ASTRAGALOSIDES MODULATES CONTRACTILE FUNCTION
OF TOAD GASTROCNEMIUS MUSCLE
INTRODUCTION
Huangqi (Radix Astragali) decoction (HQD) is a well-known traditional Chinese herbal formulation, it has been prescribed for general debilitation and chronic illnesses and to increase overall vitality for centuries (1). Astragalosides (AST) is the main active ingredient component of Huangqi. AST is used as the main component of many polyherbal formulations for treating patients with cancer and inflammation-associated diseases (2, 3). Recent studies have found that AST has various biological activities, such as anti-aging (4), immunomodulatory (5), anti-vascular disease (6), anti-neurodegeneration (7, 8) and anti-tumour (9) effects.
Skeletal muscle contractility plays an important role in human motor ability. The toad gastrocnemius muscle is a typical skeletal muscle tissue that is commonly used to study the mechanism of skeletal muscle contractility and anti-fatigue (10, 11). Toads are widely raised in various places of China. It is easy to make gastrocnemius specimen from toad. The gastrocnemius muscle can survive 12 hours in toad Ringer’s solution (RS).
Skeletal muscles contract to produce the force necessary in everyday life, but cannot contract continuously without impairment in performance (i.e. they fatigue) (12). Some evidences indicated that astragalus and astragalus polysaccharides can be used as an ergogenic aid for anti-fatigue (13, 14). AST attenuated myocardium lesions in Sprague-Dawley rats (15). Other study has shown that AST have the effects on reducing [Ca2+]i and sarcoplasmic reticulum Ca2+ load, enhancing free radical removal and decreasing lipid peroxidation in isoproterenol treated cardiomyocytes, which might account for their protective effect on myocardial injury (16). However, few reports demonstrate the effects of AST on the skeletal muscle thus far. We hypothesized that the AST might exert beneficial effect in anti-fatigue. Further studies are necessary to address these pharmacological effects. In our experiment, the modulating function of AST on the skeletal muscle contractile function was assayed on the isolated toad gastrocnemius muscle via electrical field stimulation (EFS) technique (11, 17). Our result showed AST can modulate the skeletal muscle contraction of toad gastrocnemius muscle.
MATERIAL AND METHODS
Drug preparation
Toad Ringer’s solution (RS) provides the equivalent physiological condition of the toad and is composed of 112 mM NaCl, 2 mM KCl, 1.5 mM CaCl2, 0.1 mM NaH2PO4 and 2.38 mM NaHCO3. The pH of the RS was adjusted to 7.2, and all measurements were recorded with the preparations equilibrated at room temperature (22 – 25°C) (11). Reagents used were purchased from Sigma Co. (St. Louis, MO, USA). AST with a purity of more than 98% was purchased from Aladdin Co. (Shanghai, China). AST was dissolved in RS at concentrations of 0, 10, 25, 50 mg/L, respectively.
Tissue preparation
The care and use of animals and the experimental protocol of this study were approved by the Institutional Care and Use Committee of Jiangxi Science and Technology Normal University.
The skeletal muscle tissue was prepared as described in our previously study (11). A total of 60 toads (100 ± 5g) were used in this study. Toads were euthanised by destruction of the spinal brain and decapitation. The hind limbs were skinned, and the resting length of both gastrocnemius muscles was measured to the nearest millimetre. Each muscle was carefully separated from the tibia after inserting a hook through the Achilles tendon and cutting the tendon distal to the hook. The tibia was then severed below the knee and the femur was cut near the pelvis. The weight of isolated muscle are 0.68 ± 0.05 g, the size of isolated muscle are 0.64 ± 0.04 mL. The muscles were then allowed to equilibrate in bath conditions of RS for 15 minutes. The muscles were immersed in the RS during preparation and throughout the experiment. The gastrocnemius muscle of toad is mainly fast glycolytic fibres, the rest is combined slow oxidative and fast oxidative-glycolytic fibres(18). The twitch time parameters of fast motor units in gastrocnemius muscle were shortened during the maturation (19).
