A solution to induce an acute hepatic toxicity and a rapidly progressing encephalopathy with severe seizures in rats may be a single paracetamol overdose (5 g/kg intraperitoneally) that excided the regimens previously used (1, 2, 6, 11). A counteracting agent may be the stable gastric pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, MW 1419, an anti-ulcer peptide per-orally active (12-15) that may also affect many central disturbances (16-23), efficient in inflammatory bowel disease trials (PL 14736) (24, 25) and various wound treatment (26-28), no toxicity reported (12, 13, 24, 25)) which showed various hepatoprotective effects (29-31). BPC 157 is stable in human gastric juice (more than 24 h), unlike rapidly degraded standard peptides such as h-EGF and h-TGF (12, 13), and is recognized to be a basal protectant in saliva and gastric juice (12, 13).
To demonstrate possible BPC 157 therapy capability as a paracetamol antidote in early and advanced stage of paracetamol toxicity, BPC 157 was applied intraperitoneally or intragastrically (12, 13) (i) prophylactically, immediately after paracetamol or (ii) therapeutically, after 3 hours elapsed. Presenting the generally known significance of paracetamol toxicity (1-3, 6, 11), this may reveal the role of BPC 157 (i.e., a free radical scavenger (17, 29)) against the early paracetamol lesions development, and even more importantly, when the original damaging process induced by an extreme paracetamol over-dose was highly advanced.
Animals
Male Albino Wistar (200 g) rats, were used in all of the experiments approved by the Local Ethic Committee (at least 10 rats per each experimental group per each period), assessed by observers naive about the given treatment.
Drugs
Medication, without a carrier or peptidase inhibitor, includes pentadecapeptide BPC 157 (a partial sequence of human gastric juice protein BPC, freely soluble in water at pH 7.0 and in saline; peptide with 99% (HPLC) purity (1-des-Gly peptide as impurity, manufactured by Diagen, Ljubljana, Slovenia, GEPPPGKPADDAGLV, M.W. 1419 (12,13)) and paracetamol (Plicet, Pliva).
Drugs protocol and assessment
Paracetamol (5 g/kg) was intraperitoneally applied. Then, we applied BPC 157 (dissolved in saline, 10.0 µg, 10.0 ng, 10 pg/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically as follows: (i) prophylactically, immediately after or (ii) therapeutically, after 3 hours elapsed. Histology and biochemistry assessment was carried out immediately after sacrifice after paracetamol challenge at 25 min (general seizure initiation), 3 h (at the time of maximal convulsion, used as the point of therapeutic application of BPC 157 regimens) and at 24 h post-paracetamol. Intensity of behavioral disturbances and seizure presentation was accordingly assessed. Assessment was (i) at 10, 20, 25, 30, 35, 60, 120, 240, 300, 360, 420, 480, and 1440 min following paracetamol when prophylactic regimen (BPC 157 immediately after paracetamol) was studied or (ii) when therapeutic regimen (BPC 157 at 180 min after paracetamol) was studied, the initial assessment was before therapy application, at 120 min and 180 min after paracetamol (i.e., in conditions of generalized convulsions). Then, after BPC 157 or saline therapy, the subsequent assessment was in few next minutes' intervals at 190, 200, 210, 220, 230, 235, 240, 300, 360, 420, 480 and 1440 min following paracetamol (i.e., 10, 20, 30, 40, 50, 55, 60, 120, 180, 240, 300, 1260 min after delayed application of BPC 157 or saline therapy).
