Considerable evidence has accumulated implicating alterations in lipid metabolism as contributing to the development of insulin resistance (8-10). Recently it has been demonstrated that an excess of lipid accumulation disturbs intracellular insulin signaling in skeletal muscles and cardiac myocytes (11). This lipid accumulation is mainly due to reduced rates of fatty acid (FA) oxidation (12-14). However, increased accumulation of lipids in cytosol depends also on the excessive transmembrane transport of long chain fatty acids (LCFAs) (15-17). Recent findings strongly suggest that susceptibility to increased intracellular fatty acid transport is to a large extent determined by an increase in the expression of fatty acid transporters (16-18). Several fatty acid transporters are known to be involved in regulation of protein-mediated LCFA transport into the cardiac myocytes (19-21). A number of studies have identified FAT/CD36 and FABPpm as the main myocardial fatty acid transporters facilitating LCFA movement across the plasma membrane in health and diseases (20-22). Notably, recent studies have established the role of FAT/CD36 and FABPpm in excessive LCFA transport into the cardiac myocytes in obesity (17) and in type 2 diabetes (16). However, while much is known about adipocyte FATP-1 function and expression, there is little information on its regulation in cardiac myocytes and its relationship to myocardial lipid accumulation (23).
Given that, it is of particular interest to examine whether IL-6 deficiency is associated with any changes in the myocardial expression of fatty acid transporters: FAT/CD36, FABPpm and FATP-1. Furthermore, as the myocardial fatty acid transporter expression may be a key factor in contributing to lipid accumulation in the heart, we examinated intramyocardial content of different lipid fractions in mice lacking IL-6 compared to the wild type littermates. We determined also the effects of IL-6-/- genotype on associated changes in the composition of specific myocardial lipid fractions.
FAT/CD36 and FABPpm were detected using the MO25 antibody (24) and FABPpm antisera (25), respectively. FATP-1 was detected with commercially available antibody (I-20, Santa Cruz, CA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Female mice (C57B4/6J IL6-/-tm1Kopf ) were bred on site and maintained at 22°C on a reverse light-dark cycle in approved animal holding facilities. They had unrestricted access to food and water. This study was approved by the local ethics committee on animal care.
The mice were killed by cervical dislocation and immediately samples of the left ventricle were taken. They were cleaned of any visible non-muscle tissue, freezed in liquid nitrogen and finely powdered. The powder was transferred to a glass tube and lipids were extracted using the Folch method (26) as modified according to van der Vusse et al (27). Individual fatty acid methyl esters were identified and quantified according to the retention times of standards by gas liquid chromatography (Hewlett-Packard 5890 Series II gas chromatograph, HP-INNOWax capillary column). Total free fatty acid (FFA), diacyloglicerol (DG), phospholipid (PL), triglyceride (TG) and ceramide content was estimated as the sum of the particular fatty acid species content of the assessed fraction and it was expressed in nanomoles per gram of tissue. We have also calculated the following indices of fatty acid profile of each lipid fractions examined in each heart: saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA).
Routine Western blotting procedures were used to detect proteins as described previously (23,28). The total protein expression of FAT/CD36, FABPpm and FATP-1 was determined in crude membranes of the hearts. Briefly, proteins were separated using 10% SDS-polyacrylamide gel electrophoresis. Membranes were immunoblotted with primary antibodies: MO25 (FAT/CD 36), FABPpm antiserum and FATP-1. Protein content was determined with bicinchonic acid method with BSA serving as a protein standard. Signals obtained by Western blotting were quantified by densitometry (Biorad).
All data are expressed as mean ± SEM. Statistical difference between groups was tested with analyses of variance and appropriate post-hoc tests, or with a Student t-test. Statistical significance was set at P
No changes were observed in non-fasting blood glucose concentration, serum free fatty acid concentration, the whole body weight and the weight of the heart in between IL-6 -/- and WT mice (data not shown).
Effect of IL-6 deficiency on fatty acid transporter expression (FAT/CD36, FABPpm, FATP-1)
The total myocardial FAT/CD36 protein content was higher in hearts from IL-6 -/- (+40%, P<0.05, Fig. 1A) compared to the wild type mice. The total FABPpm protein expression was also increased in hearts from IL-6 -/- compared to WT mice, although the change did not reach the level of significance (+15%, P>0.05, Fig. 1B). IL-6 deficiency has no effect on myocardial FATP-1 protein expression (P>0.05, Fig. 1C).