Electrophysiological recording techniques and skeletal muscle contractile performance testing
Muscle performance characteristics were measured by a modification of the procedures described previously (11, 17). Briefly, EFS was applied and responses of the tissue were recorded and analyzed by a powerlab data acquisition system (AD Instruments, Inc., Colorado Springs, CO, USA). Each muscle was suspended vertically in 10 mL organ baths for isometric recording of mechanical activity by securing the truncated femur in a clamp and attaching the distal tendon, via the hook and a thread ligature, to the blade of a force displacement transducer (AD Instruments, Inc., model FT-100). The muscle was gently stretched to the measured resting length under optimal tension (2 g). A pair of stimulating electrodes was inserted into the muscle belly at right angles to each other, so that virtually all fibers could be stimulated simultaneously.
An individual muscle contractile response was elicited by applying single direct current impulse. Typically, the stimulation intensity was adjusted initially to produce the maximum amplitude of contractile response with single square pulses with 5 ms in duration. Sampling of individual muscle contractile response was recorded using pulses delivered once every 30 seconds. The muscles were randomized choose to test the effect of AST or control. One muscle was used only in one condition. The different AST concentration tested in the the sequence randomized. In the experiment for the testing of the contractile force-stimulation intensity curve, the stimulation intensity of the single square pulse was gradually increased from the sub-threshold stimulation intensity to the maximum stimulation intensity (100 mV increments; 5 ms duration; 30 seconds intervals). The threshold stimulation intensity was determined by gradually increasing the intensity of the single square pulse stimulation voltage until a contraction was elicited. The contractile force gradually increased by the single-pulse stimulation intensity further increased until the maximum amplitude was reached. This stimulation intensity was determined as saturation stimulation intensity. The AST and control groups were tested in the the sequence randomized. All recordings were collected after a stable baseline was present for at least 3 minutes; if this condition did not occur, the data was excluded from the study.
To characterize the effects of AST on the long-lasting repeated stimulation-induced decrease in muscle contractile force, a repetitive stimulation with saturation stimulation intensity was delivered at 1 Hz; the times to reach 50% and 10% of the maximum amplitude (initial value) were labeled as T50% and T10, which refer to our previous work (11). The average muscle sizes were similar in the two groups. In fact, in order to achieve same maximum amplitude (initial value) of control and AST groups, we reduced the stimulus intensity of the AST group. For the post-exercise strength recovery test, a testing single-pulse (using the same stimulation intensity impulse) was subsequently applied every 30 s to 10 min when the muscle contractile force decreased to 10% of the maximum amplitude (initial value) induced by the pre repetitive stimulation. The amplitude of the last single muscle contraction of the 10th min was employed to analyze the muscle recovery status. A contractile amplitude recovery of up to 60% of the maximum amplitude (initial value) was set as an eligible recovery data threshold. The AST and control groups were tested in the the sequence randomized.
Data analysis
The effects of AST on the skeletal muscle contractile responses evoked were characterized after the use of EFS. We measure the effect of AST on skeletal muscle contraction using maximum amplitude of contractile force. In the experiment of testing the contractile force-stimulation intensity curve, recordings were carried out application of a single concentration of AST or control. All numerical data were presented as mean ± SEM. One-way ANOVA analysis followed by Bonferroni post hoc tests were conducted to compare muscle contractile responses to external stimulation( factor: concentration of AST, level: 0 mg/L 10 mg/L 25 mg/L 50 mg/L), contractile force-stimulation intensity curve (factor: concentration of AST, level: control 25mg/L) and repeated stimulation-induced decrease in muscle contractile force in control and AST groups (factor: concentration of AST, level:control 25 mg/L). A level of P < 0.05 indicates statistical significance.
