It has been recognized that organisms within the same species can differ markedly in their response to stress and various substances (9-11). Understanding why individuals exhibit differential responses to stress is important not only to make progress in research concerning stress vulnerability but also to find means for effective stress treatment and prevention of stress-related illnesses.
Outbred Sprague-Dawley rats are the maternal strain for inbred Fischer 344 and Lewis strains, the latter being known to have a deficient activity of hypothalamic-pituitary-adrenal (HPA) axis. Many studies have used these strains as models for physiological and behavioral studies (e.g. 12-15). In our previous study we found that restraint combined with cold stress differentially influenced acquisition and extinction in Sprague-Dawley and Lewis rats in passive avoidance learning task (16). In experiments exploring the effect of restraint on stress hormones, we observed a lower response of circulating corticosterone in Lewis compared to Sprague-Dawley rats (11).
The purpose of this experiment was to evaluate the influence of rat strains with different activity of HPA axis on their behavior affected by two types of restraint stress; the combination of restraint with cold (6) seems to induce a stronger physical impact than restraint alone. To assess the influence of these factors, we investigated behavior of rats in an open space, which enables the determination of spontaneous motor activity and may allow the interpretation of the registered motor patterns in terms of exploratory motivation and emotional reaction. Adult male Sprague-Dawley and Lewis rats were exposed to immobilization (restraint) alone or immobilization combined with water immersion, and behavioral parameters in the testing device were evaluated 2 and 5 hours after stressor application. The possible persistence of stressor effects was investigated one week later. In order to explore possible augmentation of stressor-induced behavioral alteration to further stressogenic event, the whole procedure was repeated once again. We hypothesized that for behavioral consequences the stress type as well as rat strain could be relevant.
Animals
Forty male Sprague-Dawley (S-D) and forty Lewis rats (LEW) (Charles-River Laboratories, Sulzfeld, Germany), with average starting body weights 235 and 215 g, respectively (age of two months), were used. Animals had free access to a standard pellet food and water. Rats were housed in a box for storage of SPF animals (FluFrance, Vissous, France) with a controlled light regime maintained on a 12-h light/dark cycle (lights on 6h and off at 18h), temperature (22 + 1 °C), relative humidity 50-70 %, and constant air pressure. Four rats were housed per cage (42 x 26 x 25 cm). Behavioral tests were performed from 8 a.m. to 1 p.m. Treatment of animals was in accordance with the Declaration of Helsinki Guiding Principles on Care and Use of Animals (DHEW Publication, NHI 80-23). The study was approved by the Ethical Review Committee on First Faculty of Medicine, Charles University in Prague.
Stress procedure
Animals of both strains were exposed to two types of acute restraint (immobilization) stressors for 60 minutes (6, 16, 17). Restraint alone (IMO) was applied by fixing forelimbs and hindlimbs of the rat with adhesive plaster; then the animal was restrained in a snug-fitting plastic-mesh. This mesh was bent to conform to the size of individual animal and a bandage fixed this shape of mesh. When combined immobilization with water immersion (IMO+C), restrained rats were immersed in the water bath (22 °C) in such a way that the upper 1/4 of the animal was outside of water. After the exposure to IMO+C the rats were dried and all rats were returned to the home cage. Both stresses were applied for 1 hour and started 2 or 5 hours before the onset of stress. Control animals remained untreated and were tested directly after their removal from the home cage.
Design
Rats of each strain were randomly assigned to one of five groups: control group (CO) - no stress; groups exposed for 1 hour to stressors 2 h before testing - IMO(2) and IMO+C(2); groups exposed for 1 hour to stressors 5 h before testing - IMO(5) and IMO+C(5). Each group consisted of eight animals. Over a period of four weeks behavioral activity of rats was tested four times in one-week intervals. During the first and third trial the animals were exposed to stressors before testing; in the second and fourth trial no stress was administered and all rats were tested directly after their removal from home cages.
Behavioral measurements
The testing apparatus (Coulbourn Instruments Inc., PA, USA) consisted of an arena (41x41x41 cm) with an automated monitoring system. One grid of infrared sensor beams was mounted 3 cm above the floor to measure the locomotor activity. The second tier of beams was mounted 16.5 cm above the floor to measure vertical (rearing) activity. The Tru Scan 99 software allows the recording of the following behavioral parameters: (1) total movement distance in cm (TMD); (2) total number of rearing including wall-leaning. Unlike some behavioral tests that were performed under intensive light (e.g. 18), all experiments were done in a dimly illuminated room with shielded 25W bulb in the corner of the room. Behavior was recorded for 15 min.