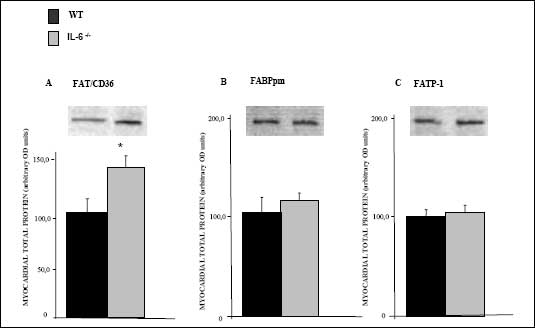 |
| Fig. 1. The effect
of IL-6 -/- genotype on myocardial total
expression of (A) FAT/CD36, (B) FABPpm and (C) FATP-1. Crude membranes
were prepared from left ventricle homogenates as described in Materials
and Methods. Data are based on 5 independent determinations for each heart
(mean ± SEM). *P<0.05, IL-6 -/- vs WT |
Effect of IL-6 deficiency on the intramyocardial lipid content
Myocardial content of diacylglicerol, free fatty acids and ceramide was significantly increased in IL-6 deficient mice compared to WT animals (+45%, +37%, +48%, respectively, P<0.05, Fig. 2). The content of triacylglycerols and phospholipids remained stable (P>0.05, Fig. 2) as well as the total intramyocardial lipid content did not differ between IL-6 -/- and WT mice (P>0.05, Fig. 2).
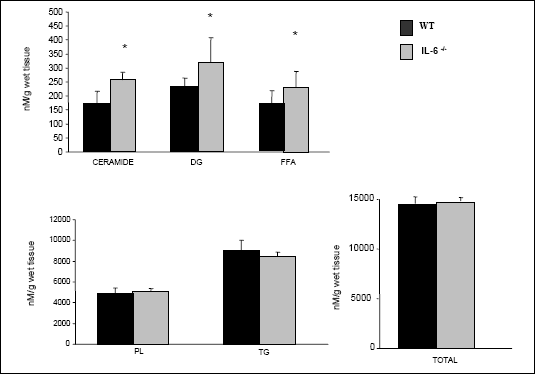 |
| Fig. 2. The effect
of IL-6 -/- genotype on the intracellular
lipid content in the myocardium. Different lipid pools were extracted
from the left ventricle homogenates as described in Materials and Methods.
Data are based on 5 independent determinations for each heart (mean ±
SEM). DG- diacylglicerols, FFA- free fatty acids, PL - phospholipids,
TG - triacylgliceroles, Total - the sum of individual lipid fractions. *P<0.05, IL-6 -/- vs WT |
Effects of IL-6 deficiency on the intramyocardial lipid composition
Although no changes in the content of myocardial PUFA as well as MUFA species were observed in between IL-6 -/- mice and WT in all lipid fractions examined, there was a trend for IL-6 deficiency to increase the amount of saturated FFA-FA, DG-FA and ceramide-FA species (8%, 12% and 10%, p=0.06 and p=0.07, respectively, Fig. 3).
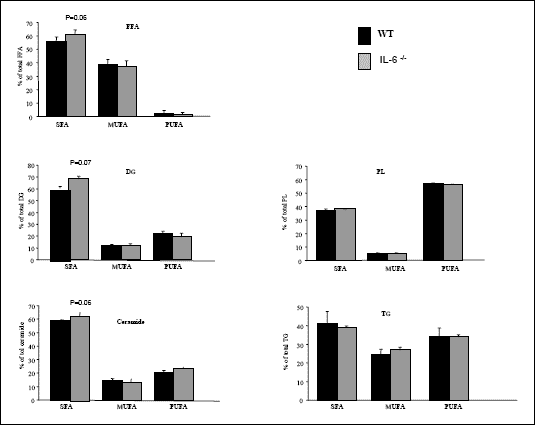 |
| Fig. 3. The effect
of IL-6 -/- genotype on the intracellular
lipid composition in the myocardium. Different lipid fractions were extracted
and the content of their fatty acid residues were summed as SFA-saturated
fatty acids, MUFA-monounsaturated fatty acids, PUFA-polyunsaturated fatty
acids in all fractions examinated as described in Materials and Methods.
Data are based on 5 independent determinations for each muscle (mean ±
SEM). FFA- free fatty acids, DG- diacylglicerols, PL - phospholipids,
TG - triacylgliceroles. *P<0.05, IL-6 -/- vs WT |
The present study revealed the effects of IL-6 deficiency on myocardial a) expression of fatty acid transporters and b) intracellular content of different lipid fractions. To the best of our knowledge, this is the first report presenting the effects of IL-6 -/- deficiency on the myocardial expression of fatty acid transporters. All of examined transporters (FAT/CD36, FABPpm and FATP-1) are expressed in many mammalian tissues, including cardiomyocytes (22,23,29,30). However, based on the present study and others (23,28,31), it appears that, only the myocardial expression of FAT/CD36 is highly regulatable. Notably, it is well recognized that FAT/CD36 plays a major role among fatty acid transport proteins and changes in its expression are highly associated with concomitant alternations in LCFA transport (28,32,33). Recent studies have shown that the increase in sarcolemmal FAT/CD36 expression is the key mechanism promoting the increased rate of LCFA uptake in obesity and type 2 diabetes (16,17,32,33). In humans, an association between FAT/CD36 deficiency and hypertrophic cardiomyopathy has also been reported and linked to impaired uptake of long chain fatty acid by the myocardium (32). Other studies have identified significant defects in myocardial LCFA uptake in CD36-deficient humans (34), although a role for CD36 deficiency in the pathogenesis of alternations in myocardial LCFA metabolism in humans remains to be established.
