitamins, and substantial amounts of growth factors located in platelet granules (7).
Osteoblasts which are subjected to growth factors possess osteogenic properties. These factors include mesenchymal stem cells (MSC), partially differentiated cells, such as preosteoblasts, fibroblasts, chondroblasts, as well as differentiated cells, such as fibrocytes and osteocytes.
The wide variety of available materials and treatment techniques can be confusing, especially in the light of increasingly aggressive marketing strategies of companies which offer this treatment. This is the reason why the knowledge of the biological aspects of the bone regeneration process is crucial, as it plays the pivotal role in planning and conducting the regeneration process.
Types of materials used for bone rebuilding
Both autologous grafts containing living cells, as well as implantable synthetic bone graft materials (allogenic, xenogenic and alloplastic), which do not contain any living cells, can be applied in bone regeneration for treatment within the oral cavity. The osteoinduction, osteoconduction and osteogenic mechanisms all regulate the regenerative potential of these materials (Fig. 1).
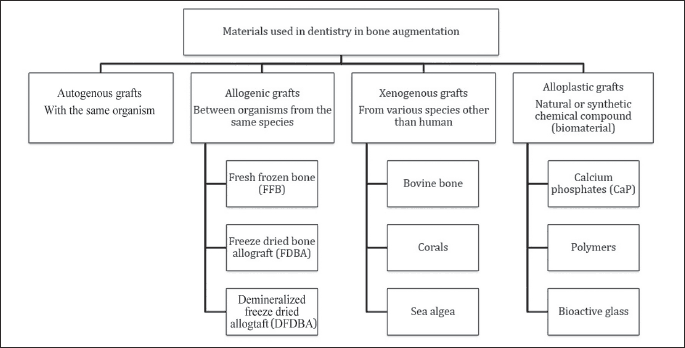
Autologous graft
Autologous bone transplantation (autotransplantation) is defined as transplanting the bone tissue from one place to another within the same organism. It is believed to be the gold standard among materials used in bone transplants (8).
Autologous bone transplants surpass other methods in terms of stimulating bone regeneration, as they have the ability to activate all the known bone regeneration mechanisms. An autologous bone contains living cells, which are able to synthesise bone tissue. The material also contains morphogenetic bone proteins, which are responsible for the growth and diversity of osteoblasts, whereas mineralised osteoid matrix, which constitutes the matrix, facilitates bone proliferation and the spread of blood vessels in the augmented place. As such, filling the cavity with autologous bone graft provides the fastest and the most comprehensive bone regeneration process. It also appears as the only available material, with osteogenic, osteoconductive and osteoinductive properties (9).
The autologous bone, which can be used in tissue transplantation, is available within the oral cavity and can be retrieved from chin, ramus of mandible, and mental tuberosity. Bigger amounts of bone tissue can be retrieved from wing of ilium, ribs and shinbone. Transplants retrieved from mandible are mostly compact bone structures, as opposed to wing of ilium, which is typically made of cancellous tissue. Compact bone can be applied in the form of block transplant or - after powdering - it can also be mixed with alloplastic materials (10).
The transplantation-related risk of intra- and post-procedural complications (bleeding, pain, hypoesthesia, hyperesthesia, infections) can be higher than 20%, which is why new materials and alternative methods of bone augmentation are continuously being searched (11, 12).
Allogenic graft
Often it is impossible to use the patient’s own tissue for medical reasons e.g. due to limitations of ambulatory practice, when only a small amount of bone tissue can be retrieved. The appropriate amount of the material can be thus provided by allogenic bone substituting materials (13).
Allogenic materials do not usually contain living cells and they can be transplanted between different organisms from the same species, and they are not genetically homogenous. Allogenic transplants, after preliminary purification and degreasing, are subsequently preserved over the course of the following processes: deep freezing, lyophilisation, and rinsing in isotonic saline. The immunogenicity of these grafts is eliminated through radiative sterilisation combined with deep freezing. There are three basic types of allogenic bones: fresh, frozen bone (FFB), frozen dried bone allograft (FDBA), and demineralized frozen dried bone allograft (DFDBA) (14).
FFB is currently not in common use due to the risk of transmitting diseases of viral etiology vertically. The advantage of DFDBA is that due to removal mineral substances it is possible to uncover the osteoid matrix, as well as that the matrix proteins, such as morphogenetic bone proteins, and they can easily diffuse to the place where the graft is implanted, playing an osteoinductive role (15).
Xenogenic graft
Xenogenic grafting is the process of tissues transplantation from one species to another. The organic matter is completely eliminated due to the risk of immunological reaction of the host and of transplanting pathogens. Inorganic elements constitute the matrix in which the new bone develops, and they are an excellent source of calcium. Bovine bone, corals and sea algae are possible sources of xenogenous material, which can be used in dental treatment.
Mineral skeletons of natural corals are mainly composed of carbonate and calcium phosphates. They are biocompatible and they form a very good foundation for proliferating cells due to the size of pores. They are also among few materials which create direct connection with both bone and soft tissues in vivo (16, 17).
Sea algae provide ceramic calcium phosphate, which has a very porous structure and rough surface. This material, when implanted to a bone defective site, enables adhesion of proteins and cells, it is resorbed slowly, and substituted with new bone tissue (18).
Bovine bone is subjected to proper cleaning and ultimately, after all modifications, it is transformed into biomaterial with no protein, which consists of calcium compounds and other elements corresponding to those which can be found in natural, hard bones. Bovine bone, after lyophilisation and deproteinization or after thermal and chemical processing, creates a good mineral substrate of high osteogenic qualities, which can be the basis for bone cells. Eliminating the protein component leaves the material free from antigenic properties, yet it does not protect from the risk of transmitting pathogens (19, 20).