Histology assessment
Four-micron sections of formalin-fixed liver, embedded in paraffin were stained with haematoxylin and eosin. Two to three complete transections of each liver were reviewed at four levels by a board-certified pathologist who was blinded to treatments and strains. Steatosis, congestion, and necrosis were graded separately over the entire submitted tissue as previously described (11). The grading system used to measure the extent of damage was the same for steatosis, necrosis, and congestion as follows: score 0: normal, no abnormality; score 1: mild, <30% of cells or lobule affected; score 2: moderate, 30-60% of cells or lobule affected; and score 3: severe, >60% of cells or lobule affected. Criteria for necrosis included karyorrhexis (loss of nucleus) and/or degeneration of cytoplasm with either coagulative or liquefactive changes. Steatosis was defined as either microvesicular, if the vacuoles were multiple within the cytoplasm and did not indent the nucleus, or macrovesicular, if there was a single vacuole with displacement and distortion of the nucleus. Steatosis progresses from microvesicular to macrovesicular with severity; however, our criteria for evaluation were the number of cells affected, not the size of the vacuole within the cell. Congestion was identified by the expansion of the sinusoids with blood cellular elements. Inability to view hepatocytes because of congestion was not equated with necrosis (11).
The brain was fixed in 10% formalin during two days. Upon fixation, the brain was grossly inspected and cut by consecutive coronal sections. Brain slabs were dehydrated in graded ethanol and embedded in paraffin. Paraffin blocks were cut into 5 µm thin slices. Paraffin slices were deparaffinated in xylene, rehydrated in graded ethanol and stained with haematoxylin and eosin. Intensity and distribution of brain lesions (red neurons) (swollen or ballooned neurons are a future of the axon reaction and a variety of disease in which perikaryal changes occur independently of axonal damage; histologically, they appear as distended, weakly-staining cells with large, realtively clear nuclei) and brain edema were described and evaluated semiquantitatively (22). While 0 generally indicated no changes, the lesions were subsequently scored as follows: 0-3, edema (1- weak diffuse and/or perifocal; 2- moderate; 3- strong and generalized); 0-4, red neurons (1- <5% red neurons, 2- 5_30% red neurons, 3- 30_50% red neurons, 4- 50% red neurons).
Biochemistry assessment
To determine serum values (IU/l) of aspartate transaminase (AST), alanine transaminase (ALT), and ammonium blood samples were centrifuged for 15 min at 3000 rpm, immediately after death. All tests were measured on an Olympus AU2700 analyzer, with original test reagents (Olympus Diagnostica, Lismeehan, Ireland).
Intensity of behavioral disturbances and seizure presentation assessment
After paracetamol application, intensity of progressing behavioral disturbances and seizure presentation was continuously monitored, and assessed and scored (score 0 - normal, score 1 - rearing on hind legs, shaking, 2 - sleepy, tottering walk, 3 - sleepy, tottering walk and repeatedly falling down, 4 - sleepy, tottering walk and repeatedly falling down, jerking of the extremities, 5 - lying down, generalized convulsions) at particular time points depending on the regimen used, as described before.
Statistical analysis
Statistical analysis of the quantified data was performed by analysis of variance (ANOVA). Post hoc comparisons were appraised using the conservative Bonferroni/Dunn test. Data are presented as meanħstandard deviation (SD). Non parametric statistic analysis was performed for categorical data using Kruskal-Wallis and post hoc Mann-Whitney U test. Values are expressed as min/med/max. Values of P<0.05 were considered statistically significant.
Liver lesions and biochemistry assessment
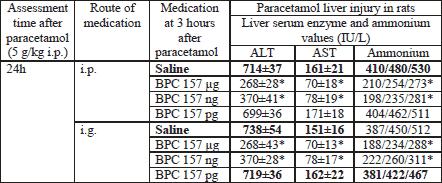
In controls, the grading system used to microscopically measure the extent of liver damage (the same for steatosis, necrosis, and congestion) showed no lesion presentation at the earliest point of 25 minutes after paracetamol (Table 1), but liver enzymes and ammonium serum values were already increased (Table 2). Then, after 3 hours, we found moderate lesions, and finally, after 24 hours severe lesions (Table 1, Fig. 1, Fig. 2) accordingly with further increase of liver enzymes and ammonium serum values (Table 2), unless BPC 157 was given within µg-ng range, intraperitoneally or intragastrically (Table 1, Table 2, Fig. 1, Fig. 2). In rats that received pentadecapeptide BPC 157 immediately after paracetamol, we found no lesions after 25 minutes or 3 hours post-paracetamol, and only mild lesions after 24 hours (Table 1) and consistently less liver enzymes and ammonium serum values (Table 2). When pentadecapeptide BPC 157 therapy was given in already advanced stage of paracetamol toxicity, such as at 3 hours after initial paracetamol application, only mild liver lesions (Table 3, Fig. 2) and markedly less liver enzymes and ammonium serum values (Table 4) could be noted at the final 24 hour post-paracetamol point.
