REGULATORY EFFECT OF DEXAMETHASONE ON TRACHEAL CALCIUM PROCESSING PROTEINS AND MUCOSAL SECRETION
INTRODUCTION
The trachea forms a continuous system that conducts air between the larynx and lungs. The trachea warm and humidifies inhaled air, resulting in an air-conditioning effect during respiration. This adaptation in the trachea prevent lung damage induced by dehydration and heat loss (1). Mucus glands present in the tracheal epithelium produce surfactants containing heterogenous mucin (MUC) proteins. To date, 21 MUC proteins have been identified, of which 14 are expressed in the airways (2). MUC proteins are produced by goblet cells embedded in the tracheal epithelium or submucosal glands present in the tracheal tissues (3). Mucosecretion is important for maintaining tracheal airflow. Moreover, the thickness of the mucus layer regulates the variations in the tracheal diameter. Therefore, the maintenance of constant mucosecretion is physiologically important. A reduction in the diameter increases air-resistance and decreases inspired airflow (4). Abnormalities in tracheal mucosecretion are associated with major respiratory diseases such as chronic bronchitis, cystic fibrosis, or asthma, which are characterized by glandular enlargement and mucus hypersecretion in the airways (5).
Calcium is an important modulator of mucosecretion in various tissues; however, the exact role of calcium in mucosecretion is not completely understood (6, 7). Several studies have suggested that calcium plays a role in accumulation of mucosubstances in the cytoplasm by providing cationic shield to the negatively charged Mucin proteins (8, 9). MUC protein granules are smaller in conjunctival goblet cells of calcium-deficient mice than in those of wild- type mice (10). In mammalian cells, intracellular calcium homeostasis is maintained by transmembrane calcium channels, sodium-calcium exchangers, calcium ATPase and cytosolic calcium binding proteins (11). In the present study, two calcium influx channel proteins, namely transient receptor potential vanilloid-4 (TRPV4) and transient receptor potential vanilloid-6 (TRPV6); one calcium-binding protein, namely, calbindin-D9k (CaBP-9k); and one calcium efflux channel protein, namely, plasma membrane Ca2+-ATPase 1 (PMCA1) were examined. TRPV4 channel has been initiating sudden calcium influx in the murine lungs upon exposure to high pressure ventilation or heat (12). TRPV6 channel protein also plays a role in calcium influx in the airway epithelium (13). TRP channels have been largely studied for their role in kidney and intestinal calcium absorption, however, no researches have been conducted on trachea (14). CaBP-9k, a cytosolic calcium binding protein, is expressed in various mammalian tissues, including the lung (15, 16). PMCA1, a transmembrane calcium pump, extrudes excessive calcium into the extracellular space (17). In many organs, the expression of these calcium processing proteins is regulated by steroid hormones such as estrogen, progesterone, or glucocorticoid hormones in a tissue-dependent manner (15, 18, 19). In the esophagus, estrogen downregulates the expression of CaBP-9k and PMCA1 proteins, whereas progesterone does not exert any regulatory effect on the expression of calcium processing proteins (20). In the lungs, glucocorticoid hormones upregulate the expression of these calcium processing proteins (21). Steroid hormone-induced regulation of the calcium processing proteins has been studied in multiple tissues, except the trachea. Steroid hormones are associated with tracheal and pulmonary diseases. Glucocorticoids have been prescribed for 50 years for treating acute asthma exacerbation because they inhibit inflammation with decreasing mucosecretion. Dexamethasone, a commonly used systemic glucocorticoid, decreases tracheal mucosecretion in rats (22). Dexamethasone exerts the regulatory effect on calcium processing genes in several tissues with tissue specific manner. In renal and duodenal tissues, dexamethasone has negative effect on the expressions of calcium processing genes and decreases serum calcium level while it has positive effect on those gene expressions in lung and decreases alveolar surfactant (21, 23). However, the influence of dexamethasone on tracheal calcium processing have never been reported. Detailed regulatory mechanism of underlying dexamethasone-induced decrease in mucosecretion is unclear. As far as studied, controlling of mucosecretion is involved with calcium mediated signaling, as well as with immune response via Jak/Stat signaling pathway (24). Therefore, the present study investigated the dexamethasone-induced regulation of calcium processing proteins and mucoscretion in the mouse trachea.
MATERIALS AND METHODS
Animals and treatments
Mature male and immature female C57BL/6 mice were obtained from Samtako (Osan, Republic of Korea). All animals were nurtured in polycarbonate cages and acclimated in an environmentally controlled room (temperature: 23 ± 2°C, relative humidity 50 ± 10%, frequent ventilation and 12 h light/dark cycle). After approximately seven days of acclimatization, to examine the effect of dexamethasone (DEX; 10 mg/kg; Sigma-Aldrich, St Louis, USA) on the calcium processing genes, mature mice (n = 10 per group, ten weeks old, 6 mice for mRNA analysis and 4 mice for histological analysis) were injected subcutaneously with corn oil as a vehicle for five consecutive days. To examine the effect of the glucocorticoid receptor (GR) antagonist treatment, mice were treated with mifepristone (RU486; 50 mg/kg; Sigma-Aldrich) 30 minutes prior to DEX treatment. To examine the effect of sex steroid hormones, immature female mice (n = 5 per group, two weeks old) were injected subcutaneously with estradiol (E2; 40 µg/kg; Sigma-Aldrich) and progesterone (P4; 4 mg/kg; Sigma-Aldrich). To determine the effect of hormones antagonists, ICI 182,780 (ICI; 10 mg/kg; Tocris, Avonmouth, UK) and RU486 (10 mg/kg) were administered 30 minutes before hormone/vehicle treatment. Mice were euthanized 24 hours after final injection.
All procedures and animal treatments were approved by the Institutional Animal Care and Use Committee of Chungbuk National University (CBNUA-1173-18-01).
RNA extraction and real-time PCR
Mouse trachea was collected by dividing cervical and thoracic tracheae with the removal of esophagus and thyroid gland. Samples were washed with cold saline and homogenized in TRIzol (Invitrogen; Thermo Fisher Scientific, Waltham, USA) according to the manufacturer’s instruction. The total RNA concentration was measured by absorbance at 260 nm by using an Epoch Microplate Spectrophotometer (BioTek Instruments, Winooski, USA). First strand complementary DNA (cDNA) was prepared by subjecting total RNA (1 µg) to reverse transcription using Moloney murine leukemia virus reverse transcriptase (Intron Biotechnology, Seongnam, Republic of Korea) and random primers (9-mers; TaKaRa Bio Inc., Kusatsu, Japan).
