Twenty-four male Wistar rats were included into the study. Animals were housed individually in transparent cages at constant temperature of 23°C. A light-dark cycle was provided for 12:12 hours. Animals had free access to standard laboratory food (Labofeed, Kcynia, Poland) and water. Body weight and food intake was measured twice a day at 8.00 AM and 8.00 PM. After two weeks of adaptation to laboratory surroundings and food, mean baseline values of food intake and body weight were taken for three consecutive days.
Animals were divided into four groups. Group A (n=6) was scheduled to MC implantation, group B (n=6) to sham operation only, group C (n=6) to MC implantation and capsaicin vagal deafferentation, and group D (n=6) to capsaicin denervation only.
Animals were anesthetized with penthobarbital 0.25mg/kg (Vetbutal, Biowet, Pulawy, Poland), and the MC was implanted subdiaphragmatically on the abdominal part of the left vagal nerve, as described previously (11). For capsaicin deafferentation, a 1% solution of capsaicin was applied locally on the right vagal nerve on a soaked swab for 30 minutes (solvent: 10% ethanol, 10% Tween 80, and 80% saline 0.9%). During sham operation, the abdominal vagal nerve was isolated and treated with the solvent for the same period of time.
Stimulation started immediately after implantation and lasted for 24 days. The MC were preprogrammed for the following parameters: frequency 0.05Hz, impulse duration 0.1s, and amplitude 0.55V (10). After a recovery period of 3 days, daily measurement of body weight and food intake started.
Serum leptin levels (pg/mL) were measured by enzyme-linked immunosorbent assay ELISA (Quantikine M, R&D System Inc.) (12). Insulin levels (uU/mL) were measured by conventional radioimmunoassay. Glucose levels (mmol/L) were measured by oxidation-reduction method.
Baseline data on food intake, body mass, and serum levels of leptin, insulin, and glucose were statistically compared to post-stimulation levels by two-way ANOVA and paired (post-hoc) t-test when appropriate, with the level of significance set at 5 % for all tests.
Ethical permission was obtained from the Ethical Committee of the Jagiellonian University Medical School.
Electrical stimulation of the left vagal nerve significantly reduced the total food intake (Fig. 1) as well as the mean daily intake (data not shown) in groups A and C in comparison to both control group B and capsaicin pretreated group D (ANOVA, F=18.55, p=0.0038). The food intake lowering effects of capsaicin per se (groups C and D) did not reach significance (F=3.85, p=0.64).
 |
| Fig. 1. Total food intake (g, mean ± SD) during the experiment in MC stimulated rats with and without concominant capsaicin denervation, in capsaicin-only treated rats, and in control animals. (** indicates p<0.05 in post-hoc t-tests) |
Post-hoc t-tests showed the differences in food intake between control groups B and D to be not significant (p>0.05) while both capsaicin pretreated and stimulated (C) as well as MC stimulated (A) rats significantly decreased food intake as compared to control (p<0.05).
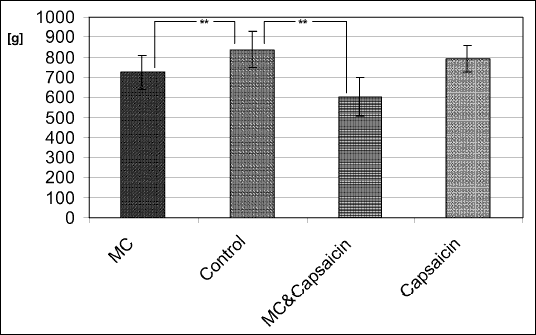
Consequently, final body weight was significantly lower in the group A (346,2 g) and C (272,7 g) then in control (B: 381,4 g) and capsaicin-only treated rats (D: 356,8 g) (F=25.68, p=0.00068). Capsaicin per se also significantly reduced body weight (F=16.97, p=0.0058) irrespective of electrical stimulation (Fig. 2).
 |
| Fig. 2. Mean body weight (g) on the last day of the experiment in MC stimulated rats with and without concominant capsaicin denervation, in capsaicin-only treated rats, and in control animals. |
The overall body weight gain (Fig. 3) demonstrates the additive effects of MC stimulation plus capsaicin treatment in comparison to MC or capsaicin denervation alone.