RESULTS
Effects of astragalosides to the muscle contractile responses
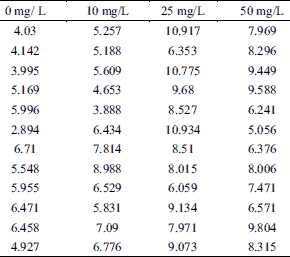
A total of 28 toads were used in this experiment. Fig. 1 illustrates the effects of AST to the contractile responses elicited via the single pulse stimulation on isolated toad gastrocnemius muscles. We used maximum amplitude of contractile force to measure the effect of AST on amplitude of muscle contraction. At concentrations of 0, 10, 25, 50 mg/L, the amplitude of contractile force were 5.19 ± 0.35 g, 6.17 ± 0.41 g, 8.83 ± 0.47 g and 7.76 ± 0.43 g, respectively (n = 12, Table 1). Application with AST (25 mg/L and 50 mg/L) profoundly increased the amplitude of muscle contraction compared to control (0 mg/L AST, P < 0.01) (Fig. 1B). As the muscle contractile responses of 25 mg/L AST was most compare to other groups (50 mg/L), this concentration was adopted for the followed tests.
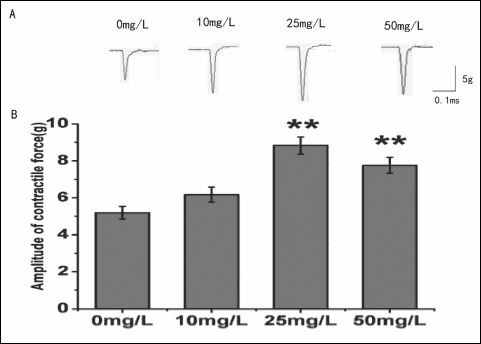 |
Fig. 1. Effects of AST on amplitude of contractile force induced via the electrical field stimulation (EFS) in isolated toad gastrocnemius muscles. (A): Sample traces (top) show the amplitude of contractile force recorded at concentrations of 0, 10, 25, 50 mg/L AST. (B): The statistical chart of different concentrations of AST (0, 10, 25, 50 mg/L) on the amplitude of skeletal muscle contraction. *P < 0.05, **P < 0.01 versus Control. |
Effects of astragalosides on the contractile force-stimulation intensity curve
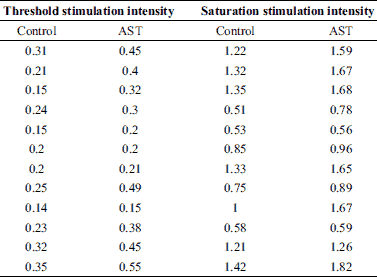
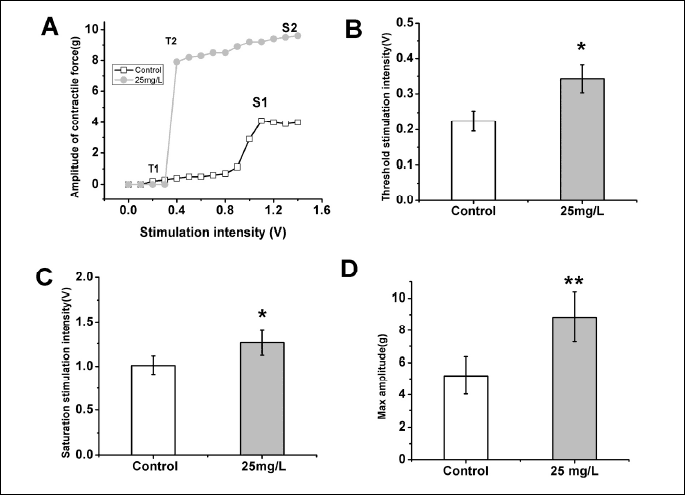
A total of 14 toads were used in this experiment. The contractile force-stimulation intensity curve of the skeletal muscles was acquired by gradually increasing the intensity of the single square pulses (100 mV increments; 5 ms duration; 30 s intervals) from sub-threshold stimulation intensity to saturation stimulation intensity (Fig. 2A). The application of AST enhanced the contractile responses to the external stimulation, as indicated by a marked increase in the threshold stimulation intensity (control: 0.23 ± 0.02 V, n = 12; AST: 0.34 ± 0.04 V, n = 12; P < 0.05) (Fig. 2A and 2B, Table 2) and saturation stimulation intensity (control: 1.01 ± 0.10 V, n = 12; AST: 1.26 ± 0.14 V, n = 12; P < 0.05) (Fig. 2A and 2C, Table 2). The maximum muscle contractile force of the skeletal muscles was markedly increased under AST application (control: 5.19 ± 1.16 g, n = 12; AST: 8.83 ± 1.50 g, n = 12; P < 0.05) (Fig. 2A and 2D, Table 1).