A mixture for quantitative real-time polymerase chain reaction (qPCR) contains 2 × SYBR Premix Ex Taq (TaKaRa), ROX reference dye, forward and reverse primers, cDNA and distilled water. The real-time PCR was performed using Applied biosystems Quant Studio 3 real-time PCR Instrument (Applied biosystems; Thermo Fisher) equipped with a 96-well optical reaction plate. The reactions were carried out for 40 cycles according to the following parameters: denaturation at 95°C for 15 s, annealing at 58°C for 30 s and extension at 72°C for 30 s. The oligonucleotide primer information is listed on the Table 1. Fluorescence intensity was measured at the end of the extension phase of each cycle. The amount of transcript is inversely related to the observed CT, and for every 2-fold dilution in the transcript, CT is expected to increase by 1. Relative expression was calculated using the equation R = 2–(ΔCTGOI – ΔCTHKG). To determine a standardized arbitrary value for gene expression, each expression levels were standardized to that of cytochrome C oxidase subunit 1 (CO1).
Immunofluorescence
Localization of four calcium processing proteins TRPV4, TRPV6, CaBP-9k, PMCA1; mucin producing protein Mucin1; and tracheal basal cell marker Keratin 14 was detected by using immunofluorescence assays. After fixing with 10% formalin, the trachea was embedded in paraffin, cut into 5 µm sections, mounted on glass slides, deparaffinized using xylene and hydrated in descending graded ethanol solutions. Antigen retrieval process was performed with citrated buffer (Dako; Agilent, Santa Clara, USA). During this process, tissues were boiled into buffer solution at 20 min. Nonspecific reactions were blocked by incubating the sections in 10% normal goat serum (Vector laboratories, Burlingame, USA) in PBS for 1 hour at room temperature. Then, tissues were incubated with primary antibodies overnight at RT with primary antibodies against TRPV4 (ACC-034, Alomone Labs, Jerusalem, Israel), TRPV6 (ACC-036, Alomone), CaBP-9k (Swant, Bellinzona, Switzerland), PMCA1 (PMCA1, Swant) and KRT14 (Ab7800, Abcam, Cambridge, UK), Mucin1 (MA5-11202, Invitrogen). All primary antibodies were diluted as 1:250 with 1% bovine serum albumin (BSA; Bioworld, St Louis Park, USA). After being washed with TBS-T, the sectioned tissues were incubated with secondary antibodies (1:500, Rabbit IgG or Hamster IgG; Vector) for an h at 37°C and wash with TBS-T. As anuclear counterstaining agent, 4’6-diamidino-2-phenylindole (DAPI; Thermo Fisher) was used, followed by applying a mounting agent (Vector).
Avidin biotin complex horseradish peroxidase (ABC-HRP) assay
Tissue-localization of TRPV4, TRPV6, CaBP-9k, and PMCA1 proteins were conducted by using immunofluorescence assays. The paraffin embedded tissues were deparaffinized with xylene and hydrated in descending graded ethanol solutions. Antigen retrieval process was performed with citrated buffer (Dako). During this process, tissues were boiled into a buffer solution at 20 min. Next, tissues were treated in TBS-T with 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Nonspecific reactions were blocked by incubating the sections in 10% normal goat serum (Vector) in PBS for 1 hour at room temperature. Samples were then probed with primary antibodies (1:250) at RT overnight, after with they were washed three times with TBS-T. The tissues-sections were then reacted with biotinylated secondary antibodies (1:500, Rabbit IgG; Vector) for 1 hour at 37°C, washed with TBS-T and exposed to a Vectastain Elite ABC HRP Kit (Vector) for 1 hour at 37°C. The sections were exposed to diaminobenzidine (DAB; Vector), counterstained with hematoxylin and mounted. Images were analyed using a fluorescence microscope (BX51 Standard Microscope, Olympus, Japan).
Alcian blue-periodic acid- Schiff (AB-PAS) staining
Paraffin fixated tissue slides were deparaffinized, hydrated and stained. For identifying mucosubstances in tracheal epithelial tissues, Alcian blue-periodic acid- Schiff (AB-PAS) at pH 2.5 was used. Stained sections were counterstained with hematoxylin and mounted in Organo/Limonene Mount (ImmunoBioScience Corp, Mukilteo, USA). Histochemical reactions were assessed to differentiate submucosal glands and goblet cells based on the chemical composition of mucopolysaccharides in cells containing acid, neutral and mixed mucopolysaccharides. Magenta-stained spots were considered to contain neutral mucopolysaccharides and polysaccharides, brightly blue stained light-blue cells contained acid mucosubstances and violet stained cells contained nuclei. Mucosubstances were quantified with using the image J software (NIH). Each three slides of tissues were used to represent each group for the quantification. Images were captured from two different spots in each image. Collected image were split into alcian blue and hematoxylin with using Colour Deconvoulution (version 1.5). Alcian blue signal was normalized with that of hematoxylin.
Data analysis
All data were analyzed by applying normality test followed by Kolmogorov-Smirnov test, Student’s t-test for comparing the means, nonparametric one-way analysis of variance followed by Turkey’s test for multiple comparisons. All experiments consisted of three separate trials. Statistical analysis was performed by using Graph Pad Prism (v.5.0; GraphPad Software, La Jolla, USA).