In marked contrast to myocardial overexpression of FAT/CD36, IL-6 -/- deficiency did not affect the expression of FABPpm and FATP-1 proteins. This may indicate that there are specific responses to IL-6 -/- deficiency (i.e FAT/CD36 vs FABPpm and FATP-1). Otherwise, it can be speculated that FABPpm and FATP-1 play a minor role in myocardial LCFA transport, as it has been suggested recently (23,28,31).
An important aspect of our study was to determine whether the IL-6 -/- deficiency, that upregulated myocardial FAT/CD36 expression, also affects cardiomyocyte lipid content. The obtained results revealed no changes in total myocardial lipid deposits due to lack of changes in the quantitatively major fractions namely, triacylglicerols and phospholipids. Surprisingly at first, as it has been reported that IL-6 exaggerates fatty acid oxidation in isolated soleus muscle (35) and thus, IL-6 deficiency could be expected to promote intracellular lipid accumulation. Several studies have dealt with alternations in fatty acid metabolism in mice lacking IL-6 gene. It has been demonstrated that, IL-6 -/- mice developed maturity onset obesity with disturbed carbohydrate and lipid metabolism (2). In contrast, Di Gregorgio et al. reported that IL-6 -/- mice do not present features of obesity or abnormal lipid metabolism although these mice on HF diet had elevated glucose levels after a GT (36). These discrepancies may be related to observed by van Hall et al. (37) changes in fat metabolism during IL-6 infusion that were probably elicited indirectly by coincidental changes in the content of other hormones such as epinephrine and cortisol rather than by a direct effect of IL-6.
In the present study we reported marked increase in intramyocardial diacylglicerol, free fatty acid and ceramide fractions. This may favor studies presenting IL-6 -/- mice as an animal model correlated with obesity related insulin resistance. In support of this view, there are studies showing plausible mechanistic links between the development of insulin resistance and accumulation of DG and ceramide in muscle without concomitant changes in intramuscular TG stores (38). Others have also pointed to elevated intramyocellular DG levels in a number of animal models of insulin resistance (39-41), while ceramide content was shown to be increased in muscle from obese insulin resistant humans (40,42,43). Recent study demonstrates also that, the changes in composition of DG and ceramide are related to the improvements of insulin sensitivity in obese subjects after endurance training (43). Specifically, in mentioned above study, endurance training reduced both total ceramide content and the content of saturated ceramide species with a trend for training to reduce both the total diacylglcyerol (DG) content and the content of saturated DG-FA species (43). Based on these reports we examined the effect of IL-6 deficiency on fatty acid composition of the myocardial lipid fractions. We found only a tendency in hearts lacking IL-6 gene for accumulation of saturated species in FFA, DG and ceramide fractions. This may be in inverse correlation with insulin resistance in cardiac myocytes, as it has been demonstrated that increase fraction of myocardial polyunsaturated fatty acids (PUFA) content exert the ability to channel fatty acids towards mitochondrial oxidation and thus direct FA away from lipid storage (44,45).
In the present study we have provided several novel observations. Firstly, we have shown in mice lacking IL-6 gene upregulation of the myocardial FAT/CD36 expression and no significant changes in FABPpm and FATP-1. Secondly, we observed that, the increase in FAT/CD36 in heart from IL-6 -/- mice was associated with the increases in the myocardial total content of diacylglicerol, free fatty acid and ceramide fractions as well as a tendency for accumulation of saturated FA species in these fractions. This lipid accumulation with concomitant trend for increase in saturation status of their fatty acid residues may, at least in part, provide a factor related to the development of intramyocardial lipotoxicity, observed in obese individuals (8-10). However, we also found lack of effects of IL-6 deficiency on myocardial content of triacylglicerol and phospholipid lipid pools.
Acknowledgements: These studies were funded by the Medical University of Bialystok (grant nr 3-18619L, 3-24589L, 2P05B01826) and Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario.