Effect of astragalosides on the repeated stimulation-induced decrease in muscle contractile force
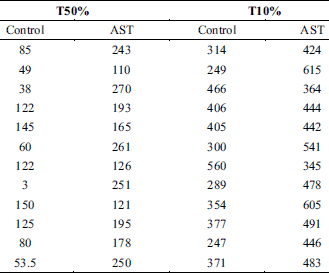
To further clarify the anti-fatigue modulation effect of AST on the skeletal muscle, the effects of AST on the repeated stimulation-induced decrease in muscle contractile force were studied using a repetitive stimulation procedure (continuous stimulation with saturation stimulation intensity at 1 Hz). A total of 18 toads were used in this experiment. As shown in the Fig. 3A, the muscle contractile force decreased gradually following the long-lasting muscle activity induced by the repetitive stimulation procedure. Interestingly, application with AST could postpone the occurrence of this process. The time of T50% (control: 86.04 ± 14.09 s, n = 12; AST: 196.92 ± 17.50 s, n = 12; P < 0.0001) (Fig 3B, Table 3) and T10% (control: 361.50 ± 27.52 s, n = 12; AST: 473.17 ± 25.04 s, n = 12; P < 0.001) (Fig. 3C, Table 3) were notably prolonged in the application of AST when compared with the control group. These results indicated that AST can alleviate the onset of repeated stimulation-induced muscle fatigue, as the continuous stimulation has been proposed as one of the mechanisms leading to muscle fatigue (20, 21).
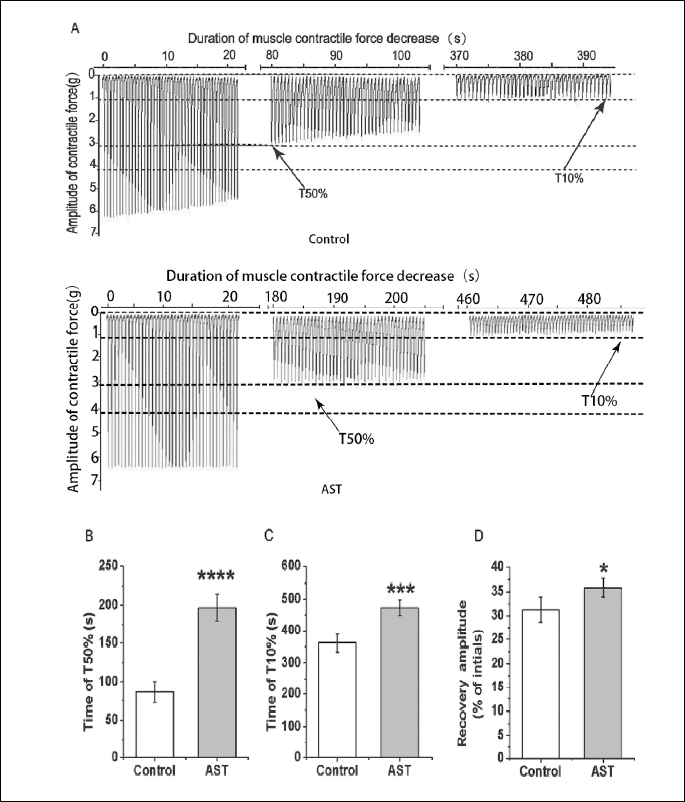
Moreover, AST also significantly enhanced the recovery after the repeated stimulation-induced decrease in muscle contractile force. The recovery amplitude of AST group (35.86 ± 1.88%, n = 12) was significantly higher than those of the control group (control: 31.29 ± 2.69%, n = 12; P < 0.05) (Fig. 3D, Table 4) indicating that AST can improve skeletal muscle recovery from long-lasting activity-induced muscle fatigue.