Alloplastic graft
Alloplastic grafts are synthetic products. Their unquestionable advantage over bone materials and bone graft substitutes lies in their limitless availability and endurance, as well as no risk of transmitting pathogens to the recipient. Alloplastic materials do not have osteoinductive and osteogenetic properties, and their intense post-operative resorption makes them a poor foundation for bone proliferation and the spread of blood vessels. This is why it is recommended to use alloplastic materials mixed with blocks or with bone chips in 1:1 ratio in order to achieve desirable and long-lasting therapeutic effects (11,21).
Treatment techniques
Regeneration of periodontal tissues and bones can be conducted with the following treatment techniques: using biomaterials which create foundations for the newly produced bone, using growth factors, and stem cells (Fig. 2).
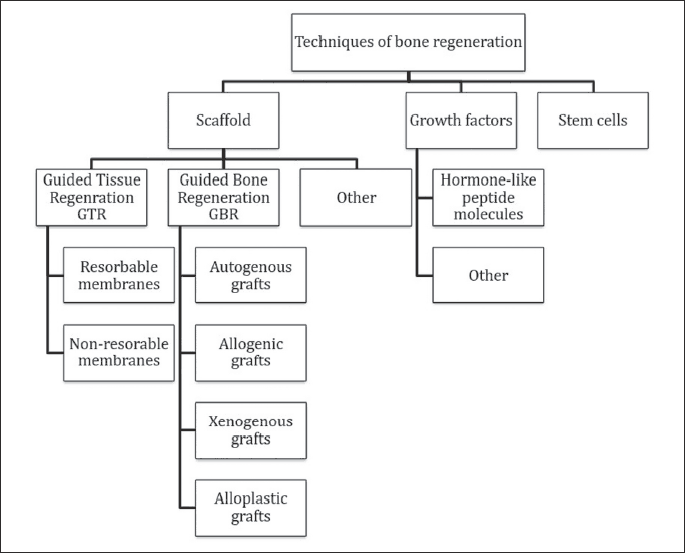
Scaffold
Treatment of bone defects includes removal of tissues affected by inflammation or necrosis, and creation of space for the new bone to grow. The most widespread technique of this generation is guided tissue regeneration (GTR) (22, 23). This method uses appropriate material in a regenerative membrane which is grafted in such a way as to prevent epithelial cells and fibroblasts from penetrating into the bone and creating a connective tissue scar. Biocompatible, resorbable membranes made from collagen or polylactic-co-glycolic acid (PLGA) copolymers, or non-resorbable membranes made from polytetrafluoroethylen (ePTFE) or titanium are used in this process (24).
GTR is thus a technique, which enables recreation of the functions and structure of the damaged tissues by taking advantage of the body’s natural ability to regenerate, and it stops the scar tissue from developing. The membranes are bioinertive and passive, which means they are unable to produce durable connection with the surrounding tissues and to stimulate osteogenic cells to produce new bone in the regenerated cavity (25).
The regeneration process is highly complex. Therefore, apart from the bioinertive membranes, which act as a matrix for cells, it is also required to use bioactive materials actively affected and integrated with the graft-surrounding tissues (guided bone regeneration, GBR). Preimplantation treatment, of which aim is to augment the size of the bone to facilitate implantation of the graft and its subsequent integration, includes distraction of osteogenesis, sinus lifting, bone splitting, and bone spreading with osteotomy techniques (26).
Growth factors
Growth factors are a well-researched group of hormone-like peptide components, which are soluble in water. Some of them may have osteoinductive properties, so they are able to recruit progenitor cells and differentiate them into mature, functional bone cells. They can also induce bone production in heterotrophic places. Molecules, which are able to stimulate the host’s cells to proliferate and/or to create new blood vessels, are critical for regenerative treatments. These molecules include: bone morphogenetic factors (BMP), transforming growth factor (TGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and growth differentiation factor (GDF). Transducing the initiation signal by injecting growth factors it is also important to mirror the natural, physiological signalling pathways, which are responsible for bone remodelling or bone morphogenesis. Growth factors are subjected to in vitro laboratory analysis (27). Table 1 presents clinically applicable molecules.
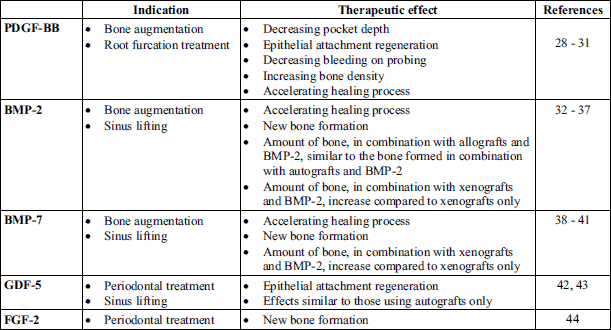
The mode of growth factors application seems to be important. Applying barrier membranes, which are used in GTR/GBR techniques, we can prevent mutual paracrine effects between neighbouring cell tissues. In Poland, there are two commercially available therapies, which use growth factors in bone, and periodontial tissues atrophy: platelet-rich plasma (PRP), and purified acidic extract retrieved from tooth bud, containing enamel matrix derivative (EMD).
PRP is autologous blood plasma in which the concentration of platelets surpasses the value of the whole blood. In PRP preparation platelets have been separated. The concentrate is obtained through centrifugation and separation of the whole blood. Due to this procedure, it is possible to obtain a high concentration of growth factors released by the platelets, which surpasses normal concentration of this substance in circulation (from pg/ml to ng/ml), in a small amount of plasma, which can be applied directly to the cavity.
