THE INFLUENCE OF THE LOW-ENERGY DEFIBRILLATING IMPULSE
ON THE ENDOTHELIN-1 CONCENTRATION IN THE LEFT HEART VENTRICLE
AND BLOOD SERUM OF THE HEALTHY RABBITS
INTRODUCTION
Despite the significant development of the intensive care during last decade, particularly, concerning the use of hypothermia, extracorporeal membrane oxygenation (ECMO) or various forms of mechanical circulatory supports, still only 7.6 to 21% of patients with out-of-hospital cardiac arrest and ROSC survive to their discharge from the hospital. The majority of deaths in the early postresuscitation period is caused by cardiac dysfunctions and heart failure. Post-cardiac arrest syndrome (PCAS) is one of the causes underlying these anomalies (1, 2). Cardiac ischemia/reperfusion injury plays a fundamental role in the pathogenesis of PCAS. In myocardial ischemia, the restoration of blood circulation and oxygen transport is of crucial importance for cardiomyocytes survival. Unfortunately, at the same time, reperfusion can lead to a number of adverse consequences in the form of intensified injury to cardiomyocytes and impairment in their contractility, an increased area of necrosis, ventricular rate abnormalities, and microcirculation dysfunctions (3). ET-1 is an important vasoactive factor influencing ‘low flow’ and ‘no-reflow’ effects in the development of cardiac ischemia/reperfusion injury. Furthermore, it was found that electrical energy delivered to the heart during defibrillation may, regardless of ischemia/reperfusion injury, influence development of post-cardiac arrest syndrome (4, 5). Eventually, myocardial damage in consequence of sudden cardiac arrest and cardiopulmonary resuscitation with defibrillation is a cumulative effect of ischemia/reperfusion injury and injury caused by electrical energy. The contribution of a shock impulse as an isolated factor in myocardial damage remains unclear. Evaluation of this damage is possible after application of energy of characteristics corresponding to a defibrillation shock impulse to a healthy heart beating at the sinus rhythm. The above observations influenced selection of the animal model for the experiments. Changes in the levels of endothelin in the course of postresuscitation disease are still not fully known, particularly in terms of a maximum increase in ET-1 levels and its duration following a low-energy defibrillation shock impulse.
Endothelin (ET) is a paracrine hormone regulating multiple organs, with three isomorphic forms: ET-1, ET-2, and ET-3, differing in their structure. It is synthesized in endothelium cells in vessels, brain, kidneys, intestines, and endocrine glands, in endometrial and placental cells, hepatocytes, type II pneumocytes, and Sertoli cells. The synthesis of these proteins is stimulated by metabolic acidosis, ischemia, catecholamines, hypoxia, hypercapnia, thrombin, insulin, lipoproteins LDL and HDL, angiotensin II, growth factors (fibroblast growth factor, epidermal growth factor, insulin-like growth factor-1), and cytokines (interleukins: IL-1, IL-2, IL-6 and tumor necrosis factor-a). Endothelin-1 (ET-1) induces the most potent vasoconstriction effect in vivo and in vitro (6-8). When administered intravenously, initially it induces vasodilation, followed by prolonged vasoconstriction. It causes constriction of renal, pulmonary and intestinal vessels, increases aldosterone, arginine and atrial natriuretic peptide levels, induces oxidative stress, and intensifies proliferation of endothelial cells and angiogenesis, and has a procoagulatory effect. These result in internal organ ischemia, vascular endothelial dysfunction, generation of reactive oxygen species, and development of oxidative stress. In the cardiac muscle, ET-1 released by endothelial cells induces coronary vasoconstriction, having simultaneous positive inotropic and chronotropic effects (9-12).
This study is aimed at evaluating time-dependent changes in ET-1 levels in serum and the heart of a healthy rabbit following the application of a low-energy two-phase shock impulse.
MATERIAL AND METHODS
Animals
The research was conducted in 35 healthy European mixed breed rabbits at the age of 36 – 42 weeks and with body mass (b.m.) ranging between 3200 and 4150 grams. During the experiment, the animals were kept in the standard laboratory conditions (air temperature 20 ± 3°C, humidity range between 50% and 70%) in 12-hour cycles (light phase: 7 a.m. – 7 p.m., dark phase: 7 p.m. – 7 a.m.). Feeding the animals was ceased in 12 hours before the experiment, with maintained access to water ad libitum.
Research protocol
Research performance was subject to the consent of the Local Ethical Committee for Experiments on Animals in Katowice, No. 34/2010 of 14 June 2010.
The animals were randomised into four groups: K0, B2J, B4J, B8J against the electrical energy dose to be applied during the experiment and premedicated with atropine sulphate mixture in the dose of 0.05 mg/kg b.m., droperidol in the dose of 0.1 mg/kg b.m. and morphine sulphate in the dose of 10 mg/kg b.m. After preparing the animals for further laboratory procedures, they were anaesthetized intramuscularly with ketamine (Bioketan) in the dose of 50 mg/kg b.m. Anaesthesia was sustained until the experiment was completed by fractionated intramuscular administration of ketamine every 30 – 50 minutes. After anaesthesia, the animals were connected to equipment for monitoring of life functions followed by right atrial catheter insertion and intracarotid cannulation into the ascending aorta. The transthoracic low-energy two-phase linear electrical impulse was applied in the studied groups of rabbits according to the scheme: B2J group - single shock impulse 2 J/kg b.m., B4J group - single shock impulse 4 J/kg b.m. and B8J group - dual shock impulse 4 J/kg b.m. within 2 minute interval. Energy volume provided during the electrical discharge complies with the 2015 European Resuscitation Committee guidelines for electrical defibrillation. During the experiment, the following life parameters of the animals were monitored with the use of BeneView T5 patient monitor with PMP module: 5-lead ECG, heart rate, respiratory rate, internal body temperature (MR 402B sensor), arterial blood pressure in the ascending aorta, blood pressure in the right atrium and peripheral blood saturation (518 A sensor). NaCl solution of 0.9% was infused intravenously in the volume of 15 ml/kg b.m. in the slow drip infusion. Passive oxygen therapy with 100% oxygen of 2.0 l/min flow was applied for arterial blood saturation levels below 94% maintained for more than 1 minute. The first blood samples in the volume of 10 ml (7 ml of venous blood and 3 ml of arterial blood) were taken in the studied groups before application of a transthoracic two-phase linear shock impulse. After 15 minutes following the application of shock impulse, the second blood sample in the volume of 10 ml (7 ml of venous blood and 3 ml of arterial blood), and after 360 minutes the third blood sample in the volume of 10 ml (7 ml of venous blood and 3 ml of arterial blood) were collected. The animals were killed afterwards, which was directly followed by sternotomy and cardiac excision. Cardiac muscle and large blood vessels were separated at the level of ascending aorta and pulmonary trunk. After blood extraction, the heart was weighed net of pericardial sac. Cardiac tissue sections from the central part of left ventricular wall myocardium were collected with biopsy forceps for biochemical tests. In the animal control group (K0), all the procedures were performed in compliance with protocol for the studied group except for shock impulse application.