Statistics
All statistical tests were conducted using the Systat 10 software (SPSS, Inc., Chicago, USA). First, two-way ANOVA was performed, the factors being rat strain (2 levels) and treatment (5 levels). Second, a one-way ANOVA followed by the Bonferroni’s method was done to compare differences among groups within a given strain for individual trials. Third, ANOVA for repeated measures was used for calculation of repeated testing during four weeks lasting experiment. Finally, t-test was performed to compare the difference between both strains within a given stress insult for individual trials. Statistical significance was accepted when p<0.05 (two-tailed). To simplify the result section statistical data for trial 1 (two-way ANOVA) are included only. Results are presented as means ± SEM.
Effect of stressors on locomotion
Fig. 1 depicts total movement distance of rats in four subsequent trials performed in one-week intervals with (trial 1 and 3) and without (trial 2 and 4) application of the stressor. A two-way ANOVA for trial 1 showed significant effects for the factor strain [F(1,70) = 4.73, p = 0.033] and factor treatment [F(4,70) = 29.9, p< 0.001]. Differences between responses due to rat strain and treatment were significant also in trials 3 and 4, but not in trial 2.
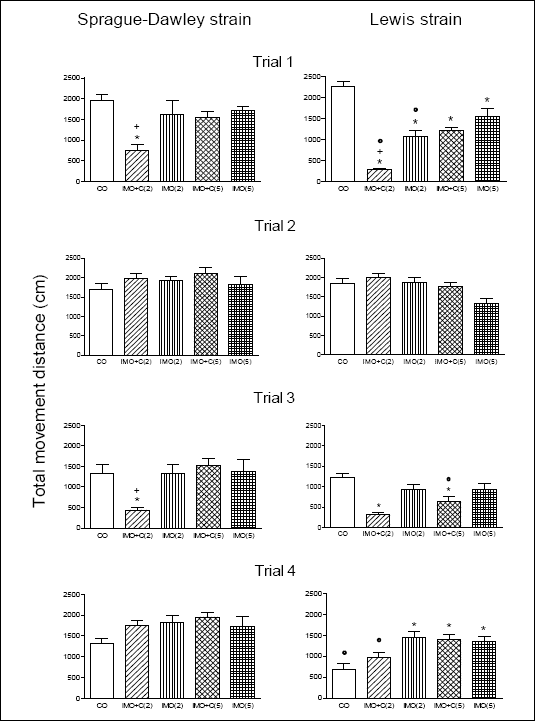 |
| Fig. 1. The effects of restraint stress (IMO) and restraint combined with cold (IMO+C) on the total movement distance (expressed in cm) in S-D and LEW rats. Four trials were performed in one-week intervals; trials 1 and 3 = with stress insult, trials 2 and 4 = without stress insult (for details see Methods). Statistical significance in all tests is for p<0.05: (a) within a given strain: * versus control group, + versus all other stressed groups, (b) within the same stressor insult: ° versus S-D rats. |
Trial 1: S-D rats subjected to IMO+C(2) exhibited a significant reduction of TMD as compared with other groups including controls (p < 0.001). All stressed LEW groups exhibited lower TMD in comparison to the control one (p < 0.001). The comparison of S-D and LEW revealed statistical differences between groups IMO+C(2) (p<0.01) and IMO(2) (p < 0.05).
Trial 2: No differences in TMD were observed among controls and stressed groups of both S-D and LEW rats.
Trial 3: When compared to controls as well as to other stressed groups a significant reduction of TMD was found again in S-D rats exposed to IMO+C(2) (p < 0.01). In LEW rats only IMO+C(2) and IMO+C(5) reduced TMD (p < 0.05). Finally, LEW rats exposed to IMO+C(5) exhibited lower TMD than S-D animals (p < 0.05).
Trial 4: In S-D rats no differences in TMD were found. Three groups of LEW rats - IMO(2), IMO+C(5) and IMO(5) - exhibited significantly higher TMD as compared to the control group (p < 0.01). In S-D rats no differences in locomotion among groups were found. Control LEW rats and those exposed to IMO+C(2) exhibited statistically lower level of locomotion as compared to S-D animals (p < 0.05).