Enamel Matrix Derivative (EMD) is a complex hydrophobic concentration of proteins of enamel matrix, which affects undifferentiated mesenchymal cells. It enables full regeneration of the tooth-supporting apparatus, which contributes to full regeneration of epithelial attachment and recreation of the lost bone tissue. The main ingredient of this material are amelogenins, which are similar and conservative in all vertebrates family. The sequence of amino-acids in porcine amelogenins and human amelogenins differ only in 4% of gene transcripts. Due to this fact, the formulation of porcine amelogenins in a solution of propylene glycol alginate can initiate the regeneration of periodontal tissues in humans without allergic reactions. Amelogenins stimulate differentiated cells to osteoinduction, and undifferentiated cells to osteogenesis. They are available in the form of convenient, easily applicable gel (45-48).
Osteoblasts
According to Lynch triad, correct and efficient bone regeneration process requires three elements. The first element is the basis, or scaffold. The second element are the signalling molecules of the healing process, such as BMP’s, adhesins, hormones, vitamins, growth factors, etc. The third element are the cells, which are affected by the so-called growth factors with potentially osteogenic properties. These are undifferentiated cells: stem cells, partially fate determined cells (preosteoblasts, fibroblasts), as well as fully differentiated cells (fibrocytes, osteocytes). Bone marrow, periosteum, bone tissue, and (less commonly) blood can all be sources of these cells. The cells can be separated and multiplied in vitro before injecting in the bone defect.
STEM CELLS
Regeneration of atrophic bone, especially in the case of large vertical cavities, is difficult to achieve with classical augmentation techniques. Bone-substitute materials, which are used in implant and prosthetic treatment, do not have osteogenic properties. Implanting them triggers bone resorption through activated osteoclasts.
Autologous cancellous bone is the only material, which has osteoconductive, osteoinductive, and osteogenic properties, and so it is the gold standard in reconstructive treatment. Autografts are the most effective biologically and clinically, they contain living bone cells which guarantees biocompatibility and does not trigger the recipient’s immunological system. It takes up to twelve months to obtain an integrated and vascularised bone, ready for subsequent modifying process.
Retrieving and preparing the graft requires an additional surgery, which weakens the bone in the place from which the material is retrieved, as well as increases the risk of complications and post-treatment afflictions. The amount of material, which is necessary for the graft, is limited, and the potential that autografts may present vary depending on the patient and the place from which the material has been retrieved.
The above-mentioned disadvantages related to bone grafts pushed for new solutions in tissue engineering, including stem cells which are able to predictably and longitudinally reconstruct the missing bone. It is believed that achieving the modern criteria for full success of implantological treatment (full integration of the implanted material and the bone, atrophy of marginal tubercule below 0.2 mm, and functioning of the prosthetic restoration based on the implanted foundation), this could be achieved only through stem cells application (49).
Clinical applications of stem cells
Stem cells can effectively improve the regenerative properties of the augmentation techniques in two ways. Firstly, they can serve for further laboratory preparation including synthetic formulation of bone or other implanted materials. Secondly, these cells can be used in preparing fresh material in the operating theatre, in which they can be directly applied (Fig. 3).
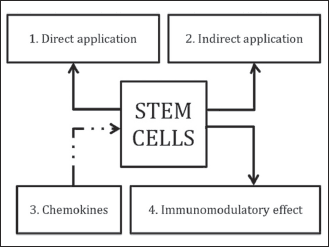 |
Fig. 3. Efficacy of augmentation procedure, applying freshly obtained stem cells directly used in the operating theatre (1), applying stem cells ex vivo propagated prior to implementation (indirect application) (2), applying endogenous stem cells when activating chemokine signalling pathways SDF-1 and MCP-1 (3) and applying immunomodulatory substances supporting stem cells survival in situ or exerting immunomodulatory and/or immunosuppressive properties of stem cells per se (4). |
Indirect application
In 2003, stem cells were used for the first time, together with bone graft, to augment a maxillary front bone (50). A year later, the same research group observed trabecular bone formation three months after the grafting, in a clinical trial conducted on 27 patients (51). In 2004, Ueda et al. administered a mixture of bone marrow stem cells (BMSC) and platelet rich plasma (PRP) during implantation (52). They used the same method to treat periodontitis (53), for bone augmentation, as well as in sinus lifting (54-56). Bone marrow stem cells’ effectiveness in oral cavity bone loss treatment, as well as their excellent osteogenic characteristics were also corroborated in studies in which BMSC were used together with hydroxyapatite (HA) (57), HA and one of the polymorphic tricalcium phosphates (TCP) - β-TCP (58), porous sponge (59), HA/TCP and recombinant PDGF (60) or frozen spongy bone (61). In addition, fat tissue stem cells (62, 63) and dental pulp stem cells (64) can be applied for bone regeneration.
Reports on the use of stem cells in clinical practice seem to sound optimistic. Yet, researchers agree on the lack of the characteristics of stem cells as well as the lack of optimal stem cell isolation and expansion protocols, which would facilitate preparation of a designed population of cells for grafting. Meijer et al. described great individual variation in bone formation in patients treated with stem cells (57). The results of randomised control trials (RCT) conducted by Kaigler et al. showed that treatment with stem cells isolated from bone marrow and enriched by cells with CD90 and CD 15 surface antigens accelerates the regeneration of alveolar process bone better than guided bone regeneration (65).
It is imperative to conduct more randomised clinical trials in order to learn whether current tissue engineering techniques may bring long-lasting, (desired) therapeutic effects. So far, there have not been any guidelines created, describing the optimal preparation of transplanted cells and selecting a proper carrier which would allow for free cell migration, easy access to nutrients and good by-products elimination.