Coronary perfusion pressure measurement
In order to measure and monitor the coronary perfusion pressure, the right cardiac atrium and ascending aorta were cannulated. The external jugular vein and internal carotid artery were dissected. The pediatric 5 Fr Arrow CS-14502 double-lumen catheter was inserted into the external jugular vein directly in the supramanumbrial area using the Seldinger method. The insertion depth of 45 mm was applied to obtain access to right atrial lumen. The internal carotid artery was cannulated directly in the supramanumbrial area with the use of 1.0/32 mm or 1.2/32 mm Becton Dickinson venflon. Pressure in right atrium and ascending aorta was measured continuously with the use of BeneView T5 patient monitor equipped with PMP module and one-off IBP transducers connected with pressure line to the cannula inserted in the carotid and jugular vessels of the rabbit. Systolic blood pressure (BPs), diastolic blood pressure (BPD) and mean ascending aortic pressure (BPM) and mean right atrium pressure (RAP) measurements were performed. Coronary perfusion pressure (CPP) was calculated as the difference between diastolic aortic pressure and diastolic right atrium pressure.
Application of low-energy two-phase shock impulse
The transthoracic electrical impulse was applied with the use of standard paediatric spoons and Zoll M Series defibrillator generating low-energy two-phase linear impulse (Zoll Rectilinear Biphasic). The Zoll M-series defibrillator has the function of active body impedance compensation ensuring high efficiency of the procedure regardless of the animal body mass. Defibrillator spoons were placed onto shaven and gel-covered skin in the right subclavian and cardiac apex areas. Electrical impulse was applied synchronously with ECG R wave. The Zoll M-series defibrillator has programmed defibrillation energy levels which imposes technical limitations when choosing the adequate energy dose per animal body mass. In defibrillation within the range between 1 and 10 J, it is possible to change the energy in 1 J increments. Defibrillation with energy above 10 J is possible only at the following programmed levels: 15 J - 20 J - 30 J - 50 J - 75 J - 100 J -120 J - 150 J - 200 J.
Determination of endothelin-1 and heart-type fatty acid binding protein using the ELISA method
Homogenates of 10% in ice-cold phosphate buffered saline (PBS) were prepared from collected cardiac tissue sections, which were then stored at –80°C before measurements. Serum samples were left at room temperature for two hours, and then centrifuged at 1000 × g for 20 minutes. Determination of ET-1 and heart-type fatty acid binding protein (h-FABP) was conducted with the ELISA method using a Uscn Life Science Inc. Wuhan kit (Wuhan, China). The ELISA tests were performed according to the manufacturer’s instructions provided with the kits. During the procedure, a Rayto RT-2600C washer and BioTek WWW ELx800 reader were used. The test sensitivity was 3.9 pg/ml. Absorbance levels were read with the use of a µQaunt (BioTek, USA) microplate reader and the results were processed with KC Junior (BioTek, USA) software. The inaccuracy of the methods equals 4.8% for ET-1 and 5.1% for h-FABP. The total protein levels in myocardial homogenates were determined using the Pyrogallol Red method (reagents from Sentinel Chemicals, Italy) and the biochemical analyser EM280 (Emapol, Gdansk, Poland) at 37°C.
Determination of the total oxidant status and nitrotyrosine levels in myocardial homogenates
The total oxidant status (TOS) was determined using the method described by Erel, based on a system containing xylene orange, o-dianisidine and Fe+2 ions. Measurements were conducted using the analyser EM 280, with the method calibrated against a series of hydrogen peroxide standard solutions (0 – 450 µmol/L). The method imprecision was 6.4%.
The nitrotyrosine level was determined with ELISA, using obtained by us nitrosine polyclonal rabbit antibodies (immunogen: hemocyanin from Megathura crenullata incubated with peroxynitrite), which were coated on the ELISA plate following immunochromatographic purification. After incubation with studied material for two hours, the plate was rinsed and bionitylated nitrotyrosine monoclonal mouse antibody (Cayman Chemicals Company, cat. No. 10006966) was added, at working dilution of 1:1000. Following another incubation with peroxidase-conjugated streptavidin (DakoCytomation, Denmark), peroxidase substrate (TMB Supersensitive, Sigma USA) was added, and after 20 – 30 minutes the reaction was arrested with 0.5 M sulfuric acid solution. The absorbance values were read with the µQaunt reader (BioTek USA). Rabbit albumin exposed to peroxynitrite was used as a standard, with the nitrotyrosine content determined on a basis of the molar absorption coefficient of 4300 M–1. The determination imprecision was 4.6%.
Examination of left ventricular myocardial specimens by transmission electron microscopy
The collected tissue sections were fixed in a 3% glutaraldehyde sodium cacodyl buffer at pH 7.2 for 2 hours at 4°C. The tissues were then rinsed three times with the same buffer for 24 hours, and then re-fixed in a 1% osmium tetroxide cacodylis-ic buffer. After dehydration in the growing alcohol series and propylene oxide, the tissues were embedded in the Epon 812 mixture. Polymerization of the tissue blocks was performed for 72 hours at increasing temperatures ranging from 35°C to 60°C. The Epone blocks were then cut into 1-µm-thick half-thin sections with Reichert OUM2 ultramicrotome, and stained with toluidine blue to observe and localize the lesions under a light microscope. Ultrathin sections with a size of 70-nm were obtained using an RMC diamond blade and copper nets, and automatically contrasted in Leica EM AC 20 with uranyl acetate and lead citrate. They were then analyzed and documented using the FEI Tecnai T12 Spirit electron microscope at 120 kV.
Statistical analysis
Statistical analyses were performed with the SAS statistical package, version 9.4 (SAS Institute Inc., Gary, NC). Frequency distributions of two qualitative variables were compared with the Fisher’s exact test. The Student’s t-test was used to compare the quantitative variables divided into two groups with distribution in both groups consistent with the normal distribution. Otherwise, the Mann-Whitney U test was applied. Where quantitative variable was divided into more than two groups, the analysis of variance (ANOVA) was used. Differences between the repeated measurement results were assessed with the use of the following: the Student’s t-test for repeated measurements or Wilcoxon’s signed-rank test depending on the normality of the distribution of differences. Furthermore, the expected values of the studied parameters were assessed with 95% confidence interval (95 % PU), followed by multi-factor analysis of variance. Correlation strength was presented using the Spearman’s rank correlation coefficient. The results of continuous measurements were subject to multi-dimensional analysis based on linear models with consideration to autoregressive structure of covariance. The parameter measurements results in the period before the electrical impulse application were averaged and introduced to the model as a base value.
RESULTS
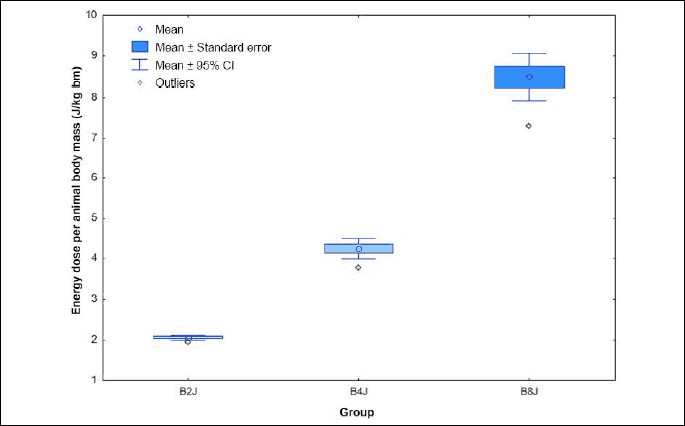
Electrical energy dose per animal body mass applied during the experiment is shown in the Fig. 1.