RESULTS
The mRNA expression of the calcium processing genes and tissue localization of the calcium processing proteins
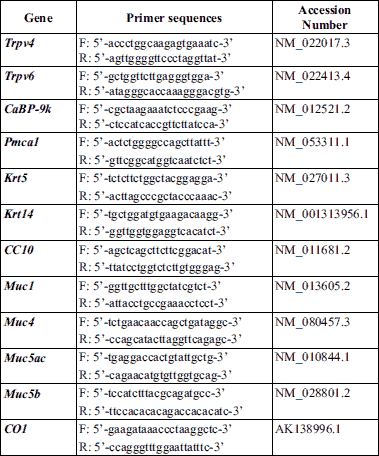
To identify the expression of calcium processing gene in mice trachea, tracheal tissues were collected from mature male mice (n = 6) of vehicle control group. The mRNA expression of the calcium processing genes was analyzed by performing real-time PCR. Trpv4 (Fig. 1A), Trpv6 (Fig. 1B), CaBP-9k (Fig. 1C) and Pmca1 (Fig. 1D) were expressed in the tracheal tissue. Remarkably, a comparison of the mRNA expression of these genes between the cervical and thoracic tracheal sections of the mice indicated that the four calcium processing genes were highly expressed in the cervical tracheal section than in the thoracic tracheal sections. Immunohistochemical labeling was performed to determine the localization of the calcium processing proteins in the tracheal tissue (Fig. 2). Result of the immunohistochemical labeling showed that all four calcium processing proteins were localized throughout the trachea, including epithelial cells (Fig. 2A, 2D, 2G and 2J, black arrow), lamina propria mucosae (Fig. 2A, 2D, 2G and 2J, blue arrow) and glands (Fig. 2A, 2D, 2G and 2J, red arrow), cricoid cartilage chondrocytes (Fig. 2B, 2E, 2H and 2K, red arrow) and perichondrial cells (Fig. 2B, 2E, 2H and 2K, black arrow) and tracheal muscles (Fig. 2C, 2F, 2I and 2L, black arrow). However, each protein showed different expression at each site in the trachea. TRPV4 showed strong expression in the tracheal muscles (Fig. 2C, black arrow) but the weak expression in the tracheal epithelial cells (Fig. 2A, black arrow). TRPV6 showed consistent expression in the tracheal cells. CaBP-9k showed strong expression in tracheal epithelial cells (Fig. 2G, black arrow) and cartilage chondrocytes (Fig. 2H, red arrow). PMCA1 was strongly expressed in submucosal glands (Fig. 2J, red arrow). The relative protein expression intensity data are summarized in Table 2.
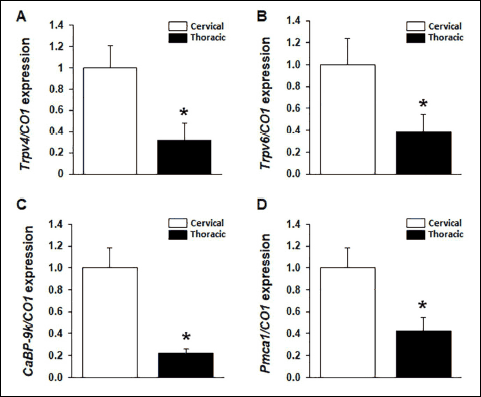 |
Fig. 1. mRNA expression of calcium processing genes in cervical and thoracic tracheal sections. Tracheal tissues were collected from mature male mice (n = 6) of vehicle control group by dividing cervical and thoracic tracheae. mRNA expression of Trpv4, Trpv6, CaBP-9k, Pmca1 genes were measured with real-time PCR in cervical and thoracic trachea. (A): Trpv4, *P < 0.05 for cervical versus thoracic tracheal section; (B): Trpv6, *P < 0.05 for cervical versus thoracic tracheal section; (C): CaBP-9k, *P < 0.05 for cervical versus thoracic tracheal section; (D): Pmca1, *P < 0.05 for cervical versus thoracic tracheal section. Each ΔCT values of the genes were normalized by that of cytochrome C oxidase subunit 1 (CO1). Data are presented as the means ± standard error of the mean. Statistical significance was determined by Student’s t-test. |
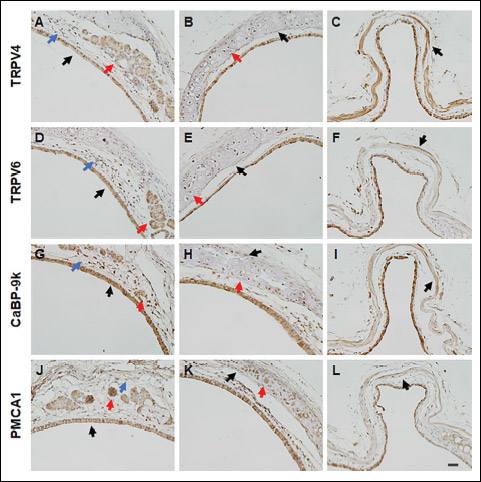 |
Fig. 2. Immunolocalization of calcium processing proteins in mouse trachea. TRPV4, TRPV6, CaBP-9k and PMCA1 proteins were localized in tracheal tissues with immunohistochemistry (A-L). Tracheal epithelial cells: (A), (D), (G), (J); black arrow. lamina propria mucosae: (A), (D), (G), (J); blue arrow. Submucosal glands: (A), (D), (G), (J); red arrow. Cartilage chondrocytes: (B), (E), (H), (K); red arrow. Perichondrial cells: (B), (E), (H), (K), black arrow. Tracheal muscles: (C), (F), (I), (L); black arrow. Scale bar, 40 µm. |
Submucosal glands located in cervical tracheal sections and their correlation with calcium-processing proteins
Results of histological analysis with Alcian blue-periodic acid-Schiff (AB-PAS) staining showed that the staining proportion of submucosal tissues was higher in the cervical trachea than in the thoracic tracheal section of the mice (Fig. 3A). Submucosal glands were detected in the cervical tracheal sections (Fig. 3B). To compare the cell types of the cervical and thoracic tracheal sections, both tracheal sections were analyzed for the expression of cell-type marker genes by performing real-time PCR. Keratin 5 (Krt5) and Keratin 14 (Krt14) are the markers of basal cells present in the submucosal glands, and club cell 10-kDa protein (CC10, secretoglobin) is a marker of club cells present in the tracheal epithelium. The mRNA expression of the Krt5 and Krt14 genes was stronger in the cervical tracheal sections than in the thoracic tracheal sections (Fig. 3E and 3F). The mRNA expression patterns of the Keratin genes were identical to that of the calcium processing genes (Fig. 1A-1D). However, the mRNA expression of CC10 was not significantly different between the cervical and thoracic tracheal sections (Fig. 3G). The calcium processing proteins were colocalized with the KRT14 (basal cell marker) in tracheal tissues. It was observed that the four calcium processing proteins were colocalized with KRT14 in the submucosal glands (Fig. 3H-3W).