| Table 1. Prophylactic effect of pentadecapeptide BPC 157 on paracetamol liver injury in rats. BPC 157 (10.0 µg, 10.0 ng, /kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically. Microscopy presentation (score 0-3), Min/Med/Max, *vs. saline (control), at least P<0.05, assessed at 25 min, 3 hours and 24 hours post-paracetamol. |
 |
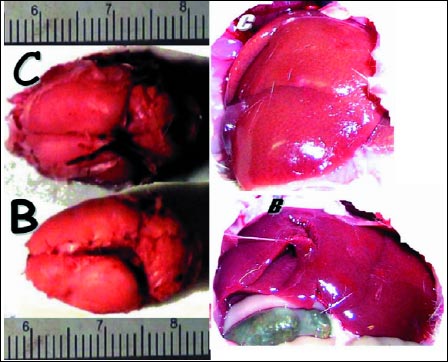 |
Fig. 1. Prophylactic effect of pentadecapeptide BPC 157 on paracetamol liver and brain injury in rats. Characteristic yellowish liver (C) and edematous brain (C) presentation in situ in control (saline (5.0 ml/kg)) or liver (B) and brain (B) presentation close to normal in BPC 157 (B) treated rats at 24 h after paracetamol application. Gross liver and brain appearance was not influenced by large vs. small dose of BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or or intraperitoneal vs. intragastrical application. |
| Table 2. Serum liver enzymes and ammonium values (IU/L) in rats that receive BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically immediately after paraceteamol. Min/Med/Max, *vs. saline (control), at least P<0.05, assessed at 25 min, 3 hours and 24 hours post-paracetamol. |
 |
 |
Fig. 2. Effect of pentadecapeptide BPC 157 on paracetamol liver injury in rats. Characteristic liver histology presentation in control (C) at 24 hours (upper) or at 3 hours (lower) post-paracetamol. Characteristic liver histology presentation at 24 hours post-paracetamol in BPC 157 rats (B) when BPC 157 had been given immediately after paracetamol (upper), or at 3 h after paracetamol (lower)). Suddan staining, 25x. BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically. Histologic appearance was not influenced by large versus small dose of BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or or intraperitoneal vs. intragastrical application. |
Intensity of behavioral disturbances and seizure presentation
Regularly, paracetamol toxicity is presenting with progressing intensity of behavioral disturbances and seizure presentation, unless BPC 157 was given.
| Table 3. Paracetamol liver injury in rats that received BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically at 3 hours after paracetamol challenge. Microscopy presentation (score 0-3), Min/Med/Max, *vs. saline (control), at least P<0.05, assessed at the final interval (24 hours post-paracetamol). |
 |
Shortly after paracetamol application, the rats started to rear on hind legs and shake. After 10 minutes, they became sleepy, with a tottering walk, repeatedly falling down while after 20 min they started to exhibit jerking of the extremities. After 25 minutes all rats were lying down, and all presented generalized convulsions, a status lasting the next 5 hours. Then, they regained posture, but were still sleepy, with a tottering walk, repeatedly falling down for next 2 hours. Then, rats started to drink excessively, then to eat and repeatedly drink water for a period of a few minutes. The rest of the time (7-24 h) the paracetamol rats survived normally although exhausted (Table 5, Table 6).