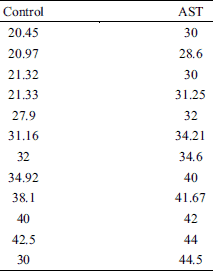
DISCUSSION
In the present study, the muscle contractile response induced by EFS on isolated toad gastrocnemius muscles was increased in the presence of AST (25 mg/L) (Fig. 1). AST also produced a leftward shift of the force -stimulation intensity relationship (Fig. 2A), suggesting that AST produces a larger contractile amplitude at the same stimulus intensity. The threshold stimulation intensity and saturation stimulation intensity in AST group were increased significantly compared with the control group (Fig. 2B and 2C). These results suggest the reduced excitability and strength of muscle fibers with the application of AST. Most notably, the maximum muscle contractile force was increased significantly with AST application under higher saturation stimulation intensity (Fig. 2A and 2D). This increase demonstrated that AST can improve the contractile function of skeletal muscle. Furthermore, this increase may be through the improvement in the muscle response sensitivity (the degree of muscle response under the same stimulus parameters), because muscle physical fitness responded to the external stimulation. The results showed that AST can delay the repeated stimulation-induced decrease in muscle contractile force via repetitive stimulation and can improve post-exercise recovery from the repeated stimulation-induced fatigue (Fig. 3). Collectively, these results demonstrate that AST can positively affect toad gastrocnemius muscle performance and provide ergogenic and prophylactic benefits in decreasing skeletal muscle fatigue. Reduced intracellular calcium release during fatigue and has been shown to help reduce strength (22). AST may increase intracellular calcium release to relieve muscle fatigue. This hypothesis will be invested in our future work.
Typically, the muscle strength is usually used as a physiological/pathological index to identify the muscle contractile function. Decreased muscle strength has been reported after fatigue in different parts of the human body (23, 24). Interestingly, our results showed that the application of AST significantly increased the muscle response sensitivity to external stimulation and markedly increased the maximum muscle contractile force under higher saturation stimulation intensity, suggesting that the muscle contractile function was modulated to better adapt to the changes in the external environment, such as temperature change. The increase in the maximum muscle contractile force also implies that AST has potential anti-fatigue effect.
Muscle fatigue is an exercise-induced decline in maximal voluntary muscle force or power, and it develops soon after the onset of sustained physical activity (25). Fatigue is common in daily life, but the cellular and physiological mechanisms involved in fatigue are not fully understood. The level of circulating brain-derived neurotrophic factor (BDNF), but not cortisol, proinflammatory (IL-6), anti-inflammatory (IL-10) or norepinephrine (NE), was associated with changes in central motor fatigue (26). Physical fitness is a major factor in preventing fatigue. However, within common physiological limits anyone may experience a state of muscle fatigue if the time period is long enough (23, 27). Therefore, long-lasting stimulation has been proposed as one of the common mechanisms leading to muscle fatigue (20, 21). In previous work, repetitive muscle compression reduces the hyperemic response to muscle contraction in human body (28). We found that AST could significantly postpone the repeated stimulation-induced decrease in muscle contractile force via repetitive stimulation and could improve post-exercise recovery from repeated stimulation-induced fatigue. This result indicates that AST has its anti-fatigue function by improving the muscle physical fitness through its modulation of muscle response sensitivity and maximum muscle strength (Fig. 1 and 2). Our result is well in accordance with the previous study, in which AST, a main component of Huangqi Jianzhong Tang, increase exhaustion time, positively influence anaerobic threshold, and also enhance recovery from fatigue of athletes (29), Astragalus membranaceus increased endurance exercise capacity and ameliorates exercise-induced fatigue in trained mice (13), and AST can prolong the swimming time in hypoxic mice, which demonstrated that AST has an obvious anti-fatigue effect (14).