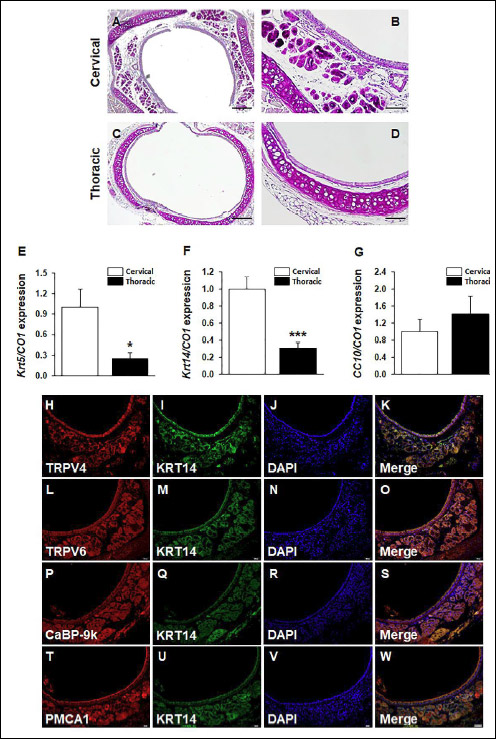 |
Fig. 3. Histological analysis on the composition of trachea by anatomic position. Tracheal tissues were collected from mature male mice (n = 6) by considering anatomic position. AB-PAS staining was conducted to detect the production of mucosubstances. The cervical tracheal sections were compared with the thoracic tracheal sections. Scale bar 200 µm in (A), (C); scale bar, 100 µm in (B) and (D). mRNA expressions of Krt5, Krt14 and CC10 were analyzed by real-time PCR. (E): Krt5, *P < 0.05 for cervical versus thoracic tracheal sections. (F): Krt14, ***P < 0.001 for cervical versus thoracic tracheal sections. (G): CC10. Statistical significance was determined by Student’s t-test. Calcium processing proteins were colocalized with basal cell marker Keratin 14 (KRT14) protein in submucosal glands of cervical tracheal sections. (H): TRPV4 (red); (L): TRPV6 (red); (P): CaBP-9k (red); (T): PMCA1 (red). (I), (M), (Q), (U): KRT14 (green). All nuclei are marked by a DAPI counterstain (J), (N), (R), (V); blue; (K): merged image of TRPV4 and KRT14; (O): merged image of TRPV6 and KRT14; (S): merged image of CaBP-9k and KRT14; (W): merged image of PMCA1 and KRT14. Scale bar, 100 µm in (W). |
Dexamethasone-regulated transcription of the calcium processing genes in the trachea
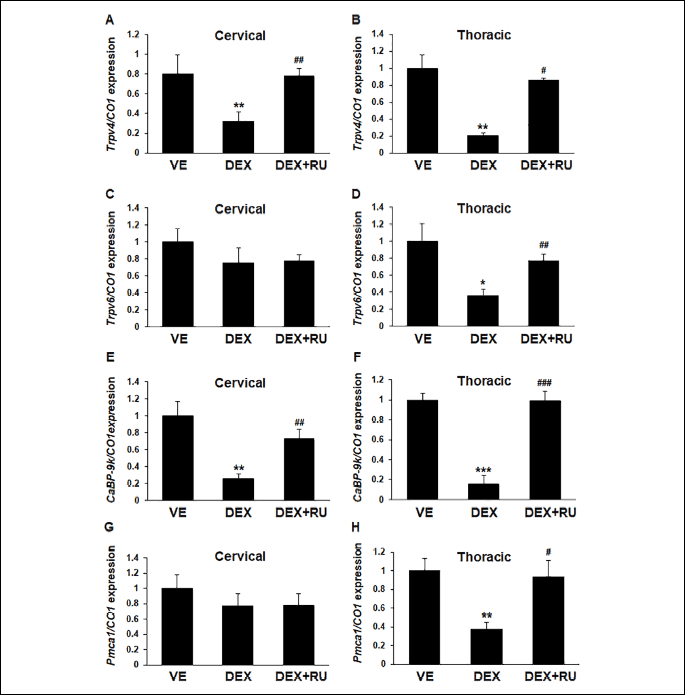
The cervical and thoracic tracheal sections were separately collected form the mice for comparing the mRNA expressions. Dexamethasone treatment affected the mRNA expression of the calcium processing genes in both the cervical and thoracic tracheal sections (Fig. 4). In the results of cervical tracheal sections, dexamethasone downregulated the mRNA expression of Trpv4 and CaBP-9k. In the results of thoracic tracheal sections, all the investigated calcium processing genes were downregulated by dexamethasone treatment. These decreases in mRNA expression induced by dexamethasone treatment was restored after treatment with a glucocorticoid receptor antagonist RU486 (Fig. 4B, 4D, 4F and 4H). Dexamethasone differently regulated the mRNA expression of the calcium processing genes between the cervical and thoracic tracheal section.
Effect of sex steroid hormones on the transcription of the calcium processing genes in the trachea
The effect of sex steroid hormones on Trpv4, Trpv6, CaBP-9k and Pmca1 mRNA expression in the mouse trachea was investigated by injecting immature female mice with estradiol and progesterone. The estradiol treatment increased uterine weight, which was recovered after ICI treatment, suggesting that the immature mice responded appropriately to the sex steroid hormones treatment (Fig. 5A). The regulatory effect of sex steroid hormone estradiol and progesterone was analyzed in the cervical and thoracic tracheal sections. However, the sex steroid hormones did not significantly affect the mRNA expression of Trpv4 (Fig. 5B-5C), Trpv6 (Fig. 5D-5E), CaBP-9k (Fig. 5F-5G) and Pmca1 (Fig. 5H-5I) genes. The expression of Pmca1 was upregulated by progesterone with RU486 treatment compared with VE. However, no significant change was observed when subjected to progesterone only (Fig. 5I).