| Table 4. Serum liver enzyme and ammonium values (IU/L) in rats that received BPC 157 (10.0 µg, 10.0 ng, /kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically at 3 hours after paracetamol challenge. Min/Med/Max, *vs. saline (control), at least P<0.05, assessed at the final interval (24 hours post-paracetamol). |
 |
This course was markedly changed when BPC 157 was given within µg-ng range, intraperitoneally or intragastrically. Generally, BPC 157 rats had a markedly attenuated course completely preventing convulsions as well leading to full reversal when applied in rats exhibiting generalized convulsions. When pentadecapeptide BPC 157 was given immediately after paracetamol, BPC 157 rats started to rear on hind legs and shake shortly after paracetamol application while after 10 minutes, they became sleepy, with a tottering walk, after 35 min they regained normal behavior, drank (but not excessively) and then ate and showed no behavioral abnormalities until their sacrifice (Table 5).
| Table 5. Prophylactic effect of pentadecapeptide BPC 157 on paracetamol convulsions in rats. BPC 157 (10.0 µg, 10.0 ng, /kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically. Intensity of behavioral disturbances and seizure presentation, Min/Med/Max, *vs. saline (control), at least P<0.05, assessed throughout the post-paracetamol period. |
 |
Moreover, presenting that the most threatening period may be the seizures-period, and the regular duration estimated to be 5 hours, and that at the time point of 3 hours following paracetamol the rats would exhibit generalized convulsions for the next two subsequent hours, it is interesting that after BPC 157 application the generalized convulsions disappeared within the next 25 minutes and the rats regained posture, still sleepy, with a tottering walk for the next 50 minutes. Then, the rats started to drink excessively, then to eat and repeatedly drink water for a period of a few minutes after which they regained normal behavior, drank and then ate and showed no behavioral abnormalities until their sacrifice (Table 6).
| Table 6. Paracetamol convulsions in rats that received BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically after 3 hours had elapsed following paracetamol challenge. Intensity of behavioral disturbances and seizure presentation, Min/Med/Max, before (120-180 min) and after (190-1440 min) therapy, *vs. saline (control), at least P<0.05, assessed throughout post-paracetamol period. |
 |
Brain lesions assessment
Regularly, after 24 hours we found a heavy interstitial edema in all rat brains, particularly in the cerebellum and more so in white than in gray mater. Neurons presented with severe damage. Severely damaged red neurons, without any inflammatory reaction, were present in all animals, in more then one half of hippocampal neurons, neurons of the dentate nucleus, Purkinje cells and other cerebellar nuclei. Other damaged neurons were in the pons and mesencephalon, particularly in the tegmental areas of these structures. Less than one third of neurons in the lateral geniculate body were changed like the red neurons. Interestingly, moderate edema was already present at 25 minutes following paracetamol application, at the time of initiation of the generalized convulsions. Damaged red neurons, without any inflammatory reaction, were present in all animals in more than 30% but less than 50% of hippocampal neurons, neurons of the dentate nucleus, Purkinje cells and other cerebellar nuclei. The severity of lesions in neurons further progressed after a 3 hour period leading to additional worsening, noted at the final 24 hour post-paracetamol point (Table 7, Table 8, Fig. 1, Fig. 3).
| Table 7. Prophylactic effect of pentadecapeptide BPC 157 on paracetamol brain injury in rats. BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically. Microscopy presentation, intensity and distribution of brain lesions (red neurons (0-4) and brain edema (0-3) were described Min/Med/Max, *vs. saline (control), at least P<0.05, assessed at 25 min, 3 hours and 24 hours post-paracetamol. |
 |
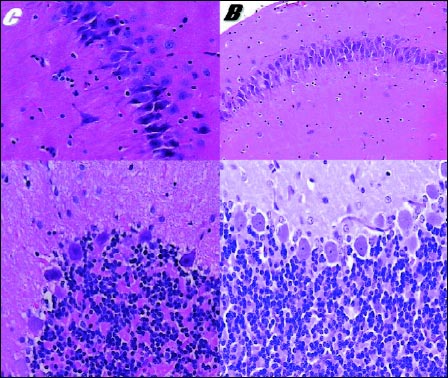 |
Fig. 3. Effect of pentadecapeptide BPC 157 on brain injury in rats. Characteristic brain histology presentation at 24 hours in control (C) and BPC 157 rats (B). Hippocampal red neurons (C, x 400), some hippocampal neurons are red (B, x 200) (upper) Purkinje cells are acidophilic (C, x 400) Regular morphology of Purkinje cells (B, x 400) (lower). HE. Histologic brain appearance was not influenced by large versus small dose of BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or intraperitoneal vs. intragastrical application. |
When pentadecapeptide BPC 157 was given immediately after paracetamol (µg-ng range, intraperitoneally or intragastrically), in accordance with a complete absence of convulsions, BPC 157 rats commonly had all alterations markedly less expressed 24 hours after paracetamol, reduced interstitial edema (only mild) and markedly less damaged neurons, without inflammatory reaction. Interestingly, these rats presented consistently less damage at the earliest points i.e., 25 minutes or 3 hours following paracetamol application, there was no edema and no damaged red neurons, without any inflammatory reaction (Table 7, Fig. 3).