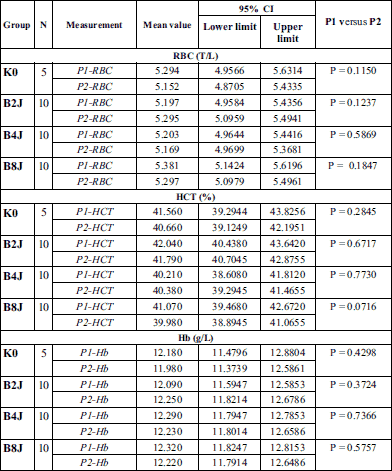
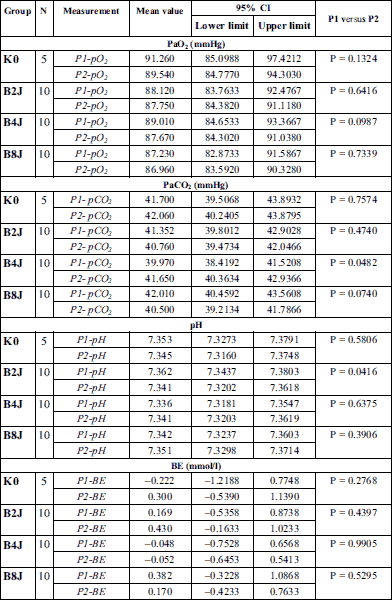
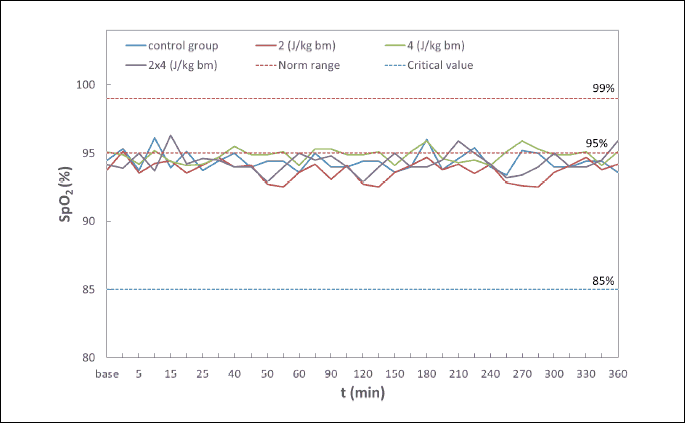
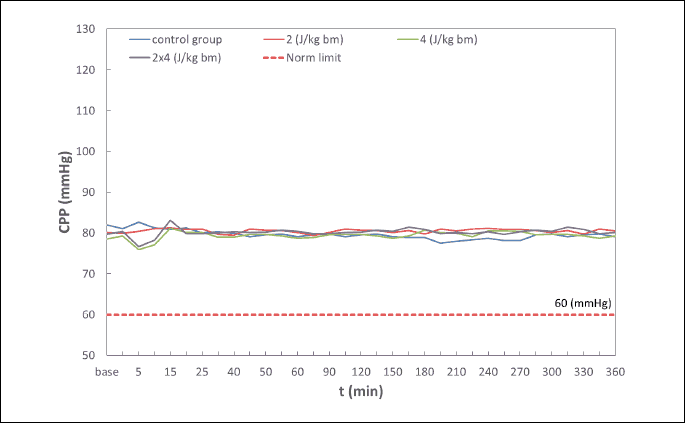
Within the scope of examined biochemical parameters (partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) in an arterial blood, pH, base exscess (BE), hematocrit (HCT), red blood cell count (RBC), hemoglobin level (Hb), arterial oxygen saturation (SpO2) and monitored life functions the disorders leading to ischemia and myocardial hypoxia that could influence the endothelin-1 levels regardless of effects of the shock impulse have not been detected in rabbits (Table 1, 2, and Fig. 2). Coronary perfusion pressure during the entire observation period was from 78 to 95 mmHg (Fig. 3). In all animals, CPP remained at a level significantly above 60 mmHg throughout the follow-up period after application of a low-energy shock impulse.
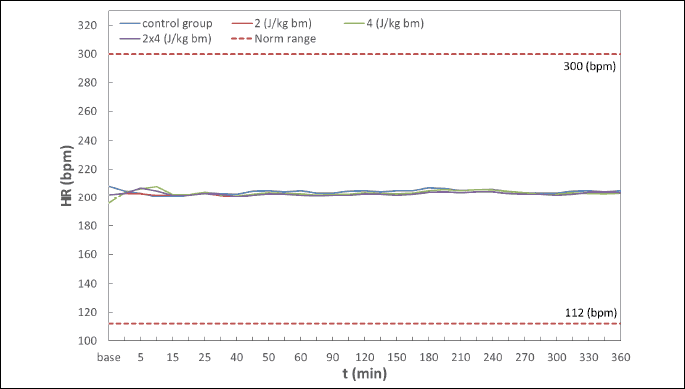
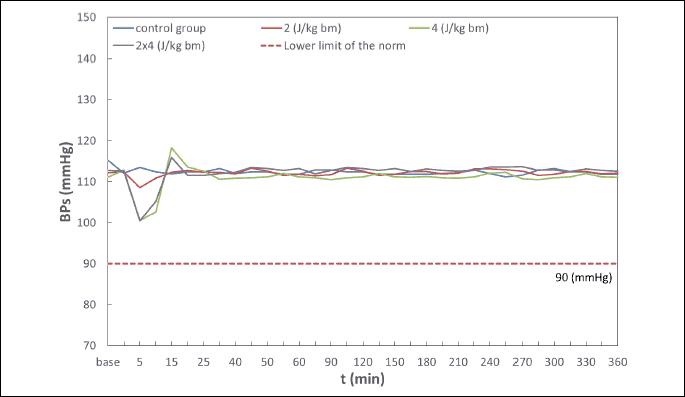
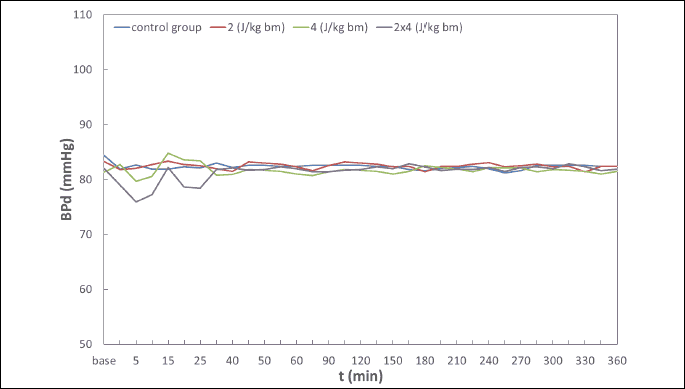
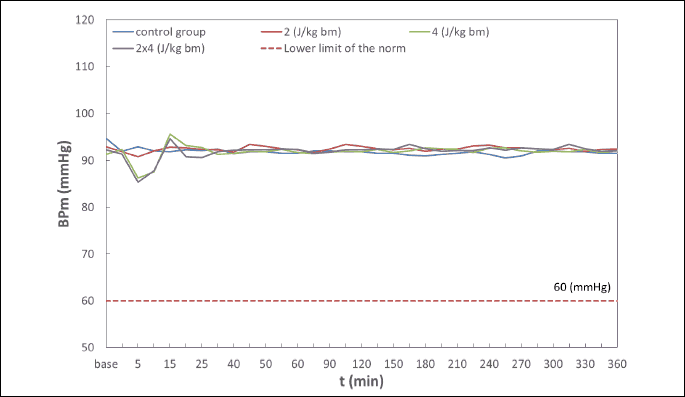
Hemodynamic disturbances in the range of heart rate (HR), systolic blood pressure (BPs), diastolic blood pressure (BPd), mean blood pressure (BPm) observed after electric pulse application were insignificant, short-term in nature, and did not reach the shock-typical critical values in any of the experimental animals (Fig. 4-7). Continuous monitoring of myocardial electrical activity showed only short-term variations of the ST segment ranging from +0.02 mV to –0.04 mV. Furthermore, in animals from all studied groups, short-term single ventricular ectopic beats occurred, in a number from 0 to 10/hour and maximum 1 per minute (Lown I A). However, no electrocardiographic features of ischemia, hypoxia, or myocardial infarction were observed in any of the experimental animals.