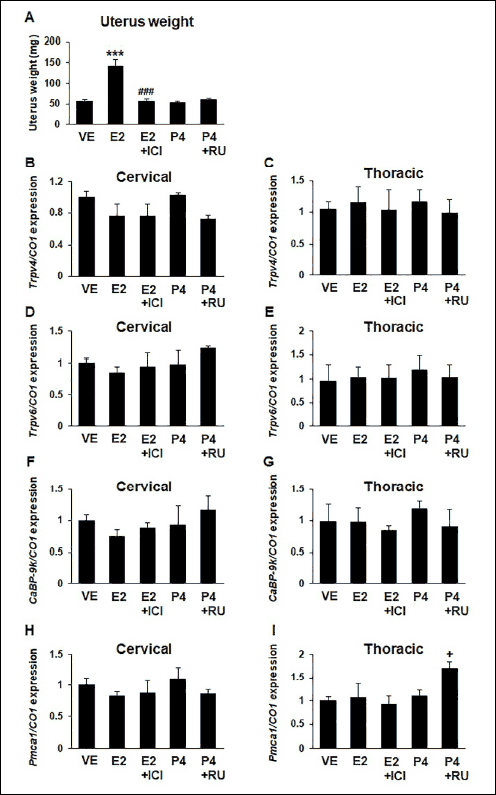 |
Fig. 5. Effect of sex steroid hormones on calcium processing genes in cervical and thoracic tracheal sections. Immature female mice (n = 5) were administered with estrogen (E2; 40 mg/kg) or E2 with ICI (10 mg/kg) or progesterone (P4; 4 mg/kg) or P4 plus RU486 (10 mg/kg) for examining the effect of sex steroid hormones and their receptor antagonists. Calcium processing genes were assessed by real-time PCR. mRNA expression was investigated in cervical and thoracic trachea. Effect of estradiol and progesterone on uterus weight. VE, vehicle group; E2, estrogen treated group; E2 + ICI, estrogen treated with ICI; P4, progesterone treated group; P4 + RU, progesterone treated with RU486 group. (A): uterus weight, F4,21 = 24.38, ***P < 0.001 for VE versus P4; ###P < 0.001 for P4 versus P4 + RU; (B): Trpv4 in cervical tracheal section; (C): Trpv4 in thoracic tracheal section; (D): Trpv6 in cervical tracheal section; (E): Trpv6 in thoracic tracheal section; (F): CaBP-9k in cervical tracheal section; (G): CaBP-9k in thoracic tracheal section; (H): Pmca1 gene in cervical tracheal section; (I): Pmca1 in thoracic tracheal section. Each DCT values of the genes were normalized by that of cytochrome C oxidase subunit 1 (CO1). Data are presented as the means ± standard error of the mean. Statistical significance was determined by one-way ANOVA with the Tukey correction test. |
Localization of mucin 1 protein and quantification of mucin gene mRNA and protein expression
The mRNA expression of Muc genes such as Mucin 1 (Muc1), Mucin 4 (Muc4), Mucin 5ac (Muc5ac) and Mucin 5b (Muc5b) of mucin secretion in the cervical and thoracic tracheal section were investigated with real-time PCR. MUC1 and MUC4 are cell surface MUC proteins and MUC5ac, and MUC5b are secretory MUC proteins. The mRNA expression of Muc genes was not significantly differ in the cervical and thoracic tracheal sections (Fig. 6A-6D). In addition, immunofluorescence staining was performed to examine mucin protein localization in the trachea. MUC1 protein was localized to evaluate the natural distribution in cervical and thoracic tracheal sections. MUC1 was detected in the tracheal epithelial layer. MUC1 protein was evenly distributed in the cervical and thoracic tracheal sections (Fig. 6E-6J). Protein distribution between the cervical and thoracic tracheal sections was compared by measuring fluorescent signals with image J software. No significant difference was observed between cervical and thoracic tracheal sections (Fig. 6K), which was consistent with the mRNA expression of Muc genes.
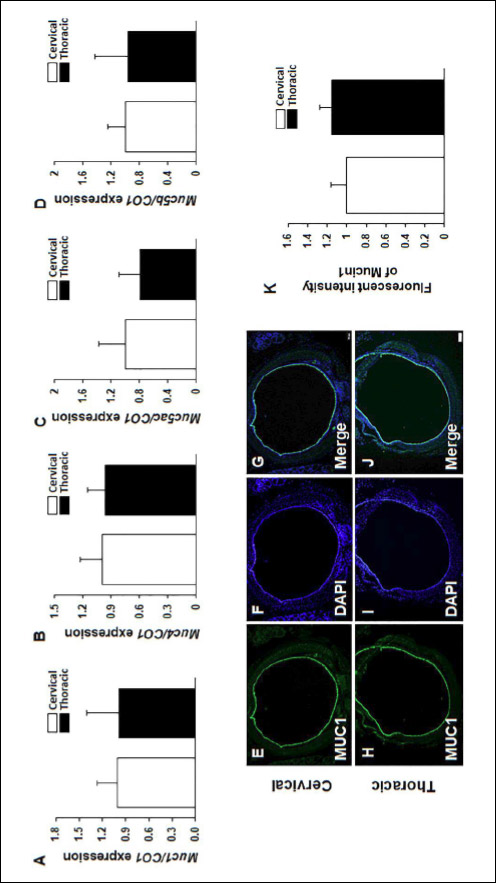 |
Fig. 6. mRNA expression of Mucin producing genes in cervical and thoracic tracheal sections. Tracheal tissues were collected from mature male mice (n = 6). Mucin producing genes such as Muc1, Muc4, Muc5ac and Muc5b genes were analyzed with real-time PCR. mRNA levels of Muc genes were measured in cervical and thoracic tracheal sections. (A): Muc1; (B): Muc4; (C): Muc5ac; (D): Muc5b. Each ΔCT values of the genes were normalized by that of cytochrome C oxidase subunit 1 (CO1). Data are presented as the means ± standard error of the mean. Statistical significance was determined by one-way ANOVA with the Tukey correction test. Immunofluorescence was conducted to visualize the distribution of MUC1 protein with anti-MUC1-CT antibody; MUC1 (E), (H), green. All nuclei are marked by a DAPI counterstain (F), (I), blue. Merged image of MUC1 and DAPI (G), (J). Scale bar, 200 µm in (E). (K): fluorescence intensity was measured with NIH image J. |
Decreased mucin gene mRNA expression and tracheal mucosecretion by dexamethasone
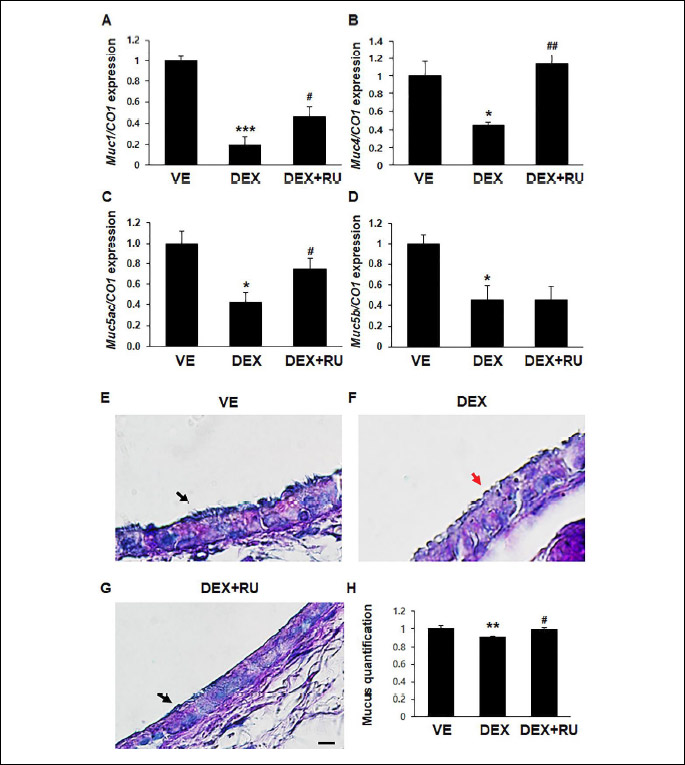
Dexamethasone downregulated the mRNA expressions of the Muc genes such as Muc1, Muc4, Muc5ac and Muc5b (Fig. 7A-7D). The dexamethasone-induced decrease in the mRNA expression of the Muc genes was restored after treatment with the glucocorticoid receptor antagonist RU486; however, RU486 did not restore the mRNA expression of Muc5b. Alcian blue-periodic acid-Schiff (AB-PAS) staining was performed to examine the effect of dexamethasone on tracheal mucosecretion. Magenta-stained areas indicated the presence of neutral mucopolysaccharides, while bright light blue-stained areas indicated the presence of acidic mucosubstances. Acidic mucosubstances were detected in the tracheal epithelial layer (Fig. 7E-7F). Dexamethasone treatment remarkably decreased tracheal mucosecretion. A thick, continuous mucus layer was detected in the trachea of the mice in vehicle and dexamethasone plus RU486 groups (Fig. 7E and 7G; black arrow), whereas a shrunk, discontinuous mucus layer was detected in the trachea of the mice in dexamethasone group (Fig. 7F; red arrow). The proportion of mucosubstances was measured using the Image J software (Fig. 7H).
DISCUSSION