We add that the comparison of changes in TMD over four repeated trials revealed a significant decrease in LEW but not in S-D controls. The comparison of CO animals in four repeated trials revealed significant differences in LEW [F(3,28) = 32,7, p < 0.001] but not in S-D rats. Bonferroni´s post-test showed a significant decrease of locomotion in controls of LEW rats in trials 3 and 4.
Effect of stressors on rearing
Fig. 2 depicts the total number of rearing of S-D and LEW rats. A two-way ANOVA for trial 1 showed significant effects for the factor strain [F(1,70) = 37.4, p<0.001] and factor treatment [F(4,70) = 25.0, p<0.001]. Differences between responses due to rat strain and treatment were significant also in trial 2, 3 and 4.
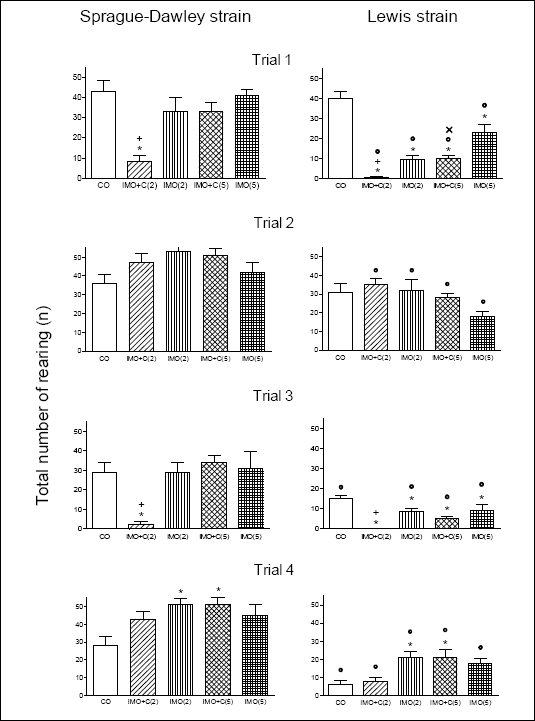 |
| Fig. 2. The effects of restraint stress (IMO) and restraint combined with cold (IMO+C) on the total number of rearing in S-D and LEW rats. Four trials were performed in one-week intervals; trials 1 and 3 = with stress insult, trials 2 and 4 = without stress insult (for details see Methods). Statistical significance in all tests is for p<0.05: (a) within a given strain: * versus control group, + versus other stress groups, x versus IMO(5); (b) within the same stressor insult: ° versus S-D rats. |
Trial 1: In S-D animals a significant reduction of rearing was found in IMO+C(2) group as compared to controls (p < 0.001). This test also showed that IMO+C(2) exposed rats exhibited lower rearing activity than in groups treated by other stressors (p<0.05). In LEW animals, all stressed groups showed a significantly lower rearing as compared to the control group (p < 0.001); the reduction in IMO+C(2) group being the strongest. Rearing activity in IMO+C(5) group was lower than that in IMO(5) group (p < 0.05). A significantly lower rearing activity was found in all stressed LEW groups as compared with S-D ones (p < 0.001).
Trial 2: All previously stressed LEW groups exhibited lower rearing activity than S-D rats (p < 0.05).
Trial 3: A significant reduction of rearing in S-D rats exposed to IMO+C(2) was found (p < 0.01). As LEW rats concerns no rearing was observed in IMO+C(2) group. Also the other stressed groups displayed reduced rearing when compared to the controls (p < 0.05). Except for IMO+C(2) group, a significant reduction of rearing was found in all stressed LEW as compared with S-D rats (p < 0.05).
Trial 4: There was an significant increase of rearing in both strains in groups IMO(2) and IMO+C(5) compared to controls (p < 0.05). All stressed LEW rats exhibited lower rearing activity than S-D animals (p < 0.05).
The comparison of CO animals in four repeated trials showed strain differences [F(1.56) = 12.9, p < 0.001] and differences due to test repetition [F(3.56) = 13.2, p < 0.001]. Repeated measurements revealed differences in LEW controls only [F(3.28) = 29.9, p < 0.001]. Bonferroni’s post-test showed a significant decrease of rearing of CO in the third and fourth trials (p < 0.05).