A significant increase in h-FABP serum levels was found in rabbits in all studied groups: B2J (P < 0.0001), B4J (P < 0.0001) and B8J (P < 0.0001). The highest h-FABP level was noted in the study group B8J, however, it was not significantly higher than in the study group B4J (P = 0.2419). After 360 minutes post application of the defibrillation shock impulse (P3hFABP) a slight increase in h-FABP serum levels in rabbits in all studied group was noted versus P2hFABP determinations. The increase in h-FABP levels was statistically significant in the study groups B4J (P = 0.0201) and B2J (P = 0.0285), while in the group B8J it was statistically insignificant (P = 0.0649) (Fig. 8).
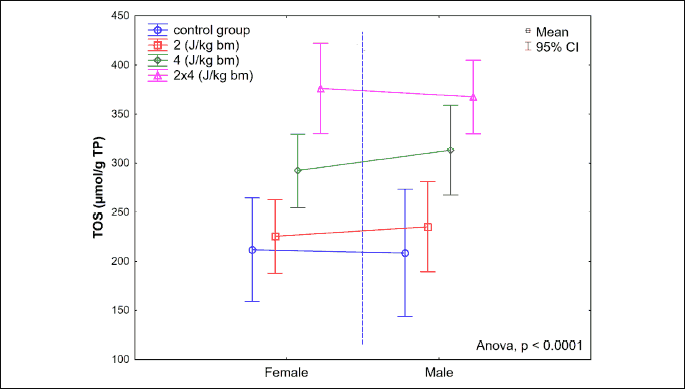
The transthoracic application of a low-energy defibrillation two-phase shock impulse, with a single impulse energy of 4 J/kg b.m., or a cumulated energy of 8 J/kg b.m., results in a statistically significant increase in the total oxidant status in the muscle of the left heart ventricle. The greatest increase in TOS was noted in the group B8J, and it was significantly higher than in the study group B4J (P < 0.0001). For studied groups B4J and B2J, differences in TOS levels in homogenate were also statistically significant (P < 0.0001). No statistically significant increase in the total oxidant status was observed following application of the defibrillation shock impulse of 2 J/kg b.m. No statistically significant gender-related differences were observed for values of the total oxidant status in homogenate of the myocardial section from the left heart ventricle (Fig. 9).
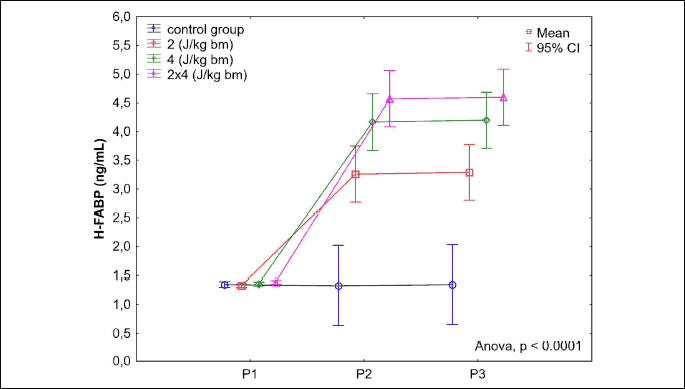
(P1 - before application, P2 - 15 minutes post application, P3 - 360 minutes post application). A significant increase in h-FABP serum levels in rabbits in all studied groups: B2J (P < 0.0001), B4J (P < 0.0001) and B8J (P < 0.0001). The highest h-FABP level in the study group B8J, however, not significantly higher than in the study group B4J (P = 0.2419). After 360 minutes post application of the defibrillation shock impulse (P3hFABP) a slight increase in h-FABP serum levels versus P2hFABP determinations: significant in the groups B4J (P = 0.0201) and B2J (P = 0.0285), statistically insignificant (P = 0.0649) in the group B8J.
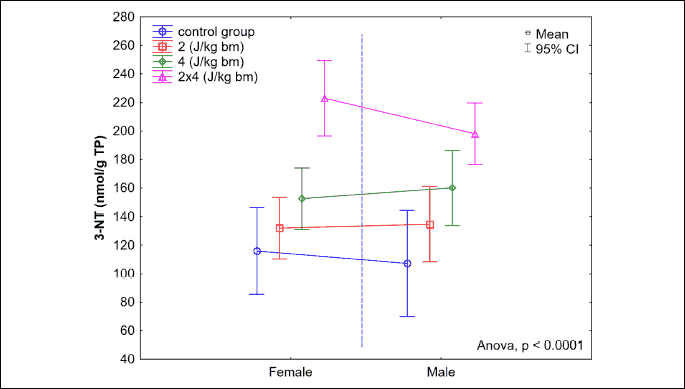
A statistically significant increase in the nitrotyrosine level in homogenate of the myocardial section from the left heart ventricle was observed. The greatest increase in the nitrotyrosine level was noted in the group B8J, and it was significantly higher than in the study group B4J (P < 0.0001). No statistically significant gender-related differences in the nitrotyrosine level in homogenate of the myocardial section from the left heart ventricle were observed (Fig. 10).
Results of measurements of endothelin-1 levels in blood serum and in homogenate of a myocardial section from the left heart ventricle in rabbits before application (P1) and 15 minutes (P2) and 360 minutes (P3) post application of a low-energy two-phase shock impulse are shown in Figs. 11 and 12. A significant increase in serum endothelin-1 levels was noted in all animals 15 minutes post application of a low-energy defibrillation two-phase shock impulse. The increase in ET-1 depends on the energy of the applied electrical impulse. Its highest value was found in the B8J group following the application of two impulses of 4 J/kg b.m. After 360 minutes from the application of the defibrillation shock impulse, a slight decrease in the serum ET-1 level was noted; however, it was still maintained at a significantly higher level versus the baseline values. A very strong correlation was found between the increase in the serum endothelin-1 levels in rabbits and the energy of the electrical impulse (ρs = 0.95, P < 0.0001).