Finally, for therapy effect of BPC 157 on paracetamol brain injury in rats that received pentadecapeptide BPC 157 later in an advanced paracetamol toxicity stage, we should consider that at the time point of 3 hours following paracetamol, as previously mentioned, after therapy these rats had initially exhibited generalized convulsions and had significant brain damage. However, they presented with markedly attenuated brain lesions at the final 24 hour post-paracetamol point. Notably, they exhibited lesser interstitial edema (only mild), markedly less damaged neurons than corresponding controls and had no inflammatory reaction (Table 8).
| Table 8. Paracetamol brain injury in rats that received therapy BPC 157 (10.0 µg, 10.0 ng/kg b.w.) or saline (5.0 ml/kg) intraperitoneally or intragastrically at 3 hours after paracetamol challenge. Microscopy presentation, intensity scored (red neurons (0-4) and brain edema (0-3)) and distribution of brain lesions were described, Min/Med/Max, *vs. saline (control), at least P<0.05, assessed at the final interval (24 hours post-paracetamol). |
 |
In an attempt to elucidate the sudden onset of encephalopathy with paracetamol overdose (3), this study established a consistent BPC 157 beneficial effect in paracetamol-rats, given in early or advanced stage of paracetamol toxicity. Also, an essential convulsive effect of paracetamol overdose may be a prime rapid and then sustain hallmark of paracetamol toxicity. With generalized convulsions, severe brain lesion and hyperammonemia and increased serum enzyme values already at 25 min post-paracetamol, it seems that toxins can rapidly reach the brain and affect its function, even before major liver lesions might appear, unless BPC 157 was given. Moreover, once started, following jerking of the extremities and coarse tremors (i.e., they had to be preceded by the already advanced significant damage in the brain), the seizures lasted for a few hours throughout the progressing brain and liver damage (as seen with worsening throughout the 3-24 h post-paracetamol period). Again, the course was completely changed with BPC 157 application (i.e., absence and reversal of convulsion). Thus, this paracetamol over-dose toxicity, therefore, would be an effective means for the progressive hepatic encephalopathy and convulsions course in rat relation, at least. Thereby, it is possible that this consistent BPC 157 beneficial effect on rat's pathology underlies the sudden onset of encephalopathy with paracetamol overdose as well as paracetamol overdose as the leading cause of acute liver failure in patients (3). Also, this BPC 157 effect on the background of antagonization of high paracetamol regimen may be relevant for anatagonization of comparably lower doses of paracetamol.
Likely, the prime commencing of generalized seizures may be related to the largely described molecular paracetamol processes underlying the progressive hepatotoxicity (1, 2) (i.e., the covalent binding of NAPQI to the thiol groups of critical cellular proteins (following glutathione depletion), oxidative stress induced directly by NAPQI or via coproduction of reactive oxygen species (superoxide radical and hydrogen peroxide) through redox cycling between the paracetamol semiiminoquinone radical and NAPQI) (1, 2), and vice versa, these processes counteraction may be involved in BPC 157 beneficial effects. Thus, by being both more rapid and persistent in causing neurological abnormalities, these processes are even more destructive for brain (i.e., paracetamol preferentially inhibited brain cyclooxygenases (32)), thereby generalized convulsions, particularly presenting brain tissue's high susceptibility to oxidative stress, leaving the glutathione in the brain defenseless to depletion by paracetamol (4, 5), unless BPC 157 was applied. Early concentrations of paracetamol in the brain (33) appear to be well-timed with the period of the rising concentration in various brain structures (33) and coincide with the noted brain damage and with the appearance of generalized seizures in the present study.