Direct application
In 2006, Smiler and Soltan described a technique of fresh stem cells application derived from iliac crest and bone blocks used as scaffold for bone regeneration. This methodology was proven to be effective in bone regeneration. In future, their authors claim, it can become not the gold, but the platinum standard in reconstructive treatment (66-68). The above-mentioned method has been widely described in a literature. It consists of preparation of a concentrated, low-volume formulation containing twice as many stem cells as in bone marrow. The bone marrow aspirate concentrate (BMAC) administered to the target site of regeneration facilitate and accelerate healing, as well as stimulate osteogenic cells’, bone tissue’s and blood vessels’ growth (69).
It has been shown that mononuclear cells concentrates together with BBM triggers tubular bone growth. The bone, made out of the spongy and compact bone, contains a net of parallel collagen fibres. It is highly mineralised and at the same time able to bear great loads caused, for instance, by dental pillars (70, 71).
Rickert et al. conducted randomised, prospective clinical trials in patients who underwent sinus lifting procedure. The results showed that the bone marrow concentrates together with BBM revealed higher osteogenic potential than the autologous bone grafts. It was also shown, in histomorphometric analyses (which allows to quantitatively describe the quality of bone tissue), that there was more bone on the site in which the stem cells had been implanted than on another one, in which the autografts were used (72). The results of randomised, control histological and clinical trials in patients, with single-blind trials, showed that the use of stem cells concentrates had no influence on the amount of bone tissue in the augmented region within 3 – 4 months after the sinus lifting procedure. Therefore, BMAC with BBM should be perceived as an alternative to autologous materials (73).
Stem cells transplanted immediately after aspiration do not trigger clinical symptoms or histological changes in cells mediating inflammatory reactions (72, 74). Fundamental scientists as well as practitioners underline the therapeutic, anti-inflammatory activities of bone marrow stem cells administered locally or intravenously (75-77). It should be mentioned that fresh preparation administered to the recipient’s body is not homogenous and may contain different types of cells: stem cells, osteogenic precursor cells, bone marrow hematopoietic cells, cells accelerating blood vessels’ growth and stromal cells.
Research areas
Stem cells seem to posses promising therapeutic implications in bone regeneration. However, bone regeneration is a relatively new area of expertise in tissue engineering and ongoing mechanisms in the body during transplantation are not yet fully known. When it comes to clinical assessment of the cells transplanted, it has not been yet stated whether the new bone is formed by transplanted stem cells themselves (osteogenic role of stem cells) or through bone-forming cells of the tissue reservoir, stimulated by the transplanted stem cells (osteoinductive role of stem cells) (78). The BMSC morphological structure is well-known but their biological characteristics have been scarcely described, especially after the cells have been administered to the recipient. BMSC cultivation and expansion may additionally change their initial biological properties. Moreover, the BMSC influence on the host’s immunological system is still poorly known.
Transplanted cells’ survival rate
Stem cells used in bone regeneration techniques are indispensable at least until the capillary blood vessels would develop. These vessels provide the transplant with oxygen and nutrients, as well as eliminate unnecessary products of metabolism.
Studies have shown that the survival rate of stem cells administered to bone losses is short-lived. Zimmermann et al. reported that transplanted BMSC were absent as early as after 14 days following the procedure of labelling them with a fluorescent dye, and placing on a scaffold made of hydroxyapatite mixed with tricalcium β-phosphate when transplanting them subcutaneously to isogenic rats (79).
These reports were corroborated by other studies conducted by Boukhechba et al. (80) in which BMSC did not survive more than 3 weeks following the transplantation. Quintavalla et al. observed a reduced number of transplanted stem cells as early as on the 7th day of their experiment (81).
The results of numerous studies conducted with patients (82-84) and/or animals (85-87) show that stem cells may significantly accelerate osteogenesis. However, if the transplanted cells were to be as source of osteoblasts responsible for bone formation, they would have to survive long enough to trigger the processes of bone matrix production and mineralisation.
Therefore, it can be assumed that transplanted stem cells are not only a source of osteoblasts responsible for bone formation but also can stimulate the host’s organism to recruit endogenous cells responsible for bone development. In addition, they may play an immunomodulatory role.
The development of capillary blood vessels is an indispensable part of a new bone formation. These vessels transport oxygen, nutrients and metabolism products, as well as progenitor cells required for osteogenesis. Transplanting endothelial progenitor cells (EPC) into a particular place is currently a promising and widely studied technique facilitating angiogenesis. These cells can turn into endothelial cells and significantly accelerate blood vessel formation. The mentioned method was reported to be effective when treating myocardial infarction in animal experimental model (88).
The recruitment of endogenous osteoprogenitor cells
While the human body, including the oral cavity, can be an excellent source of stem cells, methods using these cells for bone regeneration do not always bring satisfactory results. Alternative methods are being actively studied, including those making use out of endogenous cells (89).
Chemokines are proteins belonging to the cytokines family, with chemotactic properties. They recruit the cells with a corresponding receptors and make them able to migrate according to the chemokine gradient (90). There is about 50 chemokines and 20 chemokine receptors currently known. Chemokines can be divided into 4 classes, depending on the location of two n cysteine residues: CC, CXC, CX3C and C. Chemokine receptors have been named accordingly. It has been proved that the SDF-1 (CXCL12)/CXC4 and MCP-1 (CCL2)-CCR2 chemokine signalling pathways are crucial in the mobilisation, proliferation and survival of many cell types including osteoprogenitor stem cells (91, 92), (Fig. 3).