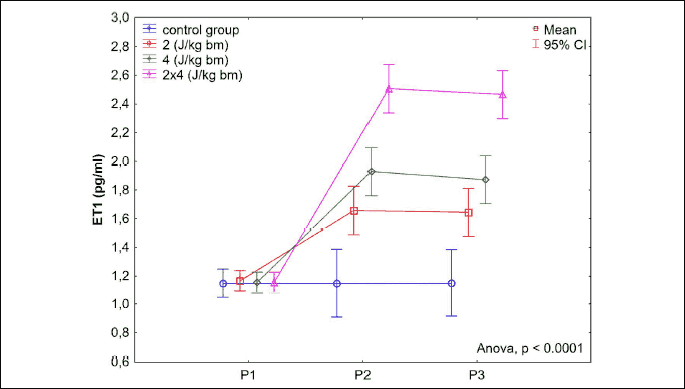
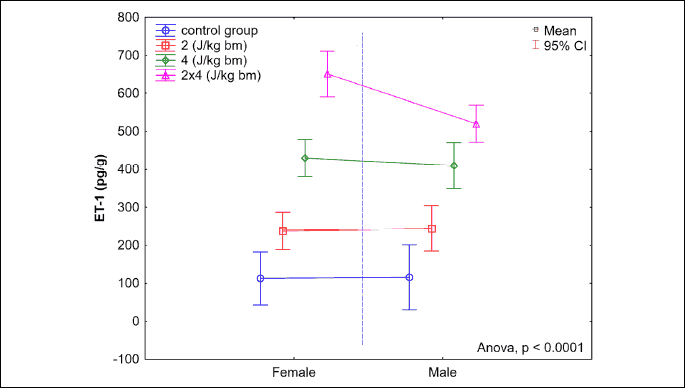
The transthoracic application of a low-energy defibrillation shock impulse of energy of 2 J/kg b.m. or 4 J/kg b.m., or a double impulse of a cumulated energy of 8 J/kg b.m., results in a significant increase in endothelin-1 levels in the muscle of the left heart ventricle in rabbits from all studied groups (P < 0.0001). The greatest increase was observed 360 minutes after the impulse application in the B8J group, and it was significantly higher versus the B4J group (P < 0.0001). For studied groups B4J and B2J, and for B2J and K0, differences in endothelin-1 levels were also statistically significant (P < 0.0001) in the discussed period. The statistically significant sex-dependant difference in the increase in the endothelin-1 levels in the heart muscle was found only in group B8J (P = 0.0019). A very strong correlation was found between the increase in the endothelin-1 levels in the left heart ventricle muscle in rabbits and the energy of the electrical impulse (ρs = 0.95, P < 0.0001).
Histological analysis under a light microscope did not show any morphological changes within the myocardium of the left ventricle following application of a low-energy RLB shock impulse. No morphological changes were found in the heart muscle, both after application of a single shock impulse of 2 J or 4 J/kg b.m., and after application of a double shock impulse of a total accumulated energy of 8 J/kg b.m. (Figs. 13-14).
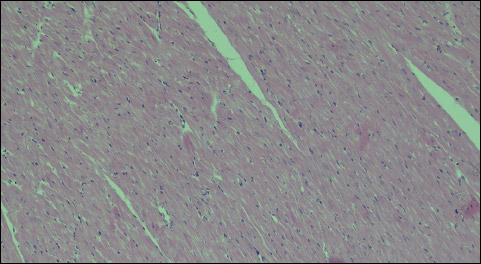 |
Fig. 13. Group B4J - normal morphological image of the myocardium after application of a single shock impulse RLB of 4 J/kg b.m., 100 × magnification. |
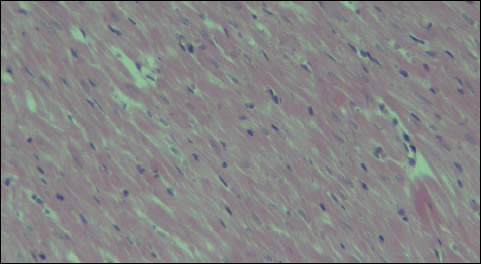 |
Fig. 14. Group B8J - normal morphological image of the myocardium after application of a double shock impulse RLB of a total accumulated energy of 8 J/kg b.m., 200 × magnification. |
The transmission electron microscopy results of left ventricle myocardial specimens are shown in Figs. 15-23. In all animals in the study groups B2J, B4J and B8J, the analysis of the ultrastructure of the muscle from the left heart ventricle disclosed focal lesions in cardiomyocytes and capillary vessels, gradually increasing with the increase in energy of the shock impulse. The observed changes in the capillary vessels had a form of swelling of endothelial cells. In the group B4J, that swelling was more pronounced and connections between endothelial cells were loosened, when compared to the group B2J. In the group B8J, significant swelling of the vascular endothelial cells was observed, which in some of them resulted in disruption of cell membranes and apoptotic changes. Lamellar structures appeared in the lumen of some vessels. Disruptions in the cardiomyocyte structure affected mitochondria, myofibrils, and endoplasmic reticulum, and had a form of: 1) mitochondria - swelling, shortening and blurring of mitochondrial cristae, matrix thinning, point loss of external membrane integrity, changes in a number and shapes of mitochondria; 2) myofibrils - loss of continuity of individual myofilaments; 3) endoplasmic reticulum - swelling.