Calcium is essential for and affects almost every aspect of various cellular functions. Calcium-mediated signal transduction regulates cell proliferation, adhesion, and exocytosis (25, 26). For example, in airways, suppression of IP3R-mediated calcium signaling contributes to the proliferation of airway smooth muscle (27). Intracellular calcium homeostasis is maintained by the coordinated activity of numerous specialized components, including specific calcium channels and intracellular calcium-binding proteins. Calcium processing proteins affect cell functions by regulating the uptake and release of calcium ions. Therefore, analysis of the expression and regulatory mechanisms of calcium processing proteins is important to understand their unique cellular functions (28). In mucosecretion, calcium plays a major role in cell secretion by regulating the final step of exocytosis of these MUC-rich substances and intracellular calcium ion is implicated in the packaging of MUC granules (29, 30). In the esophagus, downregulation of calcium efflux channel proteins (PMCA1 and Sodium-calcium exchanger 1) decreased esophageal mucus secretion (20). In the lung, the upregulation of calcium processing proteins decreased alveolar surfactant secretion (21). Therefore, alteration in the expression of the calcium processing proteins may associate with a secretory function in lung. In the present study, we investigated DEX induced alteration of the expression of tracheal calcium processing proteins and the tracheal mucosecretion.
By considering the anatomical position of the trachea, based on the blood supply, mRNA expression of the calcium processing genes and localization of the calcium processing proteins were examined (31). The cervical and thoracic tracheal region contain different cell types, with the submucosal glands being present in the cervical tracheal region (32). The present study showed that the mRNA of the calcium processing genes and tracheal basal cells marker genes were highly expressed in the cervical tracheal sections than in the thoracic tracheal sections. In addition, four calcium processing proteins were co-localized with KRT14, the basal cell marker, in the tracheal submucosal glands. In a nasal basal cell in a mouse, KRT5 and KRT14 are highly expressed in the submucosal glands and co-expressed with TRPV4 channel (33). Tracheal submucosal glands include the majority of tracheal secretory cells (2). The high mRNA expression of the calcium processing genes in the cervical tracheal sections was associated with the expression of the basal cell in the submucosal glands which present much more in cervical tracheal section than in thoracic section. These findings suggest that the calcium processing proteins might be involved with the secretory role of tracheal submucosal glands.
The steroid hormone-induced alteration on the calcium processing was investigated in the trachea. Tracheal regulatory mechanisms of the calcium processing proteins in the trachea has not been observed. However, previous studies have suggested that calcium processing genes are affected by the steroid hormones in multiple organs (34). Trpv4 gene promoter activity is reduced in the presence of progesterone in human airway and mammary epithelial cells, and the mRNA expression of CaBP-9k is regulated by estradiol in the uterus (35, 36). In the present study, the regulatory effect of systemic glucocorticoid hormone dexamethasone and sex steroid hormones estradiol and progesterone was investigated. RU486 was used as an antiglucocorticoid and antiprogesterone agent and ICI 182, 780 was used as an antiestradiol agent (37, 38). Dexamethasone treatment downregulated the mRNA expression of the calcium processing genes. However, it was observed that the sex steroid hormones did not exert any significant effect on the expression of the calcium processing genes, indicating that these hormones did not affect the transcription of the calcium processing genes in the trachea. In addition, the results of dexamethasone with RU486 treatment group suggest that, in tracheal tissues, transcriptional regulation of calcium processing genes is involved with the action of glucocorticoid receptors (37). In duodenal tissues, long-term glucocorticoid receptor indirectly regulates the transcription of Trpv6 and CaBP-9k through vitamin D receptor (VDR) (23). Dexamethasone induces vitamin D receptor by increasing transcription of Vdr gene in a glucocorticoid receptor-dependent manner (39). Cystic fibrosis, abnormal mucus secretion resulted from chloride and sodium imbalance, is involved with down-regulation of VDR (40). The present finding elucidates tissue specificity of dexamethasone on tracheal calcium processing genes, however, future direction will include the how dexamethasone modulates VDR-medicated transcriptional regulation on calcium processing genes.