Stromal cell-derived factor-1 (SDF-1), isolated in 1993, known also as the chemokine ligand 12 (CXCL12), belongs to the CXC class (93). It is the sole CXCR4 receptor ligand, however SDF-1 can also bind to CXCR7. Both receptors are G protein-coupled receptors (GPCR). SDF-1 (ligand) - CXCR4 (receptor) signalling process is very complex and delicate. It activates a number of signalling pathways in target cells. The SDF-1/CXC4 axis plays the key role in migration, proliferation, repopulation, differentiation and survival of many progenitor cell types (94). It affects cell migration from bone marrow to blood. In case of tissue damage, SDF-1 levels increase. On a SDF-1-dependent pathway, transplanted stem cells are recruited to the damaged tissue site, where they can participate in its reconstruction (95-99).
Stromal, endothelial and reticular cells located in the bone marrow perivascular niche are the main source of SDF-1. Increased SDF-1 expression has been also observed in osteoblastic progenitor cells, in contrast to the bone-forming bone marrow stem cells. CXCR4, receptor dedicated to binding with the ligand, is expressed in hematopoietic cells, osteoblasts and mesenchymal stem cells. SDF-1 cytokine concentration increases in the place of trauma. The cytokine stimulates the chemotaxis of endogenous progenitor cells (CXCR4+) which are indispensable in the repair and regeneration of many organs, including bone tissue (100).
Monocyte chemotactic protein-1 (MCP-1), a proinflammatory cytokine known also as the C-C chemokine ligand type 2 (CCL2), carries out chemotactic activity towards monocytes and basophils. The main task of CCL2 is to activate leukocytes to chemotaxis, which constitutes the key stage of immunological response (92). Chemokine receptor CCR2, also known as CD192, binds with G protein, a transmembrane receptor, taking part in transducing the extracellular signal (ligand binding) into the intracellular one (G protein activation). CCR2 binds to chemoattractants, including the MCP-1 protein (101). The chemoattractant’s activity towards the receptor initiates chemotaxis, i.e. the movement of cells towards the increasing gradient. CCR2 undergoes expression in monocytes, hematopoietic bone marrow cells and mesenchymal stem cells (102).
In their studies on mice, Ando et al. observed that transplanted stem cells take part in the recruitment of endogenous bone marrow stem cells, creating ossification centres by forming callus. The formation of callus leads to the formation of young spongy bone that subsequently turns into mature bone. MCP-1/CCR2 axis plays the crucial role in this phenomenon (103).
Immunomodulatory properties of stem cells
Stem cells are capable of immunomodulation either by immunostimulation or by immunosuppression. MSC inhibit the proliferation of T lymphocytes, which is based on the activity of transforming growth factor β1 (TGF-β1) and hepatocyte growth factor (HGF) in vitro (104). They decrease IL-2, IFN-γ and TNF-α cytokine secretion, while increase the production of IL-4 (105), (Fig. 3).
Stem cells keep T lymphocytes in the G0-G1 phase and inhibit the expression of D2 protein which regulates the length of particular cell cycle phases (106). MSC secrete indoleamine 2,3-dioxygenase (IDO), a chemical compound responsible for tryptophan degradation, while tryptophan concentrations negatively correlate with proinflammatory cytokines while positively with C-reactive protein (CRP) concentrations. IDO induction also means the production of tryptophane catabolite - kynurenine, which consequently leads to T lymphocytes’ proliferation inhibition. Activation the kynurenine pathway, is associated with osteoblastogenesis and can be implicated in the occurrence of bone diseases. Oxidation products like kynurenine stopped proliferation of bone marrow mesenchymal stem cells. This may result in inhibition of osteoblastic proliferation and differentiation. The ratio of kynurenin/tryptophan is also an indicator for the activity of IDO (107). Nitrogen oxide produced by stem cells inhibits the phosphorylation of signal transducers and activators of transcription-5 (STAT-5) protein which belongs to the group of proteins that participate in transmission from the cell surface to nucleus activating the gene transcription (108). Increased human leukocyte antigen-G5 (HLA-G5) expression on stem cells’ surface seems to be an important mechanism that damages cell immune defence ability by decreasing the activity of cytotoxic lymphocytes and NK cells, as well as by inhibiting T CD4+ lymphocytes’ proliferation and inducing their differentiation into T regulatory lymphocytes. All these mechanisms trigger immunosuppressive regulatory arm and, consequently, lead to immunological tolerance (109). Some researchers also reported that MSC were able to inhibit the activity of immunological system cells by direct cell-to-cell contact (110-112).
Interactions between stem cells and B lymphocytes are not yet fully recognized, however the mechanisms of their mutual interaction have been already described. MSC block the activity of proinflammatory and prothrombotic CD40 – CD40, receptor - ligand system, by means of the anti-CD40L monoclonal antibody. They also inhibit the production of antibodies and CXCR4, CXCR5 and CXCR7 chemokine receptors (113).
MSC affect CD4+ T helpers’ (Th) activity by stimulating T regulatory cells (Treg) to proliferate and suppressing Th17 cells which constitute specific subpopulation of T helper cells. Foxp3 transcription factor plays an important role in the development and differentiation of regulatory lymphocytes (Treg). The results of numerous studies indicated an increased Foxp3 expression in T CD4+CD25+ lymphocytes, triggered by the activity of mesenchymal stem cells (114-117).
A characteristic property of Th17 lymphocytes is an interleukin 17 secretion (IL-17A), hence their name. IL-17 plays an important role in the pathogenesis of autoimmunological, inflammatory and allergic diseases. So far, neither negative nor positive impact of these cells on cancer development has been observed. In in vitro trials, MSC inhibited T CD4+ lymphocyte differentiation towards Th17, as well as the production of IL-17, IL-22, IFN-γ and TNF-α by not fully differentiated Th17 cells. Inflammatory cytokines, T helper 17 cells and activator of nuclear factor kappa B ligand-interleukin-17 are responsible for osteoclast activation and can lead to bone loss. Mesenchymal stem cells are potent immunomodulators and may inhibit the immune response by suppressing T cells, inducing regulatory T cells and converting dendritic cells and macrophages into a regulatory phenotype. This therapeutic role of MSC may be useful in tissue engineering, especially in the treatment of periodontal diseases (118).