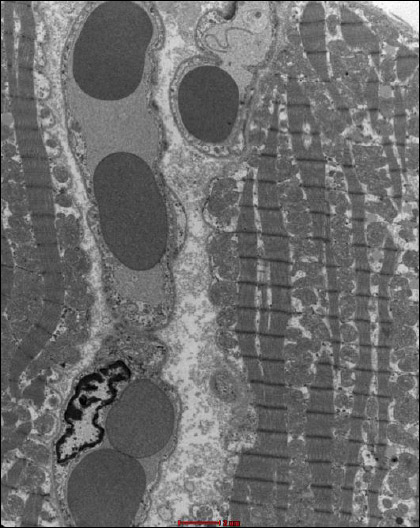 |
Fig. 15. Group K0 - normal ultrastructure of the myocardium. |
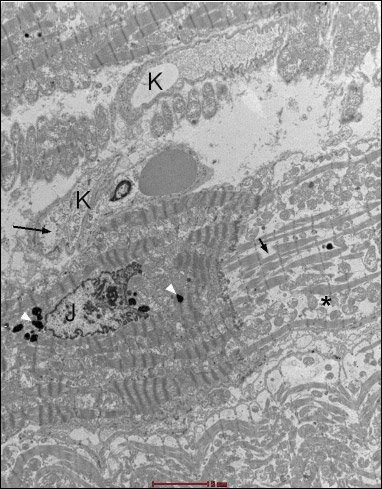 |
Fig. 16. Group B2J. A fragment of the heart muscle, forming a connection between two adjacent cardiomyocytes in a row. A nucleus (J), with electron dense grains of lipofuscin (white arrows) and numerous contraction nodes visible in one of them (the left one). In the second (right), focal decomposition of myofibrils (short black arrow), expanded space between myofibrils (*) and relatively numerous mitochondria are noticeable. Visible capillary vessels (K) in the interstitial tissue. The endothelium of one of the vessels has signs of oedema (longer black arrow). |
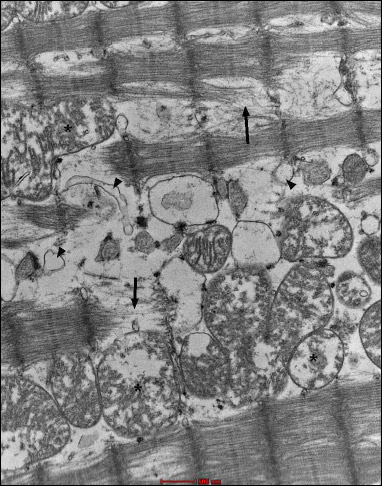 |
Fig. 17. Group B2J. Lost continuity of microfilaments (black arrow), oval mitochondria with the damaged inner membrane (residual cristae) (*), and single cisterns of smooth reticulum (black arrows). |
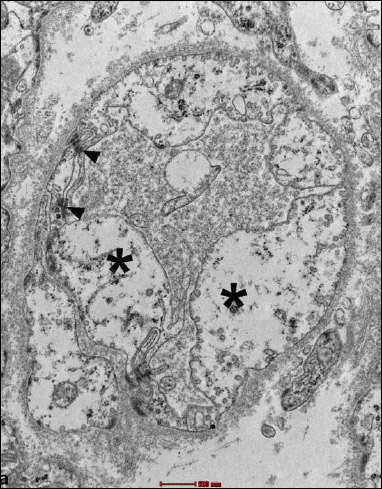 |
Fig. 18. Group B4J. Intensified swelling lesions - swollen endothelial cells of irregular surface, expanded cellular connections (arrow), highly diluted cytoplasm (*) which is practically devoid of organelles, and a single caveolae is visible. |
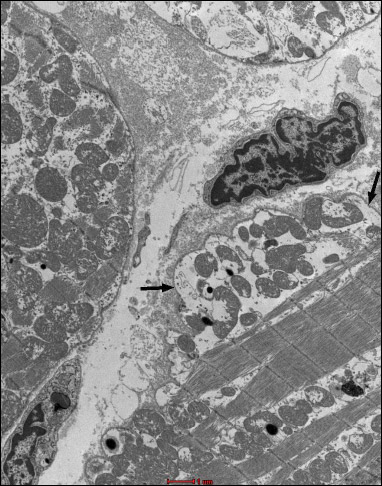 |
Fig. 19. Group B4J. Subsarcolemmal swelling (black arrows). |
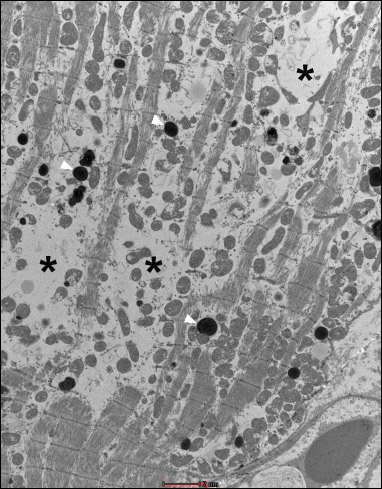 |
Fig. 20. Group B4J. Visible changes in the form of atrophy of myofibrils (*) and mitochondrial disorders, and numerous lysosomes (white arrows). Endothelial swelling is moderate. |
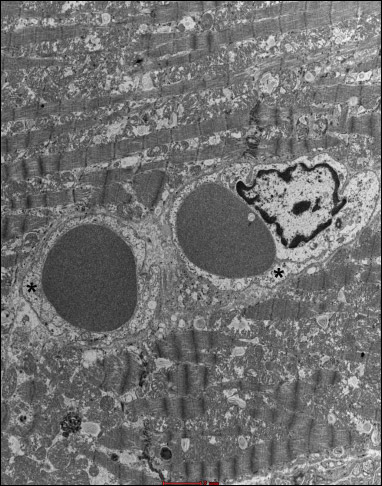 |
Fig. 21. Group 8J. Mild endothelial swelling in capillary vessels (*). |
In the group B4J, the mitochondrial changes and subsarcolemmal swelling were more pronounced, when compared to the group B2J. The most pronounced mitochondrial changes were observed in the group B8J. Mitochondrial damage increases with the increase in energy of the individual defibrillation shock impulse and the amount of cumulated energy.
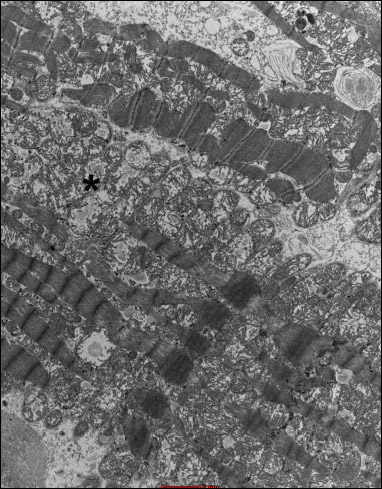 |
Fig. 22. Group 8J. Intensified swelling changes in mitochondria (*). |
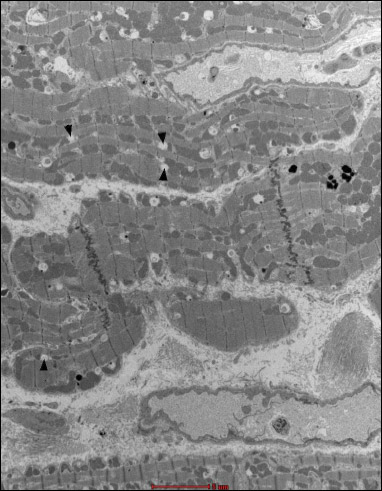 |
Fig. 23. Group 8J. Fragments of cardiomyocytes with signs of expanded smooth endoplasmic reticulum (black arrows). In the right bottom and top part of the figure, capillaries with normal endothelium are visible. |
DISCUSSION
The studies conducted by the authors demonstrated that a low-energy two-phase shock impulse of parameters corresponding to a defibrillation impulse causes a significant increase in ET-1 levels in serum and the myocardium of the left ventricle of a healthy rabbit heart beating at the sinus rhythm. The increase in the ET-1 level depends on energy of an individual electric impulse and on cumulative energy. Furthermore, an increase in the total oxidant status in the myocardium of the left ventricle was observed. A simultaneous increase in the myocardial nitrotyrosine levels indicates a developing free radical injury.
The heart fatty acid binding protein is a sensitive and early biomarker of cardiomyocyte damage. This protein is responsible for the transport of fatty acids from the cell membrane to mitochondria for their oxidation in metabolic processes. An increase in heart fatty acid binding protein levels found by the authors in the serum of healthy rabbits following the application of a low-energy shock impulse indicates that cardiomyocytes were damaged. Even a single transthoracic defibrillation two-phase shock impulse of 2 J/kg b.m. leads to an increase in h-FABP within 15 minutes after its application, which was maintained for at least 360 minutes. The increase in the h-FABP serum levels depends on the energy of the single shock impulse applied. At the same time, h-FABP does not rise with an increase in the total accumulated energy to 8 J/kg b.m. following the application of a double impulse of 4 J/kg b.m. Guensch et al. (13) found an increase in h-FABG levels in the serum of pigs during five hours after five applications of a low-energy shock impulse of 200 J. However, Sbarouni (14) did not observe a significant increase in the h-FABG serum levels in patients requiring cardioversion for atrial fibrillation.