Alteration of tracheal mucosecretion, when dexamethasone suppressed the calcium processing genes mRNA expression, was examined with real-time PCR and AB-PAS staining. To estimate the normal level of mucin secretion in the cervical and thoracic trachea, the mRNA expression of Muc genes Muc1, Muc4, Muc5ac, and Muc5b were investigated. The mRNA expression of Muc genes was similar in the cervical and thoracic tracheal section. In addition, MUC1 protein was localized to evaluate the natural distribution in cervical and thoracic tracheae. The similar distribution of MUC proteins is observed in cervical and thoracic tracheae. Subsequently, the effect of dexamethasone on tracheal mucosecretion was investigated. mRNA expression of Muc genes were investigated. In the previous in vitro studies, dexamethasone has been upregulated or downregulated the transcription of Muc genes, with tissue-specific manner. In human prostate cell lines, dexamethasone increased the mRNA expression of Muc1, and decreased Muc5ac mRNA expression in the human lung cell line (41, 42). In the trachea, dexamethasone has a decreasing effect on the mRNA expression of Muc1, Muc4, Muc5ac and Muc5b genes which showed similar results with the previous study (43). In addition, AB-PAS staining showed that dexamethasone decreased the levels of acidic mucosubstances in tracheal epithelium. These results suggest that dexamethasone treatment decreased tracheal mucosecretion. These findings are critical for clinical applicability of corticosteroid treatment in asthma. Airway mucus control is an important issue in airway disorders. In airway disease models, corticosteroids have been studied by previous researchers. In ovoalbumin-induced bronchial asthma model, dexamethasone reduced the specific airway resistance and reactivity with decreasing proinflammatory cytokines such as interleukin 4 (IL-4) and interleukin 5 (IL-5) (44). In addition, budesonide, which is a systemic corticosteroid, exerted a synergistic effect with exogenous surfactant therapy on meconium-induced lung injury by decreasing anti-oxidative stress (45). As far, corticosteroids have been focused on an indirect decrease of airway mucosecretion through cytokines or immune response, however, this finding would suggest direct evidence that corticosteroid decreases mucosecretion by affecting a mucus production.
Thus, the present study shows the expression and localization of the calcium processing proteins in the trachea. The salient findings of the present study can be summarized as follows: (1) dexamethasone treatment suppresses the expression of the calcium processing genes and (2) dexamethasone treatment decreased the mRNA expression of the Muc genes and mucosecretion in the trachea by simultaneously downregulating the mRNA expression of the calcium processing genes. These results suggest that the dexamethasone-induced downregulation of the calcium processing genes might be associated with the tracheal mucosecretion. Previous studies have indicated that alteration on intracellular calcium ions and calcium processing proteins is related to the secretory function in multiple tissues. TRPV4-mediated calcium release from the endoplasmic reticulum is a prerequisite for saliva secretion (46). In pancreas, insulin secretion is contributed by cellular calcium signals and CaBP-9k ablation caused abnormality in the insulin secretion (47). However, data obtained in the present study are insufficient to establish an association between the expression of the calcium processing genes and the inhibition of tracheal mucosecretion. Therefore, further studies are needed to understand the exact role of the calcium processing proteins in tracheal mucosecretion.
This result highlights the importance of the calcium processing gene regulation in tracheal tissues. In addition to the previous reporting of calcium processing genes in alveolar tissues, the present findings provides a wider understnading of calcium processing in the airway (21). Major pulmonary diseases related respiratory secretion are involved with calcium processing genes. For instance, cystic fibrosis, associated with Trpv4 mutation (48). In addition, in a primary cultured human epithelial cell line from cystic fibrosis, increased activation of TRPV6 channel resulted in an abnormally high (85%) calcium influx compared to cells from non-cystic fibrosis ones (49). For a reason, findings from the present study may provide helpful information for understanding the secretory mechanism and tracheal disease.
Acknowledgments: This work was supported by a Global Research and Development Center (GRDC) Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2017K1A4A3014959 and NRF-2017R1A2B2005031).
Conflict of interests: None declared.
REFERENCES
- McRae RD, Jones AS, Young P, Hamilton J. Resistance, humidity and temperature of the tracheal airway. Clin Otolaryngol Allied Sci 1995; 20: 355-356.
- Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet cells. Chest 2018; 154: 169-176.
- Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope 2007; 117: 932-938.
- Epstein SK. Anatomy and physiology of tracheostomy. Respir Care 2005; 50: 476-482.
- Finkbeiner WE. Physiology and pathology of tracheobronchial glands. Respir Physiol 1999; 118: 77-83.
- Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol 2006; 291: L596-L601.
- Mitrovic S, Nogueira C, Cantero-Recasens G, et al. TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. Elife 2013; 2: e00658. doi: 10.7554/eLife.00658
- Kuver R, Klinkspoor JH, Osborne WR, Lee SP. Mucous granule exocytosis and CFTR expression in gallbladder epithelium. Glycobiology 2000; 10: 149-157.
- Verdugo P, Aitken M, Langley L, Villalon MJ. Molecular mechanism of product storage and release in mucin secretion. II. The role of extracellular Ca++. Biorheology 1987; 24: 625-633.
- Paz HB, Tisdale AS, Danjo Y, Spurr-Michaud SJ, Argueso P, Gipson IK. The role of calcium in mucin packaging within goblet cells. Exp Eye Res 2003; 77: 69-75.
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem 1987; 56: 395-433.
- Hamanaka K, Jian MY, Weber DS, et al. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 2007; 293: L923-L932.
- Borthwick LA, Neal A, Hobson L, Gerke V, Robson L, Muimo R. The annexin 2-S100A10 complex and its association with TRPV6 is regulated by cAMP/PKA/CnA in airway and gut epithelia. Cell Calcium 2008; 44: 147-157.
- Nijenhuis T, Hoenderop JG, Bindels RJ. TRPV5 and TRPV6 in Ca(2+) (re)absorption: regulating Ca(2+) entry at the gate. Pflugers Arch 2005; 451: 181-192.
- Choi KC, Jeung EB. Molecular mechanism of regulation of the calcium-binding protein calbindin-D9k, and its physiological role(s) in mammals: a review of current research. J Cell Mol Med 2008; 12: 409-420.
- Dupret JM, L’Horset F, Perret C, Bernaudin JF, Thomasset M. Calbindin-D9K gene expression in the lung of the rat. Absence of regulation by 1,25-dihydroxyvitamin D3 and estrogen. Endocrinology 1992; 131: 2643-2648.