MSC increase the amount of Treg in plasma indirectly. This influence is rooted in the activity of IDO, PGE2, TGFβ1 produced by these cells, as well as in the direct cell-to-cell contact. Precise mechanisms of immunological modulation by affecting Treg and Th17 activities are still understudied.
It is believed that the successful integrated dental treatment in a long-term perspective will only be possible due to the application of stem cells. The use thereof in order to enlarge the dimensions of the bone may proceed producing a fresh material, administered to the patient of its own or together with a bone substitutes. Alternatively, they may be used indirectly with prior preparations in laboratory conditions ex vivo or commercially available on request.
It has been proven that stem cells are not only able to transform into more differentiated cells, being a source of osteoblasts, but can also stimulate mobilization of endogenous stem and progenitor cells to enhance osteoblasts number, osteoid volume, and bone volume. Immunostimulating and immunosuppressive function may likewise play an important role in the supporting of bone regeneration process.
Conflict of interests: None declared.
REFERENCES
- Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920-926.
- Meyer U, Meyer T, Handschel J, Wiesmann HP. The history of tissue engineering and regenerative medicine in perspective. In: Fundamentals of Tissue Engineering and Regenartive Medicine. Springer-Verlag Berlin Heidelberg 2009, 5-12.
- Tonelli P, Duvina M, Barbato L, et al. Bone regeneration in dentistry. Clin Cases Miner Bone Metab 2011; 8: 24-28.
- Abou Neel EA, Chrzanowski W, Salih VM, Kim HW, Knowles JC. Tissue engineering in dentistry. J Dent 2014; 42: 915-928.
- Amrollahi P, Shah B, Seifi A, Tayebi L. Recent advancements in regenerative dentistry: a review. Mater Sci Eng C Mater Biol Appl 2016; 69: 1383-1390.
- Hing KA. Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Trans A Math Phys Eng Sci 2004; 362: 2821-2850.
- Miron RJ, Zhang YF. Osteoinduction: a review of old concepts with new standards. J Dent Res 2012; 91: 736-744.
- Mao JJ, Stosich MS, Moioli EK, et al. Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng Part B Rev 2010; 16: 257-262.
- Martuscelli R, Toti P, Sbordone L, Guidetti F, Ramaglia L, Sbordone C. Five-year outcome of bone remodelling around implants in the maxillary sinus: assessment of differences between implants placed in autogenous inlay bone blocks and in ungrafted maxilla. Int J Oral Maxillofac Surg 2014; 43: 1117-1126.
- Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg 2012; 23: 323-327.
- Kumar P, Vinitha B, Fathima G. Bone grafts in dentistry. J Pharm Bioallied Sci 2013; 5 (Suppl. 1): S125-S127.
- Nazirkar G, Singh S, Dole V, Nikam A. Effortless effort in bone regeneration: a review. J Int Oral Health 2014; 6: 120-124.
- McMahon RE, Wang L, Skoracki R, Mathur AB. Development of nanomaterials for bone repair and regeneration. J Biomed Mater Res B Appl Biomater 2013; 101: 387-397.
- Stanford JW. Bone-inducing materials: their place in dentistry. Int Dent J 1987; 37: 162-168.
- Young MP, Worthington HV, Lloyd RE, Drucker DB, Sloan P, Carter DH. Bone collected during dental implant surgery: a clinical and histological study. Clin Oral Implants Res 2002; 13: 298-303.
- Guillemin G, Patat JL, Fournie J, Chetail M. The use of coral as a bone graft substitute. J Biomed Mater Res 1987; 21: 557-567.
- Pollick S, Shors EC, Holmes RE, Kraut RA. Bone formation and implant degradation of coralline porous ceramics placed in bone and ectopic sites. J Oral Maxillofac Surg 1995; 53: 915-922; discussion 922-923.
- Le Guehennec L, Layrolle P, Daculsi G. A review of bioceramics and fibrin sealant. Eur Cell Mater 2004; 8: 1-10; discussion 10-11.
- McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol 2007; 78: 377-396.
- Jazayeri HE, Tahriri M, Razavi M, et al. A current overview of materials and strategies for potential use in maxillofacial tissue regeneration. Mater Sci Eng C Mater Biol Appl 2017; 70: 913-929.
- Sukumar S, Drizhal I. Bone grafts in periodontal therapy. Acta Medica (Hradec Kralove) 2008; 51: 203-207.
- Gottlow J, Nyman S, Lindhe J, Karring T, Wennstrom J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol 1986; 13: 604-616.
- Nyman S, Gottlow J, Lindhe J, Karring T, Wennstrom J. New attachment formation by guided tissue regeneration. J Periodontal Res 1987; 22: 252-254.
- Retzepi M, Donos N. Guided bone regeneration: biological principle and therapeutic applications. Clin Oral Implants Res 2010; 21: 567-576.
- Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 1988; 81: 672-676.
- Hammerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000 2003; 33: 36-53.
- Pilipchuk SP, Plonka AB, Monje A, et al. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent Mater 2015; 31: 317-338.
- Jayakumar A, Rajababu P, Rohini S, et al. Multi-centre, randomized clinical trial on the efficacy and safety of recombinant human platelet-derived growth factor with b-tricalcium phosphate in human intra-osseous periodontal defects. J Clin Periodontol 2011; 38: 163-172.
- Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent 2003; 23: 213-225.