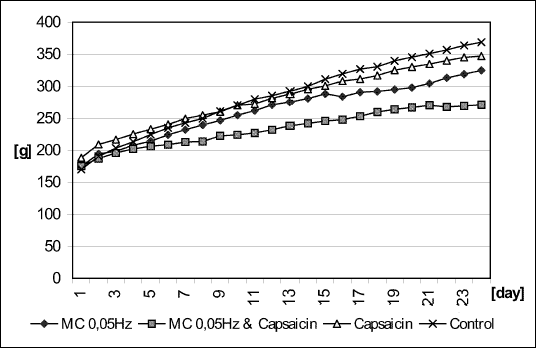 |
| Fig. 3. Body weight change (g) during the experiment in MC stimulated rats with and without concominant capsaicin denervation, in capsaicin-only treated rats, and in control animals. (** indicates p<0.05 in post-hoc t-tests) |
Leptin level decreased in the group C (165 pg/mL) in comparison to groups A (625 pg/mL), B (677 pg/mL), and D (612 pg/mL) (p<0,05), mainly due to MC stimulation (F=7.27, p=0.019) (Fig. 4). No effect of capsaicin on leptin levels was seen (data not shown). There were no changes in insulin levels with MC and capsaicin (C), but glucose level was decreased significantly with MC stimulation (A) (F=5.55, p=0.036) - by 11 % with MC alone (A) and by 16% with MC stimulation combined with capsaicin (C) (Fig. 5). Only the leptin levels correlated significantly (r=0.75, p<0.05) with the final body weight.
 |
| Fig. 4. Blood leptin level (uU/L) in MC stimulated rats with and without concominant capsaicin denervation, in capsaicin-only treated rats, and in control animals. (** indicates p<0.05 in post-hoc t-tests) |
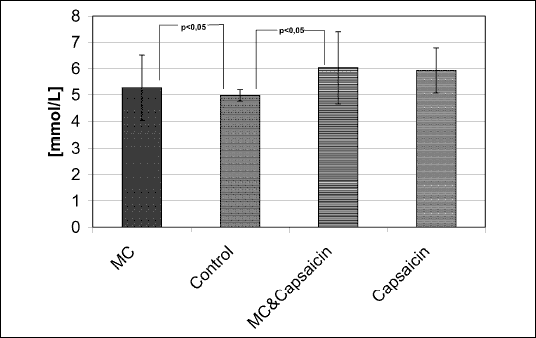 |
| Fig. 5. Blood glucose level (mmol/L) in MC stimulated rats with and without concominant capsaicin denervation, in capsaicin-only treated rats, and in control animals. (** indicates p<0.05 in post-hoc t-tests) |
Vagal afferents conduct signals form the stomach to nucleus tractus solitarius carring information about the size and chemical composition of a meal, which is transmitted by other specific connections to the satiety center in the hypothalamus and the collateral ventro-medial nucleus. The hypothesis could be stated that electrical stimulation of the ventral vagal nerve increases the afferent traffic and by influencing the function of satiety centers, at the central level decreases food intake and body weight (11). This idea is partly supported by studies of Forster who stimulated the antral part of the stomach with a low energy signal and achieved a decrease in the severity and frequency of nausea and vomiting. He confirmed that processing afferent vagal information could be modulated not only by vagal but also by gastric stimulation (13). The possibility to affect food intake and body weight with elecrical stimulation has already been reported. As was shown by Cigaina chronic antral stimulation with high-energy signal influences the alimentary behaviour in pigs and is believed by author to introduce antiperistaltic gastric waves which were responsible for decreased food intake (14). Also chronic stimulation of ventral vagus in rabbits resulted in food intake reduction with consequences of body weight loss (15). These studies confirm our hypothesis that vagal stimulation with the MC mimics a full stomach.