It has been sufficiently proved that various rat strains behave in a different way when submitted to a particular experimental situation (e.g. 15, 19-22), including exposure to a stressor (16, 23-26). The present study confirmed that the same holds true for S-D and LEW rats. When tested for the spontaneous behavior in a rectangular arena enabling registration of locomotion and rearing, no differences between the control rats were found during the first session. In contrast, the four times repeated exposure revealed a significant activity decline in LEW rats only, indicating habituation to the unfamiliar environment in this strain. In experiments using the open field test, LEW rats did not differ in the locomotor activity from S-D and Fischer 344 rats, the difference being found in movement patterns (27). On the other hand, LEW rats demonstrated a clear between-sessions habituation, i.e. reduction of the overall activity from day to day, while no such phenomenon was observed in Fischer 344 rats (14).
IMO+C and IMO decreased locomotion and rearing behaviors in both tested strains. However, while in LEW rats a decrease in these behavioral parameters was found in all stressed groups, in S-D rats only group subjected to IMO+C(2), representing the strongest stressogenic effect, displayed the detrimental effect. In the study comparing restraint alone and restraint performed at 4 °C a decrease of spontaneous motility in S-D rats was observed only after exposure to cold stressor (26). Another evidence of a higher sensitivity to the stressors in LEW, compared to S-D rats, comes from the stronger suppression of locomotion in IMO+C(2) and IMO(2) and of rearing in all stressed LEW groups. The second exposure to the stressors resulted in a similar decrease of locomotion and rearing in S-D and LEW rats in relation to controls. These results (trial 3) suggest more pronounced suppression of behavioral performance in LEW than in S-D rats, the difference reaching significance in animals subjected to IMO+C(5) and IMO(5). Although the recorded activities appear more suppressed after the second stress application, especially in LEW rats, the lower control values in trial 3 do not allow the comparison with the results in trial one. Taken together, the present results show that behavioral consequences are observable 1 and 4 hours after the termination of a single acute exposure to both stressors in LEW rats, while only IMO+C(2) induced analogous behavioral alterations in S-D rats. The difference between IMO and IMO+C was found also in the previous study performed in Wistar rats: restraint combined with cold induced stronger suppression of locomotion and rearing in the open field than restraint alone (5).
The role of time elapsed between the stress termination and behavioral testing was most pronounced as a stronger suppression of exploratory behavior after IMO+C(2) than after IMO+C(5) stressor in both strains. In S-D rats IMO+C(2) was the only stressogenic insult inducing the behavioral deficit. LEW rats displayed reduced locomotion and rearing even after IMO+C(5) application; there were either no or minor differences in the effects of restraint alone at the two used intervals. These results support the notion of the role of physical impairment of joint and muscle function as well as general motor coordination in animals shortly after the cold exposure (26).
In trial 2 performed one week after stressor presentations, the locomotor score of both strains returned to control values. A decrease in rearing in comparison with controls also disappeared in both strains, however, the recovery in LEW rats did not reach that of S-D ones. In trial 4, in contrast to deficit in locomotion and rearing induced by acute stress exposure, we observed a tendency to enhanced locomotion in the previously stressed rats of both strains reaching significance in LEW rats in all groups except for IMO+C(2). Higher rearing frequency occurred in both strains in groups IMO+C(5) and IMO(2). Probably due to stronger tendency to habituate, shown in the controls (28), the locomotion and especially rearing values were lower in LEW compared to S-D rats.
The open field test is commonly used and pharmacologically validated test for evaluating anxiety in animals in an unfamiliar environment (29-31) and the stress-induced reduction of exploration is considered as being related to increased fear and/or anxiety. Single or repeated sessions of immobilization reduced exploration in laboratory rodents (5, 32-34), however, also no changes have been reported (35-37). The disparate results may be related to many variables in the used experimental design, the intensity, duration and chronicity of the applied stressor, together with constitutional factor. In the present study we observed significant differences between S-D and LEW rats, on the other hand, the two stressors differentiated in the locomotory reduction in the shortest stress-testing interval only. It has been reported that LEW rats more often avoid the central zone of the open field, the open arms of the elevated plus maze and the white compartment of the black and white box than SHR animals; the findings have been interpreted in terms of more anxiety-related behavior of LEW strain (12, 31). LEW rats also exhibited greater stress-induced increases in anxiety-like behaviors than S-D or Fischer 344 rats (38). Here, the control LEW and S-D rats exhibited similar locomotion and rearing in the first trial and the differences could be observed after the stress exposure only.
Various types of stressors have been shown to induce behavioral alterations lasting for a long period after the stress exposure (8, 39-41). Long-term locomotion reduction was observed also after immobilization (32, 33, 42). In the present study effects of IMO and IMO+C on locomotion and rearing disappeared during one-week interval after the first stress exposure, but testing performed one week after the repeated stress session (trial 4) revealed an increase of exploratory behavior in several groups of stressed S-D and LEW rats. These long lasting stress effects were more pronounced in LEW rats: they displayed increased locomotion as well as rearing score, while S-D rats exhibited only enhanced rearing in IMO(2) and IMO+C(5) groups.