- Nevins ML, Camelo M, Schupbach P, Nevins M, Kim SW, Kim DM. Human buccal plate extraction socket regeneration with recombinant human platelet-derived growth factor BB or enamel matrix derivative. Int J Periodontics Restorative Dent 2011; 31: 481-492.
- Nevins ML, Reynolds MA, Camelo M, Schupbach P, Kim DM, Nevins M. Recombinant human platelet-derived growth factor BB for reconstruction of human large extraction site defects. Int J Periodontics Restorative Dent 2014; 34: 157-163.
- Boyne PJ, Marx RE, Nevins M, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent 1997; 17: 11-25.
- Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg 2009; 67: 1947-1960.
- Kao DW, Kubota A, Nevins M, Fiorellini JP. The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int J Periodontics Restorative Dent 2012; 32: 61-67.
- Torrecillas-Martinez L, Monje A, Pikos MA, et al. Effect of rhBMP-2 upon maxillary sinus augmentation: a comprehensive review. Implant Dent 2013; 22: 232-237.
- Freitas RM, Spin-Neto R, Marcantonio Junior E, Pereira LA, Wikesjo UM, Susin C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: a systematic review. Clin Implant Dent Relat Res 2015; 17 (Suppl. 1): e192-e201.
- Cochran DL, Jones AA, Lilly LC, Fiorellini JP, Howell H. Evaluation of recombinant human bone morphogenetic protein-2 in oral applications including the use of endosseous implants: 3-year results of a pilot study in humans. J Periodontol 2000; 71: 1241-1257.
- Fiorellini JP, Buser D, Riley E, Howell TH. Effect on bone healing of bone morphogenetic protein placed in combination with endosseous implants: a pilot study in beagle dogs. Int J Periodontics Restorative Dent 2001; 21: 41-47.
- Corinaldesi G, Piersanti L, Piattelli A, Iezzi G, Pieri F, Marchetti C. Augmentation of the floor of the maxillary sinus with recombinant human bone morphogenetic protein-7: a pilot radiological and histological study in humans. Br J Oral Maxillofac Surg 2013; 51: 247-252.
- Fu HD, Wang HR, Li DH. BMP-7 accelerates the differentiation of rabbit mesenchymal stem cells into cartilage through the Wnt/β-catenin pathway. Exp Ther Med 2017; 14: 5424-5428.
- Chen F, Bi D, Cao G, et al. Bone morphogenetic protein 7-transduced human dermal-derived fibroblast cells differentiate into osteoblasts and form bone in vivo. Connect Tissue Res 2017; Jul 11: 1-10. doi: 10.1080/03008207.2017.1353085
- Windisch P, Stavropoulos A, Molnar B, et al. A phase IIa randomized controlled pilot study evaluating the safety and clinical outcomes following the use of rhGDF-5/β-TCP in regenerative periodontal therapy. Clin Oral Investig 2012; 16: 1181-1189.
- Koch FP, Becker J, Terheyden H, Capsius B, Wagner W. A prospective, randomized pilot study on the safety and efficacy of recombinant human growth and differentiation factor-5 coated onto β-tricalcium phosphate for sinus lift augmentation. Clin Oral Implants Res 2010; 21: 1301-1308.
- Kitamura M, Akamatsu M, Machigashira M, et al. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res 2011; 90: 35-40.
- Sasaki S, Shimokawa H. The amelogenin gene. Int J Dev Biol 2003; 39: 127-133.
- Gestrelius S, Lyngstadaas SP, Hammarstrom L. Emdogain - periodontal regeneration based on biomimicry. Clin Oral Investig 2000; 4: 120-125.
- Smith CE, Poulter JA, Antanaviciute A, et al. Amelogenesis imperfecta; genes, proteins, and pathways. Front Physiol 2017; 8: 435. doi:10.3389/fphys.2017.00435
- Weikard R, Pitra C, Kuhn C. Amelogenin cross-amplification in the family Bovidae and its application for sex determination. Mol Reprod Dev 2006; 73: 1333-1337.
- Aghaloo TL, Tuan RS, Schmitz JP, Aboud M, Amet E, Cardaropoli G. The academy of osseointegration silver anniversary summit: impact of biological and technological advances on implant dentistry (stem cell therapy group report). Int J Oral Maxillofac Implants 2011; 26 (Suppl.): 64-69.
- Schmelzeisen R, Schimming R, Sittinger M. Making bone: implant insertion into tissue-engineered bone for maxillary sinus floor augmentation-a preliminary report. J Craniomaxillofac Surg 2003; 31: 34-39.
- Schimming R, Schmelzeisen R. Tissue-engineered bone for maxillary sinus augmentation. J Oral Maxillofac Surg 2004; 62: 724-729.
- Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: from basic research to clinical case study. Cell Transplant 2004; 13: 343-355.
- Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: a clinical case report. Int J Periodontics Restorative Dent 2006; 26: 363-369.
- Hibi H, Yamada Y, Ueda M, Endo Y. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg 2006; 35: 551-555.
- Yamada Y, Nakamura S, Ito K, et al. Injectable tissue-engineered bone using autogenous bone marrow-derived stromal cells for maxillary sinus augmentation: clinical application report from a 2 – 6-year follow-up. Tissue Eng Part A 2008; 14: 1699-1707.
- Yamada Y, Nakamura S, Ueda M, Ito K. Osteotome technique with injectable tissue-engineered bone and simultaneous implant placement by cell therapy. Clin Oral Implants Res 2013; 24: 468-474.
- Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials 2008; 29: 3053-3061.
- Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: 203-209.
- Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A 2010; 16: 2809-2820.
- Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg 2012; 40: 2-7.