- Brini M, Carafoli E. The plasma membrane Ca²+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 2011; 3: a004168. doi: 10.1101/cshperspect.a004168
- van Goor MKC, Hoenderop JGJ, van der Wijst J. TRP channels in calcium homeostasis: from hormonal control to structure-function relationship of TRPV5 and TRPV6. Biochim Biophys Acta Mol Cell Res 2017; 1864: 883-893.
- Bhargava A, Meijer OC, Dallman MF, Pearce D. Plasma membrane calcium pump isoform 1 gene expression is repressed by corticosterone and stress in rat hippocampus. J Neurosci 2000; 20: 3129-3138.
- Kim K, Lee D, Ahn C, et al. Effects of estrogen on esophageal function through regulation of Ca. J Gastroenterol 2017; 52: 929-939.
- An JY, Ahn C, Kang HY, Jeung EB. Inhibition of mucin secretion via glucocorticoid-induced regulation of calcium-related proteins in mouse lung. Am J Physiol Lung Cell Mol Physiol 2018; 314: L956-L966.
- Kitano M, Ishinaga H, Shimizu T, Takeuchi K, Majima Y. Effects of clarithromycin and dexamethasone on mucus production in isografted rat trachea. Pharmacology 2011; 87: 56-62.
- Kim MH, Lee GS, Jung EM, Choi KC, Jeung EB. The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder. Life Sci 2009; 85: 146-152.
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010; 363: 2233-2247.
- Sheng L, Leshchyns’ka I, Sytnyk V. Cell adhesion and intracellular calcium signaling in neurons. Cell Commun Signal 2013; 11: 94. doi: 10.1186/1478-811X-11-94
- Kiessling V, Kreutzberger AJB, Liang B, et al. A molecular mechanism for calcium-mediated synaptotagmin-triggered exocytosis. Nat Struct Mol Biol 2018; 25: 911-917.
- Song X, Zhang Y, Wang H, et al. Stereoselectivity of tradinterol’s inhibition on proliferation of airway smooth muscle cells induced by acetylcholine through suppressing Ca(2+) signalling. J Physiol Pharmacol 2016; 67: 363-375.
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 2016; 96: 911-973.
- Ambort D, Johansson ME, Gustafsson JK, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA 2012; 109: 5645-5650.
- Rossi AH, Salmon WC, Chua M, Davis CW. Calcium signaling in human airway goblet cells following purinergic activation. Am J Physiol Lung Cell Mol Physiol 2007; 292: L92-L98.
- Salassa JR, Pearson BW, Payne WS. Gross and microscopical blood supply of the trachea. Ann Thorac Surg 1977; 24: 100-107.
- Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA, Dorin JR. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Respir Cell Mol Biol 1999; 20: 1181-1189.
- Ueda T, Hoshikawa M, Shibata Y, Kumamoto N, Ugawa S. Basal cells express functional TRPV4 channels in the mouse nasal epithelium. Biochem Biophys Rep 2015; 4: 169-174.
- Lee GS, Choi KC, Jeung EB. Glucocorticoids differentially regulate expression of duodenal and renal calbindin-D9k through glucocorticoid receptor-mediated pathway in mouse model. Am J Physiol Endocrinol Metab 2006; 290: E299-E307.
- Hong EJ, Park SH, Choi KC, Leung PC, Jeung EB. Identification of estrogen-regulated genes by microarray analysis of the uterus of immature rats exposed to endocrine disrupting chemicals. Reprod Biol Endocrinol 2006; 4: 49. doi: 10.1186/1477-7827-4-49
- Jung C, Fandos C, Lorenzo IM, et al. The progesterone receptor regulates the expression of TRPV4 channel. Pflugers Arch 2009; 459: 105-113.
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 1997; 48: 129-156.
- Robertson JA, Zhang Y, Ing NH. ICI 182,780 acts as a partial agonist and antagonist of estradiol effects in specific cells of the sheep uterus. J Steroid Biochem Mol Biol 2001; 77: 281-287.
- Hidalgo AA, Deeb KK, Pike JW, Johnson CS, Trump DL. Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J Biol Chem 2011; 286: 36228-36237.
- Coughlan CA, Chotirmall SH, Renwick J, et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am J Respir Crit Care Med 2012; 186: 999-1007.
- Imai M, Hwang HY, Norris JS, Tomlinson S. The effect of dexamethasone on human mucin 1 expression and antibody-dependent complement sensitivity in a prostate cancer cell line in vitro and in vivo. Immunology 2004; 111: 291-297.
- Chen Y, Nickola TJ, DiFronzo NL, Colberg-Poley AM, Rose MC. Dexamethasone-mediated repression of MUC5AC gene expression in human lung epithelial cells. Am J Respir Cell Mol Biol 2006; 34: 338-347.
- Martinez-Anton A, de Bolos C, Alobid I, et al. Corticosteroid therapy increases membrane-tethered while decreases secreted mucin expression in nasal polyps. Allergy 2008; 63: 1368-1376.
- Mokry J, Urbanova A, Medvedova I, et al. Effects of tadalafil (PDE5 inhibitor) and roflumilast (PDE4 inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs. J Physiol Pharmacol 2017; 68: 721-730.
- Mikolka P, Kopincova J, Tomcikova Mikusiakova L, et al. Effects of surfactant/budesonide therapy on oxidative modifications in the lung in experimental meconium-induced lung injury. J Physiol Pharmacol 2016; 67: 57-65.
- Derouiche S, Takayama Y, Murakami M, Tominaga M. TRPV4 heats up ANO1-dependent exocrine gland fluid secretion. FASEB J 2018; 32: 1841-1854.
- Ahn C, Lee D, Lee JH, Yang H, An BS, Jeung EB. Calbindin-D9k ablation disrupt glucose/pancreatic insulin homeostasis. PLoS One 2016; 11: e0164527. doi: 10.1371/journal.pone.0164527
- Arniges M, Vazquez E, Fernandez-Fernandez JM, Valverde MA. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem 2004; 279: 54062-54068.
- Vachel L, Norez C, Jayle C, Becq F, Vandebrouck C. The low PLC-d1 expression in cystic fibrosis bronchial epithelial cells induces upregulation of TRPV6 channel activity. Cell Calcium 2015; 57: 38-48.
A c c e p t e d : February 28, 2019