The deficits in exploratory activity produced by stressors like restraint or inescapable foot-shock have been interpreted as being related to increased fear and/or anxiety (7, 8, 33, 34, 42). However, also an initial unsustained increase in locomotor activity was observed (39, 40). The increase of locomotion and rearing seen in the present study several days after the acute post-stressor reduction indicates changes in behavioral responsiveness to the identical environmental stimuli. Symptoms that arise in the aftermath of a traumatic event include increased psychophysiological arousal (43), which may underlie changes in the internal excitability of stressed animals and consequently determine the behavioral response to the environmental challenge. Changes from reduction to increase may be interpreted as bi-directional manifestations of the long-term stress effect. The stressor-induced biobehavioral alterations displaying bi-directional expression have been included in proposed criteria for the animal model of posttraumatic stress disorder (PTSD) (43, 44).
In summary, the findings show that the used plastic-mesh restrainer induced stress of sufficient severity to induce both acute and long-term behavioral alterations. The results also support the view that S-D and LEW rats may respond differentially to the stressors. LEW rats exhibited a more pronounced decrease in locomotion and rearing after acute stressors than the S-D rats, and only LEW rats exhibited the increase in locomotion in the last session one week after the second stressor exposure. Notably, the only behavioral change seen in S-D rats exposed to IMO+C(5), IMO(2) and IMO(5) was the increased rearing in the last trial expressing thus the long-term aftermath of these stressors.
Acknowledgements: The study was supported by grants MSM 0021620806, GACR 305/03/H148 and GACR 309/06/0121.
- Chrousos GP, Gold PW. The concept of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA 1992; 267:1244-1252.
- Pacák K. Heterogenous nerochemical responses to different stressors: a test of Selye’s doctrine of nonspecifity. Am J Physiology 1998; 44: R1247-1255.
- Pacák K. Stressor-specific activation of the hypothalamic-pituitary-adrenal axis. Physiol Res 2000; 49 (Suppl 1): S11-57.
- Sida P, Koupilova M, Hynie S, Krejci I, Hlinak Z, Klenerova V. Effects of two types of restraint stress on the learned behaviour in rats. Acta Medica (Hradec Kralove) 2003; 46:153-156.
- Trnečková L, Šída P, Hynie S, Krejčí I, Hliňák Z, Klenerová V. Effects of two types of restraint stress on the spontaneous behaviour in rats. Acta Medica (Hradec Kralove) 2004; 47:177-180.
- Klenerova V, Jurcovicova J, Kaminsky O et al. Combined restraint and cold stress in rats: effects on memory processing in passive avoidance task and on plasma levels of ACTH and corticosterone. Behav Brain Res 2003; 142:143-149.
- Pijlman FTA, Herremans AHJ, van de Kieft J, Kruse CG, van Ree JM. Behavioural changes after different stress paradigms: prepulse inhibition increases after physical, but not emotional stress. Europ Neuropsychopharmacol 2003; 13:369-380.
- Pijlman FTA, Woltering G, van Ree JM. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behav Brain Res 2003; 139:131-138.
- Faraday MM. Rat sex and strain differences in responses to stress. Physiology and Behavior 2002; 75:507-522.
- Kacew S, Festing MFW. Role of rat strain in the differential sensitivity to pharmaceutical agents and naturally occurring substances. J Toxicol Environ Health 1996; 47:1-30.
- Klenerova V, Sida P, Hynie S, Jurcovicova J. Rat strain differences in responses of plasma prolactin and PRL mRNA expression after acute amphetamine treatment or restraint stress. Cell Mol Neurobiol 2001; 21:91-100.
- Ramos A, Mormede P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 1998; 22:33-57.
- Sternberg EM, Glowa JR, Smith MA, Calogero AE, Listwak SJ, Aksentijevich S, Chrousos, GP, Wilder, RL, and Gold, PW. Corticotropin releasing hormone related behavioral and neuroendoendocrine responses to stress in Lewis and Fischer rats. Brain Res 1992; 570:54-60.
- Stöhr T, Wermeling S D, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacol Biochem Behav 1998; 59:813-818.