- Lee J, Sung HM, Jang JD, Park YW, Min SK, Kim EC. Successful reconstruction of 15-cm segmental defects by bone marrow stem cells and resected autogenous bone graft in central hemangioma. J Oral Maxillofac Surg 2010; 68: 188-194.
- Kulakov AA, Goldshtein DV, Grigoryan AS, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med 2008; 146: 522-525.
- Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 2009; 38: 201-209.
- Kadar K, Kiraly M, Porcsalmy B, et al. Differentiation potential of stem cells from human dental origin - promise for tissue engineering. J Physiol Pharmacol 2009; 60 (Suppl. 7): 167-175.
- Kaigler D, Pagni G, Park CH, et al. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant 2013; 22: 767-777.
- Smiler D, Soltan M. Bone marrow aspiration: technique, grafts, and reports. Implant Dent 2006; 15: 229-235.
- Smiler D, Soltan M, Lee JW. A histomorphogenic analysis of bone grafts augmented with adult stem cells. Implant Dent 2007; 16: 42-53.
- Soltan M, Smiler D, Prasad HS, Rohrer MD. Bone block allograft impregnated with bone marrow aspirate. Implant Dent 2007; 16: 329-339.
- Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9: 641-650.
- Wongchuensoontorn C, Liebehenschel N, Schwarz U, et al. Application of a new chair-side method for the harvest of mesenchymal stem cells in a patient with nonunion of a fracture of the atrophic mandible - a case report. J Craniomaxillofac Surg 2009; 37: 155-161.
- Sauerbier S, Stricker A, Kuschnierz J, et al. in vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the FICOLL method or the BMAC method. Tissue Eng Part C Methods 2010; 16: 215-223.
- Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res 2011; 22: 251-258.
- Sauerbier S, Rickert D, Gutwald R, et al. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial - per-protocol analysis. Tissue Eng Part A 2011; 17: 2187-2197.
- McAllister BS, Haghighat K, Gonshor A. Histologic evaluation of a stem cell-based sinus-augmentation procedure. J Periodontol 2009; 80: 679-686.
- Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005-1011.
- Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery 2004; 55: 1185-1193.
- Zhou Y, Wang S, Yu Z, et al. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem Biophys Res Commun 2009; 390: 902-907.
- Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med 2007; 4: e9. doi: 10.1371/journal.pmed.0040009
- Zimmermann CE, Gierloff M, Hedderich J, Acil Y, Wiltfang J, Terheyden H. Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Eng Part A 2011; 17: 1147-1156.
- Boukhechba F, Balaguer T, Bouvet-Gerbettaz S, et al. Fate of bone marrow stromal cells in a syngenic model of bone formation. Tissue Eng Part A 2011; 17: 2267-2278.
- Quintavalla J, Uziel-Fusi S, Yin J, et al. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials 2002; 23: 109-119.
- Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001; 344: 385-386.
- Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone 2007; 40: 522-528.
- Nagata M, Hoshina H, Li M, et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: coordinated activation of bone formation and resorption. Bone 2012; 50: 1123-1129.
- Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic. Experiments in rats. Acta Orthop Scand 1989; 60: 334-339.
- Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res 1998; 16: 155-162.
- Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 2000; 49: 328-337.
- Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001; 103: 634-637.
- Herrmann M, Verrier S, Alini M. Strategies to stimulate mobilization and homing of endogenous stem and progenitor cells for bone tissue repair. Front Bioeng Biotechnol 2015; 3: 79. doi:10.3389/fbioe.2015.00079
- Baggiolini M. Chemokines and leukocyte traffic. Nature 1998; 392: 565-568.
- Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA 1994; 91: 2305-2309.
- Fuentes ME, Durham SK, Swerdel MR, et al. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol 1995; 155: 5769-5776.
- Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science 1993; 261: 600-603.
- Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med 1998; 187: 753-762.
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977-988.
- Yano T, Liu Z, Donovan J, Thomas MK, Habener JF. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes 2007; 56: 2946-2957.
- Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res 2008; 1195: 104-112.
- Mukherjee D, Zhao J. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res 2013; 3: 46-57.
- McIver SC, Loveland KL, Roman SD, Nixon B, Kitazawa R, McLaughlin EA. The chemokine CXCL12 and its receptor CXCR4 are implicated in human seminoma metastasis. Andrology 2013; 1: 517-529.
- Xu X, Zhu F, Zhang M, et al. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs 2013; 197: 103-113.
- Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 2007; 101: 135-146.
- Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One 2012; 7: e35685. doi:10.1371/journal.pone.0035685
- Ando Y, Matsubara K, Ishikawa J, et al. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014; 61: 82-90.
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843.
- Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 2009; 5: 392-399.
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105: 2821-2827.
- Michalowska M, Znorko B, Kaminski T, Oksztulska-Kolanek E, Pawlak D. New insights into tryptophan and its metabolites in the regulation of bone metabolism. J Physiol Pharmacol 2015; 66: 779-791.
- Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol 1998; 160: 5729-5734.
- Morandi F, Ferretti E, Bocca P, Prigione I, Raffaghello L, Pistoia V. A novel mechanism of soluble HLA-G mediated immune modulation: downregulation of T cell chemokine receptor expression and impairment of chemotaxis. PLoS One 2010; 5: e11763. doi:10.1371/journal.pone.0011763
- Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 2007; 28: 219-226.
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008; 8: 726-736.
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 2010; 28: 585-596.
- Tamaoki N, Takahashi K, Tanaka T, et al. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res 2010; 89: 773-778.
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822.
- Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005; 90: 516-525.
- Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008; 26: 212-222.
- Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 2008; 36: 309-318.
- Racz GZ, Kadar K, Foldes A, et al. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontics. J Physiol Pharmacol 2014; 65: 327-339.
A c c e p t e d : February 15, 2018