Therefore, the severe interstitial edema, more expressed in white than in gray mater, and severe neuron loss in hippocampal neurons, neurons of dentate nucleus, Purkinje cells and other cerebellar nuclei and less severe damage in the pons, the mesencephalon and lateral geniculate body could be a specific paracetamol effect and these territories could be involved in the occurrence of maximal paracetamol induced seizures. Besides, consistently higher paracetamol regimen may minimize all problems related to possible variations from animal to animal and from time to time in a given animal and invariably link paracetamol action with seizures, severely damaged brain tissue, hepatomegaly, fatty liver and necrosis, breakdown of liver function with profoundly increased ammonia, AST and ALT levels. Also, it may be more relevant for extreme paracetamol toxicity in patients (3).
Thus, when given to paracetamol-rats, BPC 157 would be confronted with the all processes simultaneously occurring that eventually lead to all mentioned disturbances in paracetamol over-dose-rats (1, 2). However, we shown that pentadecapeptide BPC 157, as an antiulcer peptide (12-15), may consistently counteract all paracetamol disturbances. This may also indicate that these disturbances are also interconnected throughout BPC 157 background. Moreover, considering the used paracetamol (5 g/kg i.p.)/BPC 157 (10 µg, 10 ng, 10 pg/kg i.p. or i.g., and effectiveness within µg-ng range) ratio (12, 13), it may be reasonably to assume that these therapy effects may indicate a likely role of BPC 157 in controlling, and then, counteracting one or more causative process(es).
Unfortunately, the more specific targets (1, 2) for these BPC 157 effects remained outside of the present investigation, but we should assume that in few hours period such a high paracetamol over-dose may be able also to reflect the disturbances that would otherwise require much longer period. Thus, the targets of BPC 157 counteraction may be at least partly approached within the frame of obtained paracetamol-damages. For instance, the most likely possibility may be, as mentioned before, the liver presenting BPC 157 beneficial effect on various liver lesions including CCl4 hepatotoxicity (29-31). Counteracted hepatomegaly, fatty liver and necrosis and pronounced elevation of liver enzymes (AST and ALT) and hyperammonemia, and consequently, counteraction of paracetamol seizures and brain damages by BPC 157 may be also perceived in this context. Besides, BPC 157 as an anti-ulcer peptide antagonizes hepatomegaly, fatty liver, increased AST, ALT and amylase serum values, breakdown of liver glycogen with profound hypoglycemia, along with calcium deposition after huge insulin over-dose application (31). Also, in chronically alcohol drinking rats, BPC 157 may prevent and reverse portal hypertension (30). However, another mechanism may be that the pentadecapeptide BPC 157 can directly protect against paracetamol induced brain damage.