Our study was conducted in a healthy heart, not burdened by acute ischemia following a sudden cardiac arrest. The sole stimulus was a single, or repeated after 2 minutes electrical defibrillation shock impulse of varying energy. At the same time, no anomalies were found in the rabbits, showing ischemia and/or hypoxia in the heart muscle, reflected in studied biochemical parameters and monitored body functions, which could influence endothelin-1 levels regardless of the electrical impulse effect. Kern et al. (15) observed a 50% reduction in blood flow in cardiac capillaries maintained for at least 4 hours after a sudden cardiac arrest. On the other hand, Doherty et al. (16) using perfusion scintigraphy with thallium, Tl-201, and sodium pyrophosphate99mTc did not find any blood flow anomalies in a muscle of a heart of a dog maintaining a sinus rhythm following a direct application of an electrical impulse of 30 J. Similarly, DiCola et al. (17) did not observe any microcirculatory abnormalities in the heart following 1 to 3 transthoracic electrical impulses of 400 J. The researchers analysed possible blood flow anomalies in the microcirculation without a simultaneous analysis of substances released by endothelium.
The coronary flow and myocardial microcirculation are maintained at a constant level within a wide range of coronary perfusion pressure by continuous autoregulation. Only a drop in diastolic pressure below the lower limit of autoregulation results in a reduction in the coronary flow that is proportional to a drop in coronary perfusion pressure. A crucial role in maintaining resting vascular muscle tone and autoregulation of flow in cardiac vessels is played by nitrogen oxide and endothelin-1 (18). During homoeostasis, endothelin-1, acting through ETB receptors located on vascular endothelium cells, increases nitric oxide (NO) and prostaglandin I2 (PGI2) release, counteracting vasoconstriction induced by stimulation of the ETA receptor (19-21). At the same time, the increase in nitrogen oxide levels inhibits, by feedback, ET-1 release. In their previously published study the authors observed a drop in NO levels in rabbit serum in a period from 15 minutes to 360 minutes post application of a low-energy impulse of parameters corresponding to a defibrillation shock impulse (22). A simultaneous increase in ET-1 levels and a drop in NO levels, observed from 15 minutes post application of the low-energy defibrillation shock impulse and maintained for up to 6 hours, dependant on the number and energy of electrical impulses, leads to abnormalities of various degree in the endothelial balance in the cardiac microcirculation. It was demonstrated that a drop in NO level or its reduced bioavailability might increase oxidative stress and deteriorate the myocardial function (23). The mechanism behind the contribution of endothelin-1 to ischemia-reperfusion injury may be related to an increased coronary vasoconstrictor effect of ET-1, increased accumulation of neutrophils, macrophages in the reperfused myocardium, reduced expression of endothelial NO synthase and production of reactive oxygen species (20, 24). At the same time, oxidative stress results in the transformation of NO reacting with superoxide anion radical into nitric superoxide anion. These reactions result in an increase in the 3-nitrotyrosine levels in cardiac muscle cells observed after low-energy defibrillation (22). It should be noted that changes in endothelin-1 levels, as well as changes in NO levels are observed during the most intense abnormalities of the cardiovascular function post low-energy defibrillation (22).
The authors found that transthoracic application of a low-energy RLB shock impulse of ≥ 2 J/kg b.m. leads to a significant increase in nitrotyrosine levels in the myocardium of the left ventricle of the healthy rabbit heart maintaining a sinus rhythm. The increase in nitrotyrosine levels implies a development of oxidative stress and free-radical damage by reactive oxygen and nitrogen species in the heart muscle. A peroxynitrite anion formed following the free-radical reactions causes cardiolipin degradation in the inner mitochondrial membrane and an irreversible stopping of the respiratory chain (25). Many authors note that protein nitrosylation during oxidative stress leads to a significant limitation in the bioavailability of NO due to its reaction with a superoxide radical anion (24, 26, 27). Disruptions in NO synthesis and bioavailability are considered to be one of the causes underlying post-defibrillation dysfunctions in the cardiovascular system. Zhang et al. (28) demonstrated that the peroxynitrite anion contributed to the damage of the heart muscle and development of its dysfunction following defibrillation with a low-energy single-phase shock impulse.
The transthoracic application of a low-energy RLB shock impulse leads to the increase in the TOS in the myocardium of the left ventricle of a healthy rabbit heart maintaining a sinus rhythm. A significant increase is visible only in animals that were applied a single or a double shock impulse of 4 J/kg b.m.
In 1996, Caterine et al. (29) used electron paramagnetic resonance spectroscopy to demonstrate that a transthoracic single-phase shock impulse of 200 J caused an increase in the ascorbic free-radical (AFR) levels in the myocardium. The increase in the AFR levels was noted from 3 minutes post application of the shock impulse, with a peak at 12 minutes. The increase in the AFR level depends on the energy of an individual impulse. At the same time, the total amount of energy and the ventricular fibrillation preceding defibrillation do not influence the AFR levels. The studies by Caterine et al. showed that high-energy shock defibrillation leads to an increase in production of oxygen free radicals in the heart muscle, resulting in its dysfunctions. The free radical mechanism of post-defibrillation damage to the heart muscle was also confirmed in studies by other authors (30-32). In our observations, contrary to Caterine et al. (29) the increase in the total oxidant status in the myocardium of the left ventricle in rabbits depends not only on the energy of a single impulse, but also on the total accumulated energy.
The concentration of ET-1 in the blood serum and in the homogenate of the left ventricular myocardium after the application of the electric pulse increases with the size of single discharge energy and the amount of cumulated energy. Concurrently, ultrastructural lesions of endothelial cells in the capillary vessels intensified along with the magnitude of the energy of the electrical pulse. Complete damage to the endothelial cells in the form of membrane disruption, apoptosis, and the occurrence of lamellar structures prevented the production of ET-1. However, such severe ultrastructural damage is not extensive and is only noted focally. Ultrastructural disorders are dominated by endothelial cell edema, which increases with defibrillation pulse energy, accompanied by an increase in ET-1 production.
An increase in ET-1 serum levels during cardiopulmonary resuscitation was observed. Similarly, an increase in vasopressin and angiotensin II levels was observed. A positive correlation between serum levels of endogenous vasoconstrictor peptides and aortic blood pressure indicates their potentially therapeutic role, due to their synergistic effect with epinephrine. The studies of Hilwig et al. (33) demonstrated that exogenous endothelin-1 causes an increase in blood pressure and coronary perfusion pressure during cardiopulmonary resuscitation. However, despite this mechanism, a decrease in myocardial blood flow and its ischemic injury occur. This injury is caused by a strong vasoconstrictor effect of ET-1 in microcirculatory vessels and by disrupted autoregulation. Eventually, the high level of endothelin-1 results in poorer prognosis in post-cardiac arrest disease. Oxidative stress developing in response to defibrillation may additionally intensify myocardial microcirculation dysfunctions and influence deterioration in its function (34).
In conclusions, our data demonstrate that transthoracic low-energy defibrillation shock impulse is causes a long-term increase in ET1 levels in heart muscle and blood serum in healthy rabbit. The increase in ET-1 levels results from the effect of electrical energy, independently of consequences of the ischemia/reperfusion injury. The increase in ET-1 levels may lead to capillary blood flow abnormalities in the heart, contributing to the development of its dysfunction in the course of post-cardiac arrest syndrome.