We choose vagal stimulation as a method of afferent vagal neuromodulation for two reasons: first it is less energy consuming than direct stimulation of the gastric smooth muscles, and secondly it may be more precise for long term used. Local vagal deafferentation with capsaicin, a selective neurotoxin of unmyelinated C-fibers, attenuates a short-term satiety loop determined mainly by cholecystokinin release (6). However, capsaicin deafferentation does not affect food intake in long-term energy balance regulation (7), which is supported by our data. Differences in food intake between control and capsaicin pretreated rats in our studies were not significant. In long-term stimulation no metabolic effects could be elicited.
Capsaicin causes chemical lesion of visceral afferents carrying information to terminate meals by a negative feedback signal (6). This is supported by authors who observed attenuated suppression of food intake produced by intestinal infusion of nutrient or CCK administration in capsaicin treated rats (16, 17). These results suggest that transient intervention in vagal afferents may cause alteration in alimentary behaviour. Roth et al. (18) stimulated the rats vagal nuclei through a period of several months with 2 deoxyglucose (2DG). 2DG increased efferent activity, reduced glucose fluxes and decreased peripheral insulin level but did not change food intake. In our study low frequency of vagal afferent stimulation reduced both body weight gain and food intake without affecting the insulin level - probably because the long-term stimulation resulted in engagement of an anti-insuline hormonal counterbalancing mechanisms.
On the other hand in a group of rats pretreated with capsaicin the effects of vagal stimulation of ventral vagus on food intake were augmented by afferent dorsal nerve denervation with capsaicin. This suggests that in this experimental set-up the dorsal vagus could compensate somehow central effects of ventral vagal stimulation. Similar bilateral compensation was observed in esophageal motility (19). Continuous electrical stimulation on one side increases the afferent traffic but probably also induces compensatory response from the opposite nerve, which inhibits the effects of this peripheral neuromodulation because of bilateral hypothalamic projections of both nerves. Consequently, afferent denervation of the dorsal vagus attenuates compensation and potentiates primary effects of the stimulation with MC. Satiety effects may be also related to direct or indirect effects via vagus-released hormonal satiety signals like GLP, as described by Hellstrom (20). Thus, only part of the vagal afferents is desensitised by capsaicinas as wasshown by Blackshaw, the remaining gastrointestinal receptors still being active and supply additional control of food intake (21). In our opinion, capsaicin denervation clears the oncoming afferent activity in such way that the false microchip-signal from deep gastric tension receptors can become more effective.
The main hormone which conveys feedback information from adipose tissue to the brain and which is produced by the adipose tissue is leptin. Leptin expression and leptin level are consistently associated with body adiposity and the body mass index. Leptin is not a short-term satiety factor (22). In our experiments long-term vagal stimulation induced satiety and decreased body weight gain through a decrease in plasma leptin level. Chang et al. showed that the canine vagus stores and releases CCK-8 upon electrical vagal stimulation. CCK stimulates vagus activity and satiety by acting on CCK-1 receptors on vagal afferents (23) Our data suggest also a mechanism of MC action similar to CCK, namely augmenting the afferent vagal activity. Capsaicin augments the MC action on food intake by destroying vagal afferents presenting CCK-A receptor (16). However besides the central, peripheral effects of vagal stimulation must be also stressed. We observed that vagal stimulation with the same frequency decreased the amplitude but not frequency of gastric contraction, which was reversed by L-NMA (24). Our results were partly confirmed by Takahashi and Owyang who showed relaxatory effects of CCK on proximal stomach in rats (25). They showed that CCK stimulates vagal and splanchnic afferents resulting in vago-splanchnic and splanchno-splanchnic reflexes via alpha-2 and beta-adrenergic receptors located on gastric smooth muscles. Thus, probable mechanisms of MC neuromodulation are mediated not only by capsaicin-sensitive vagal afferents but also by others like splanchnic circuits, suggesting redundancy of the nervous control mechanisms of food intake (5).
In summary it could be concluded that vagal MC neuromodulation results in central and peripheral effects leading to decrease of food intake partially dependent upon capsaicin-sensitive afferents with both vaguses involved.
Acknowledgements: This study was supported by grants from KBN 6P05C02420 (State Committee for the Scientific Research) Warsaw, Poland.
- Lutz TA, Althus J, Rossi R, Scharrer E. Anorectic effect is not transmitted by capsaicin-sensitive nerve fibers. Am J Physiol 1998; 274(6pt2): R1777-1782.