What's more, the premise that when given peripherally, BPC 157 may have a particular beneficial effect on CNS (i.e., markedly less damaged neurons in most severely injuried areas) is in accord with: its neuroprotective properties (22, 34), consistent antagonization of different central disturbances (16-23, 34), brain 5-HT synthesis and antagonization of serotonin-syndrome in rats based on region-specific influence on the brain given peripherally either acutely or chronically (i.e., dorsal thalamus, hippocampus, lateral geniculate body, hypothalamus, dorsal raphe nucleus, substantia nigra, medial anterior olfactory nucleus, lateral caudate, accumbens nucleus, superior olive)) (shown by the very precise alpha-[14C]methyl-L-tryptophan (alpha-MTrp) autoradiographic method) (16, 20). Presenting the suggested significance of substantia nigra for controlling seizures (35), it may be important that BPC 157/substantia nigra relation may be particularly substantiated: the increased 5-HT synthesis in substantia nigra was the most prominent one (20), counteracted 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) parkinsonian syndrome as well as the lethal outcome due to particular substantia nigra damage (17), maintained dopamine system function (BPC 157 may counteract akinesia, catalepsy induced by neuroleptics or reserpine as well as amphetamine-stereotypy behavior) (17-19). Also, BPC 157 was found to have anti-convulsive properties (16, 17, 21). Also very recently, with the respect of K+-ATP channels in the substantia nigra as a predisposing factor for seizure development, BPC 157 inhibits K+ conductance in WT HEK293 cells (36). Besides, after an induced traumatic brain injury in mice, BPC 157 regimens (corresponding to those used in the present study) demonstrated a marked attenuation of damage with an improved early outcome and a minimal postponed mortality throughout a 24 h post-injury period. Ultimately, the traumatic lesions (subarachnoidal and intraventricular haemorrhage, brain laceration, haemorrhagic laceration) were less intense and consecutive brain edema had considerably improved (22).
In conclusion, we showed the progressive hepatic encephalopathy, accompanied by severe seizures with a very early onset, in paracetamol-rats (i.e., the full threatening circuit in paracetamol's acute hepatic toxicity). This was consistently counteracted by the stable gastric pentadecapeptide BPC 157, showing this peptide as an effective antidote therapy (i.e., given in µg- and ng-dose regimens in either stage of paracetamol intoxication). Also, when given parenterally or per-orally (i.e., stable in human gastric juice for more than 24 h (12, 13)), in the same dose-regimens, BPC 157 protected against various agents or procedures that would otherwise have lead to severe liver lesions (29-31) or CNS disturbances (16-23). It was effectively applied either immediately (prophylactically) after paracetamol challenge or (therapeutically) administered after a 3 h period had elapsed (note, the maximum depletion of hepatic glutathione occurred 3 h after acute paracetamol dosing (37)). Finally, BPC 157 counteracted a particular overdose toxicity that excided regular paracetamol regimens (i.e., 1, 2, 6, 11), at either of the assessed intervals (i.e., 25 min, 3 hours and 24 hours). On the other hand, the demonstration of paracetamol's progressive hepatic encephalopathy with severe seizures at least partly overruled the general discordance of NSAIDs' adverse effects and therapeutic application, paracetamol in particular as previously mentioned (1-10). Also, the significance of this peptide's therapeutic benefit against amplified and advanced paracetamol toxicity remains to be further postulated, particularly considering the extensive investigation of gastrointestinal peptides in gastrointestinal and liver physiology (38), physiological mediators in NSAIDs-induced impairment of gastric mucosal defense and adaptation (39), and brain-gut peptide regulation (40). However, very recent novel evidence suggested that just the stable gastric pentadecapeptide BPC 157 may have possible significance and implications for novel mediator of both Robert's cytoprotection and adaptive cytoprotection (41), and thereby potential to counteract paracetamol and other NSAIDs (over)-toxicity.
Conflict of interests: None declared.
- Bergman K, Muller L, Weberg Teigen S. The genotoxicity and carcinogenicity of paracetamol: a regulatory (re)view. Mut Res 1996; 349: 263-288.
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 2006; 89: 31-41.
- Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am 2008; 92: 761-794, viii.
- Nencini C, Giorgi G, Micheli L. Protective effect of silymarin on oxidative stress in rat brain Phytomedicine 2007; 14 : 129-135.
- Floyd RA. Antioxidant oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 1999; 222: 236-245.
- Scorticati C, Prestifilippo JP, Eizayaga FX, et al. Hyperammonemia, brain edema and blood-brain barrier alterations in prehepatic portal hypertensive rats and paracetamol intoxication. World J Gastroenterol 2004; 10: 1321-1324.
- Steinhauer HB, Hertting G. Lowering of the convulsive threshold by non-steroidal anti-inflammatory drugs. Eur J Pharmacol 1981; 69: 199-203.