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998; 83:847-850
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002; 8:75-79
- Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997; 40:1286-1292
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280:E745-751
- Carey AL, Lamont B, Andrikopoulos S, Koukoulas I, Proietto J, Febbraio MA. Interleukin-6 gene expression is increased in insulin-resistant rat skeletal muscle following insulin stimulation. Biochem Biophys Res Commun 2003; 302:837-840
- Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol 2001; 533(Pt 2):585-591
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280:E745-751
- Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta 2005; 1734:112-126
- Ghosh S, An D, Pulinilkunnil T, Qi D, Lau HC, Abrahani A, Innis SM, Rodrigues B. Role of dietary fatty acids and acute hyperglycemia in modulating cardiac cell death. Nutrition 2004; 20:916-923
- Zhou YT. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad SCi USA 2000; 97: 1784-1789
- Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord 2000; 24 Suppl 4:S28-32
- Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 2002; 51: 2587-2595
- Corr PB, Gross RW, Sobel BE. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res 1984; 55:135-154
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 2000; 279: E1039-E1044
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 1999; 277(6 Pt 1):E1130-1141
- Luiken JJFP, Arumugam Y, Bell RC, et al. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab 2002; 283:E612-621
- Luiken JJFP, Arumugam Y, Dyck DJ, et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem 2001; 276:40567-40573
- Bonen A, Glatz JF Luiken JJFP. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem 2002; 239: 181-192.
- Schaffer JE. Fatty acid transport: the roads taken. Am J Physiol Endocrinol Metab 2002: 282: E239-E246
- Bonen A, Campbell SE, Benton CR, et al. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc 2004; 63:245-249
- Luiken JJ, Turcotte LP, Bonen A. Protein-mediated palmitate uptake and expression of fatty acid transport proteins in heart giant vesicles. J Lipid Res 1999; 40:1007-1016
- Koonen DP, Glatz JF, Bonen A, Luiken JJ. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta 2005; 1736:163-180
- Chabowski A, Coort SL, Calles-Escandon J, et al. The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by AICAR. FEBS Lett 2005; 579:2428-2432
- Matsuno K, Diaz-Ricard M, Montgomery RR, Aster T, Jamieson GA, Tandon NN. Inhibition of platelet adhesion to collagen by monoclonal anti CD36 antibodies. Br J Haematol 1996; 92: 960-967
- Calles-Escandon J, Sweet L, Ljungqvist O, Hirshman MF. The membrane associated 40 kDa fatty acid binding protein is present in human skeletal muscle. Life Sci 1996; 58: 19-28
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497-509
- Van der Vusse, GJ, Roemen, THM, Reneman RS. Assessment of fatty acids in dog left ventricular myocardium. Biochim Biophys Acta 1980; 617: 347-352
- Chabowski A, Coort SL, Calles-Escandon J, et al. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am J Physiol Endocrinol Metab 2004; 287 (4): E781- E789
- Lavrentyev EN, He D, Cook GA. Mistaken identity or yet another case of species difference? Am J Physiol Heart Circ Physiol 2005; 288: H448-H450
- Bonen A, Miskovic D, Kiens B. Fatty acid transporters (FABPpm, FAT, FATP) in human muscle. Can J Appl Physiol 1999; 24: 515-523
- Gimeno RE, Ortegon AM, Patel S, et al. Characterization of a heart-specific fatty acid transport protein. J Biol Chem 2003; 278:16039-16044
- Coort SLM, Hasselbaink DM, Koonen DYP, et al. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese Zucker rats. Diabetes 2004; 53: 1655-1663
- Bonen A, Parolin ML, Steinberg GR, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 2004; 18: 1144-1146
- Tanaka T, Sohmiya K, Kawamura K. Is CD36 deficiency an etiology of hereditary hypertrophic cardiomyopathy. J Mol Cell Cardiol 1997; 29:121-127
- Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab 2004; 287(4):E616-21
- Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab 2004; 287:E182-187
- Van Hall G, Steensberg A, Sacchetti M, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 2003; 88: 3005-3010
- Cooney GJ, Thompson AL, Furler SM, Ye J, Kraegen EW: Muscle long-chain acyl CoA esters and insulin resistance. Ann N Y Acad Sci 2002; 967:196-207
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglyceol, protein kinase C, and I?B-?.. Diabetes 2002; 51:2005-2011
- Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 1999; 274:24202-24210
- Schmitz-Peiffer C, Browne CL, Oakes ND, et al. Alterations in the expression and cellular localization of protein kinase C isoforms ? and ? are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes 1997; 46:169-178
- Adams JM, II., Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004; 53:25-31
- Bruce CR, Thrush AB, Mertz VA, et al. Endurance training in obese humans improves glucose tolerance, mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 2006 in press
- Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993; 328:238-244
- Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr Rev 2004; 62:333-339