In conclusion, to the best of our knowledge, the present study is the first to provide direct evidence on the capacity of AST to modulate skeletal muscle contraction in isolated toad gastrocnemius muscles. Our results revealed that AST can positively affect muscle performance. AST also has an anti-fatigue effect on skeletal muscle contraction. These novel findings indicate that AST can provide ergogenic and prophylactic benefits in decreasing skeletal muscle fatigue.
S.-S. Wei and Y.-W. Zou contributed equally to this study.
Acknowledgements: This work was supported by the National Natural Science Foundation of China (31660275, 31860605, 31660292, 3176007), Jiangxi Outstanding Youth Talent Cultivation Program of Jiangxi Provincial Department of Science and Technology (20171BCB23077), the Science and Technology Program of Department of Education of Jiangxi Province (GJJ170675,GJJ180594), Key Construction Laboratory Program of Jiangxi Science and Technology Normal University (2017ZDPYJD004).
Conflict of interests: None declared.
REFERENCES
- Lee DY, Noh HJ, Choi J, et al. Anti-inflammatory cycloartane-type saponins of Astragalus membranaceus. Molecules 2013; 18: 3725-3732.
- Qi Y, Gao F, Hou L, Wan C. Anti-inflammatory and immunostimulatory activities of Astragalosides. Am J Chin Med 2017; 45: 1157-1167.
- Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med 2016; 44: 1-22.
- Liu P, Zhao H, Luo Y. Anti-aging implications of Astragalus membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis 2017; 8: 868-886.
- Kong X, Hu Y, Rui R, Wang D, Li X. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int Immunopharmacol 2004; 4: 975-982.
- Yu JM, Zhang XB, Jiang W, Wang HD, Zhang YN. Astragalosides promote angiogenesis via vascular endothelial growth factor and basic fibroblast growth factor in a rat model of myocardial infarction. Mol Med Rep 2015; 12: 6718-6726.
- Li WZ, Wu WY, Huang DK, et al. Protective effects of astragalosides on dexamethasone and Abeta25-35 induced learning and memory impairments due to decrease amyloid precursor protein expression in 12-month male rats. Food Chem Toxicol 2012; 50: 1883-1890.
- Huang XP, Tan H, Chen BY, Deng CQ. Combination of total Astragalus extract and total Panax notoginseng saponins strengthened the protective effects on brain damage through improving energy metabolism and inhibiting apoptosis after cerebral ischemia-reperfusion in mice. Chin J Integr Med 2017; 23: 445-452.
- OuYang Y, Huang J, OuYang Z, Kang J. Enrichment and purification process of astragalosides and their anti-human gastric cancer MKN-74 cell proliferation effect. Afr Health Sci 2014; 14: 22-27.
- Park BS, Henning PC, Grant SC, et al. HMB attenuates muscle loss during sustained energy deficit induced by calorie restriction and endurance exercise. Metabolism 2013; 62: 1718-1729.
- Yao LH, Meng W, Song RF, et al. Modulation effects of cordycepin on the skeletal muscle contraction of toad gastrocnemius muscle. Eur J Pharmacol 2014; 726: 9-15.