- Sobocki J, Thor PJ, Krolczyk G, Uson J, Diaz-Guemes I, Lipinski M. The cybergut. An experimental study on permanent microchip neuromodulation for control of gut function. Acta Chir Belg 2002; 102: 11-13.
- Parker APJ, Polkey CE, Binne CD, Madigan C, Ferrie CD, Robinson R. Vagal nerve stimulation in epileptic encephalopathies. Pediatrics 1999; 103(4): 788-792.
- Thor PJ, Sobocki J. Neuromodulation of vago-vagal reflex. Przegl Lek 2000; 57(Suppl 5): 79-81.
- Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 2000; 16(10): 866-873.
- South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 1988; 9(3): 601-612.
- Chavez M, Kelly L, York DA, Berthound HR. Chemical lesion of visceral afferents causes overconsumption of unfamiliar high-fet diets in rats. Am J Physiol 1997; 272(5pt2): R1657-1663.
- Falchi M, Ferrara F, Gharib C, Dib B. Intracerebroventricular capsaicin and food intake in the rat. Drugs Exp Clin Res 2001; 27(2): 61-67.
- Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol 1998; 274: 1236-1242.
- Mei N. Intestinal chemosensitivity. In: Advances in the Innervation of the Gastrointestinal Tract, GE Holle, JD Wood (eds). Amsterdam, Elsevier, 1992, pp.271-281.
- Krolczyk G, Zurowski D, Sobocki J et al. Effects of continuous microchip (MC) vagal neuromodulation on gastrointestinal function in rats. J Physiol Pharmacol 2001; 42(4pt1); 705-715.
- Jaworek J, Bonior J, Pierzchalski P et al. Leptin protects the pancreas from damage induced by caerulein overstimulation by modulating cytokine production. Pancreatology 2002; 2(2): 89-99.
- Forster J, Sarosiek I, Delcore R, Lin Z, Raju GS, McCallum RW. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg 2001; 182(6): 676-681.
- Cigaina V, Saggioro A, Rigo V, Pinato GP, Ischia S. Long -term effects of gastric pacing to reduce feed intake in swine. Obes Surg 1996; 6(3): 250-253.
- Sobocki J, Thor PJ, Usson J, Dias-Guemes I, Calles C, Pascual S. Microchip vagal pacing reduces food intake and body mass. Hepatogastroenterology 2001; 48/42: 1783-1787.
- Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol 1988; 255(4 Pt 2): R569-R574.
- Tamura CS, Ritter RC. Intestinal capsaicin transiently attenuates suppression of sham feeding by oleate. Am J Physiol 1994; 267(2 Pt 2): R561-568.
- Roth HS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann NY Acad Sci 2001; 928: 305-315.
- Gidda JS, Goyal RK. Influence of successive vagal stimulations on contractions in esophageal smooth muscle of opossum. J Clin Invest 1983; 71(5): 1095-1103.
- Hellstrom PM, Naslund E. Interaction between gastric emptying and satiety, with special reference to glucagon-like peptide. Physiol Behav 2001; 74 (4-5): 735-741.
- Blackshaw LA, Page AJ, Partosoedarso ER. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience 2000; 96(2): 407-416.
- Krolczyk G, Zurowski D, Thor PJ. Interstitial cells of Cajal and their pacemaker function. Folia Med Cracov 2001; 42(1-2): 45-51.
- Chang TM, Thagesen H, Lee KY, Roth FL, Chey WY. Canine vagus nerve stores cholecystokinin-58 and -8 but releases only cholecystokinin-8 upon electrical vagal stimulation. Regul Pept 2000; 87(1-3): 1-7.
- Krolczyk G, Sobocki J, Laskiewicz J, Thor PJ. Electrical neuromodulation of vagal activity inhibits gastric motility in rats. XXII Congress of the Polish Physiological Society. J Physiol Pharmacol 2002; 53 (suppl. 1): 50.
- Takahashi T, Owyang C. Mechanism of cholecystokinin-induced relaxation of the rat stomach. J Auton Nerv Syst 1999; 75(2-3): 123-130.