- Windle RJ, Wood SA, Lightman SL, Ingram CD. The pulsatile characteristic of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential response. Endocrinology 1998; 139:3844-3852.
- Klenerova V, Kaminsky O, Sida P, Krejci I, Hlinak Z, Hynie S. Impaired passive avoidance acquisition in Spraque-Dawley and Lewis rats after restraint and cold stress. Behav Brain Res 2002; 136:21-29.
- Klenerova V, Sida P. Changes in beta-adrenergic receptors in the neurohypophysis and intermediate lobe of rat hypophysis exposed to stress. Physiol Res 1994; 43:289-292.
- Klejbor I, Ludkiewicz B, Domaradzka-Pytel B, Wójcik S, Moryś J. Open field stress and neurons containing calcium-binding proteins in the piriform cortex of the rat. J Physiol Pharmacol 2005; 56:223-231.
- Asano Y. Characteristics of open field behavior of Wistar and Sprague-Dawley rats. Exp Anim 1986; 35:505-508.
- Gentsch C, Lichtsteiner M, Feer H. Open field and elevated plus maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effect of chlordiazepoxide. Behav Brain Res 1987; 25:101-107.
- Schmitt U, Hiemke C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav 1998; 59:807-811.
- Treit D, Menrad J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav 1993; 44:463-469.
- Dhabhar FS, McEwen BS, Spencer RI. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels – a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res 1993; 616:89-98.
- Dhabhar FS, McEwen BS, Spencer RI. Adaptation to prolonged or repeated stress – comparison rats strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 1997; 65:360-368.
- Gadek-Michalska A., Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J Physiol Pharmacol 2003; 54:449-459.
- Stillman MJ, Shukitt-Hale B, Levy A, Lieberman HR. Spatial memory under acute cold and restraint stress. Physiol Behav 1998; 64:65-69.
- Paulus MP, Geyer MA, Sternberg E. Differential movement patterns but not amount of activity in unconditioned motor behavior of Fischer, Lewis and Sprague-Dawley rats. Physiol Behav 1998; 65:601-606.
- Lat J. Analysis of habituation. Acta Neurobiol Exp 1973; 33:771-789.
- Boguszewski P, Zagrodzka J. Emotional changes related to age in rats – a behavioral analysis. Behav Brain Res 2002; 133:323-332.
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev 2001; 25: 117-142.
- Ramos A, Correia EC, Izidio GS, Brüske GR. Genetic selection of two new rat lines displaying different levels of anxiety-related behaviors. Behav Genetics 2003; 33:657-668.
- Carli M, Prontera C, Samanin R. Effects of 5-HT1A agonists on stress induced deficit in open field locomotor activity of rats: evidence that this model identifies anxiolytic-like activity. Neuropharmacology 1989: 28:471-476.
- Shinba T, Shinozaki T, Mugishima G. Clonidine immediately after immobilization stress prevents long-lasting locomotion reduction in the rat. Prog Neuro-Psychopharmacol & Biol Psychiat 2001; 25:1629-1638.
- Tsuji M, Takeda H, Matsumiva T. Different effects of 5-HT1A receptor agonists and benzodiazepine anxiolytics on the emotional state of naive and stressed mice: a study using the hole board test. Psychopharmacology (Berl) 2000; 152:157-166.
- Dunn AJ, Swiergiel AH. Behavioral responses to stress are intact in CRF-deficient mice. Brain Res 1999; 845:14-20.
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 2005; 156:105-114.
- Mercier S, Canini F, Buguet A, Cespuglio R, Martin S, Bourdon L. Behavioural changes after an acute stress: stressor and test types influences. Behav Brain Res 2003; 139:167-175.
- Cohen H, Zohar J, Gidron Y et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 2006; 12:1208-1218.
- El-Hage W, Belzung C. Unavoidable predatory stress in mice: an animal model of posttraumatic stress disorder. Université de Tours 2002: www.univ-tours.fr/ed/edsst/comm 2002/elhage.pdf, 1-4.
- Pynos RS, Ritzman RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry 1996; 39:129-134.
- Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav 2003; 43:205-213.
- Van Dijken HH, Mos J, van der Heyden JAM, Tilders FJH. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiol Behav 1992; 52:945-951.
- Garrick T, Morrow N, Shalev AY, Spencer E. Stress induced enhancement of auditory startle: an animal model of posttraumatic stress disorder. Psychiatry 2001; 64:346-355.
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of post-traumatic stress disorder. Biol Psychiatry 1993; 33:479-486.