- Place N, Bruton JD, Westerblad H. Mechanisms of fatigue induced by isometric contractions in exercising humans and in mouse isolated single muscle fibres. Clin Exp Pharmacol Physiol 2009; 36: 334-339.
- Yeh TS, Chuang HL, Huang WC, Chen YM, Huang CC, Hsu MC. Astragalus membranaceus improves exercise performance and ameliorates exercise-induced fatigue in trained mice. Molecules 2014; 19: 2793-2807.
- Zhang G, Zhou SM, Zheng SJ, Liu FY, Gao YQ. Astragalus on the anti-fatigue effect in hypoxic mice. Int J Clin Exp Med 2015; 8: 14030-14035.
- Chen XJ, Meng D, Feng L, et al. Protective effect of astragalosides on myocardial injury by isoproterenol in SD rats. Am J Chin Med 2006; 34: 1015-1025.
- Meng D, Chen XJ, Bian YY, Li P, Yang D, Zhang JN. Effect of astragalosides on intracellular calcium overload in cultured cardiac myocytes of neonatal rats. Am J Chin Med 2005; 33: 11-20.
- Ziganshin AU, Kamaliev RR, Grishin SN, Ziganshina LE, Zefirov AL, Burnstock G. The influence of hypothermia on P2 receptor-mediated responses of frog skeletal muscle. Eur J Pharmacol 2005; 509: 187-193.
- Wilson RS, James RS, Kohlsdorf T, Cox VM. Interindividual variation of isolated muscle performanceand fibre-type composition in the toad Bufo viridus. J Comp Physiol B 2004; 174: 453-459.
- Dobrzynska Z, Celichowski J. Changes in contractile properties and action potentials of motor units in the rat medial gastrocnemius muscle during maturation. J Physiol Pharmacol 2016; 67: 139-150.
- Vukova TI, Dimitrov V, Radicheva N. Three methods for estimation of changes in frequency characteristics of potentials elicited by long-lasting (fatiguing) activity of isolated muscle fibres. Gen Physiol Biophys 2010; 29: 243-254.
- Robin G, Allard B. Major contribution of sarcoplasmic reticulum Ca(2+) depletion during long-lasting activation of skeletal muscle. J Gen Physiol 2013; 141: 557-565.
- Allen DG, Kabbara AA, Westerblad HK. Muscle fatigue: the role of intracellular calcium stores. Can J Appl Physiol 2002; 27: 83-96.
- Lopes-Martins RA, Marcos RL, Leonardo PS, et al. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 2006; 101: 283-288.
- Marcellis RG, Lenssen AF, de Vries J, Drent M. Reduced muscle strength, exercise intolerance and disabling symptoms in sarcoidosis. Curr Opin Pulm Med 2013; 19: 524-530.
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 2008; 586: 11-23.
- Verbickas V, Baranauskiene N, Eimantas N, et al. Effect of sprint cycling and stretch-shortening cycle exercises on the neuromuscular, immune and stress indicators in young men. J Physiol Pharmacol 2017; 68: 125-132.
- Borsa PA, Larkin KA, True JM. Does phototherapy enhance skeletal muscle contractile function and postexercise recovery? A systematic review. J Athl Train 2013; 48: 57-67.
- Messere A, Turturici M, Millo G, Roatta S. Repetitive muscle compression reduces vascular mechano-sensitivity and the hyperemic response to muscle contraction. J Physiol Pharmacol 2017; 68: 427-437.
- Chen KT, Su CH, Hsin LH, Su YC, Su YP, Lin JG. Reducing fatigue of athletes following oral administration of huangqi jianzhong tang. Acta Pharmacol Sin 2002; 23: 757-761.
A c c e p t e d : February 28, 2019
Dr. Li-hua Yao, School of Life Science, Jangxi Science and Technology Normal University, Nanchang, Jangxi 330013, P.R.China e-mail: yaolh7905@163.com