Conflict of interests: None declared.
REFERENCES
- Mongardon N, Dumas F, Ricome S, et al. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care 2011; 1: 45. doi: 10.1186/2110-5820-1-45
- Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008; 79: 350-379.
- Wojcik B, Knapp M, Gorski J. Ischemic heart preconditioning. J Physiol Pharmacol 2018; 69: 173-184.
- Gazmuri RJ, Deshmukh S, Shah PR. Myocardial effects of repeated electrical defibrillations in the isolated fibrillating rat heart. Crit Care Med 2000; 28: 2690-2696.
- Schirmer U, Hemmer W, Lindner KH, Anhaupl T, Wieser T. Ultrastructural alterations in the right and left ventricular myocardium following multiple low energy endocardial countershocks in anesthetized dogs. Pacing Clin Electrophysiol 1997; 20: 79-87.
- Rapoport RM, Merkus D. Endothelin-1 regulation of exercise-induced changes in flow: dynamic regulation of vascular tone. Front Pharmacol 2017; 8: 517. doi: 10.3389/fphar.2017.00517
- Bellien J, Iacib M, Monteil C, et al. Physiological role of endothelin-1 in flow-mediated vasodilatation in humans and impact of cardiovascular risk factors. J Hypertens 2017; 35: 1204-1212.
- Slomiany BL, Slomiany A. Role of endothelin-1-dependent up-regulation of leptin in oral mucosal repair. J Physiol Pharmacol 2005; 56: 531-541.
- Trindade M, Oigman W, Fritsch M. Potential role of endothelin in early vascular aging. Curr Hypertens Rev 2017; 13: 33-40.
- Gudddeti RR, Prasad A, Matsuzawa Y, et al. Role of endothelin in microvascular dysfunction following percutaneous coronary intervention for non-ST elevation acute coronary syndromes: a single-centre randomised controlled trial. Open Heart 2016; 3: e000428. doi: 10.1136/openhrt-2016-000428.
- Venkata GK, Forder JR, Clark D, et al. Ventricular fibrillation-induced cardiac arrest results in regional cardiac injury preferentially in left anterior descending coronary artery territory in piglet model. Biomed Res Int 2016; 2016: 5958196. doi: 10.1155/2016/5958196
- Nadar S, Blann AD, Lip GY. Endothelial dysfunction: methods of assessment and application to hypertension. Curr Pharm Des 2004; 10: 3591-3605.
- Guensch DP, Yu J, Nadeshalingam G, Fischer K, Shearer J, Friedrich MG. Evidence for acute myocardial and skeletal muscle injury after serial transthoracic shocks in healthy swine. PLoS One 2016; 11: e0162245. doi: 10.1371/journal.pone.016224510.1371
- Sbarouni E, Georgiadou P, Chaidaroglou A, Degiannis D, Voudris V. Heart-type fatty acid binding protein in elective cardioversion of atrial fibrillation. Clin Biochem 2011; 44: 947-949.
- Kern KB, Zuercher M, Cragun D, et al. Myocardial microcirculatory dysfunction after prolonged ventricular fibrillation and resuscitation. Crit Care Med 2008; 36: 418-421.
- Doherty PW, McLaughlin PR, Billingham M, Kernoff R, Goris ML, Harrison DC. Cardiac damage produced by direct current countershock applied to the heart. Am J Cardiol 1979; 43: 225-232.
- DiCola VG, Freedman GS, Downing SE, Zaret BL. Myocardial uptake of technetium-99m stannous pyrophosphate following direct current countershock. Circulation 1976; 54: 980-986.
- Polese A, De Cesare N, Montorsi P, et al. Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation 1991; 83: 845-853.
- Houde M, Desbiens L, D’Orleans-Juste P. Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol 2016; 77: 143-175.
- Kurzelewski M, Duda M, Stanley WC, Boemke W, Beresewicz A. Nitric oxide synthase inhibition and elevated endothelin increase oxygen consumption but do not effect glucose and palmitate oxidation in the isolated rat heart. J Physiol Pharmacol 2004; 55: 27-38.
- Jochem J, Zwirska-Korczala K, Gwozdz B, Walichiewicz P, Josko J. Cardiac and regional haemodynamic effects of endothelin-1 in rats subjected to critical haemorrhagic hypotension. J Physiol Pharmacol 2003; 54: 383-396.
- Zurawinski W, Sosada K, Muc-Wierzgon M, Kokot T. Impact of low-energy shock impulse on nitric oxide serum levels in healthy rabbits. J Biol Regul Homeost Agents 2017; 31: 377-382.
- Ahmad A, Sattar MA, Rathore HA, et al. Enhanced expression of endothelial nitric oxide synthase in the myocardium ameliorates the progression of left ventricular hypertrophy in l-arginine treated Wistar-Kyoto rats. J Physiol Pharmacol 2016; 67: 31-44.
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 1996; 79: 363-380.
- Dungel P, Teuschl AH, Banerjee A, Paier-Pourani J, Redl H, Kozlov AV. Impact of mitochondria on nitrite metabolism in HL-1 cardiomyocytes. Front Physiol 2013; 4: 101-112.
- Brady A, Warren J, Poole-Wilson P, Williams T, Harding S. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol 1993; 265: 176-182.
- Guterbaum TJ, Braunstein TH, Fossum A, Holstein-Rathlou NH, Torp-Pedersen CT, Dominguez H. Endothelial nitric oxide synthase phosphorylation at threonine 495 and mitochondrial reactive oxygen species formation in response to a high H2O2 concentration. J Vasc Res 2013; 50: 410-420.
- Zhang Y, Boddicker KA, Rhee BJ, Davies LR, Kerber RE. Effect of nitric oxide synthase modulation on resuscitation success in a swine ventricular fibrillation cardiac arrest model. Resuscitation 2005; 67: 127-134.
- Caterine MR, Spencer KT, Pagan-Carlo LA, Smith RS, Buettner GR, Kerber RE. Direct-current shocks to the heart generate free radicals: an electron paramagnetic resonance study. J Am Coll Cardiol 1996; 28: 1598-1609.
- Hackenhaar FS, Fumagalli F, Li Volti G, et al. Relationship between post-cardiac arrest myocardial oxidative stress and myocardial dysfunction in the rat. J Biomed Sci 2014; 21: 70-76.
- Yasuda S, Shishido T, Goto Y. Severe diastolic dysfunction with preserved energy conversion efficiency after countershock. Am J Physiol 1997; 273: 583-592.
- Pagan-Carlo LA, Garcia LA, Hutchison JL, Buettner GR, Kerber RE. Captopril lowers coronary venous free radical concentration after direct current cardiac shocks. Chest 1999; 116: 484-487.
- Hilwig RW, Berg RA, Kern KB, Ewy GA. Endothelin-1 vasoconstriction during swine cardiopulmonary resuscitation improves coronary perfusion pressures but worsens postresuscitation outcome. Circulation 2000; 101: 2097-2102.
- Toklu HZ, Muller-Delp J, Sakarya Y, et al. High dietary fructose does not exacerbate the detrimental consequences of high fat diet on basilar artery function. J Physiol Pharmacol 2016; 67: 205-216.
A c c e p t e d : February 28, 2019