- Hori S, Kizu J, Kawamura M. Effects of anti-inflammatory drugs on convulsant activity of quinolones: a comparative study of drug interaction between quinolones and anti-inflammatory drugs. J Infect Chemother 2003; 9: 314-320.
- Fetveit A. Assessment of febrile seizures in children. Eur J Pediatr 2008; 167: 17-27.
- Wallenstein MC. Differential effects of prostaglandin synthetase inhibitors on EEG in rat. Eur J Pharmacol 1985; 111: 201-209.
- Yohe HC, O'Hara KA, Hunt JA, Kitzmiller TJ, et al. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol 2006; 290: G1269-G1279.
- Sikiric P, Petek M, Rucman R, et al. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J Physiol (Paris) 1993; 87: 313-327.
- Sikiric P, Seiwerth S, Brcic L, et al. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology 2006; 14: 214-221.
- Sikiric P, Siwerth S, Grabarevic Z, et al. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesion induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promoters and gut peptides. Life Sci 1994; 54: 63-68.
- Sikiric P, Seiwerth, S, Grabarevic Z, et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol 1997; 332: 23-33.
- Boban Blagaic A, Blagaic V, Mirt M, et al. Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. Eur J Pharmacol 2005; 512: 173-179.
- Sikiric P, Marovic A, Matoz W, et al. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson's disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J Physiol (Paris) 1999; 93: 505-512.
- Jelovac N, Sikiric P, Rucman R, et al. A novel pentadecapeptide, BPC 157, blocks the stereotypy produced acutely by amphetamine and the development of haloperidol-induced supersensitivity to amphetamine. Biol Psychiatry 1998; 43: 511-519.
- Jelovac N, Sikiric P, Rucman R, et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats. Eur J Pharmacol 1999; 379: 19-31.
- Tohyama Y, Sikiric P, Diksic M. Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: alpha-methyl-L-tryptophan autoradiographic measurements. Life Sci 2004; 76: 345-357.
- Blagaic AB, Blagaic V, Romic Z, Sikiric P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. Eur J Pharmacol 2004; 499: 285-290.
- Tudor M, Jandric I, Marovic A, et al. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regul Pept 2010; 160: 26-32.
- Boban Blagaic A, Turcic P, Blagaic V, et al. Gastric pentadecapeptide BPC 157 counteracts morphine-induced analgesia in mice. J Physiol Pharmacol 2009; 60(Suppl. 7): 177-181.
- Veljaca M, Pavic-Sladoljev D, Mildner B, et al. Safety, tolerability and pharmacokinetics of PL 14736, a novel agent for treatment of ulcerative colitis, in healthy male volunteers. Gut 2003; 51: A309.
- Ruenzi M, Stolte M, Veljaca M, Oreskovic K, Peterson J. Ulcerative Colitis Study Group. A multicenter, randomized, double blind, placebo-controlled phase II study of PL 14736 enema in the treatment of mild-to-moderate ulcerative colitis. Gastroenterology 2005; 128: A584.
- Staresinic M, Petrovic I, Novinscak T, et al. Effective therapy of transected quadriceps muscle in rat: gastric pentadecapeptide BPC 157. J Orthop Res 2006; 24: 1109-1117.
- Tkalcevic VI, Cuzic S, Brajsa K, et al. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol 2007; 570(1-3): 212-221.
- Hrelec M, Klicek R, Brcic L, et al. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide, prophylaxis and therapy. J Physiol Pharmacol 2009; 60(Suppl. 7): 161-165.
- Sikiric P, Seiwerth S, Grabarevic Z, et al. Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci 1993; 53: PL291-PL296.
- Prkacin I, Separovic J, Aralica G, et al. Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine. J Physiol (Paris) 2001; 95: 315-324.
- Ilic S, Brcic I, Mester M, et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J Physiol Pharmacol 2009; 60(Suppl 7): 107-114.
- Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the antipyretic activity of acetaminophen. Nature 1972; 240: 410-411.
- Courade JP, Besse D, Delchambrec, et al. Acetaminophen distribution in the rat central nervous system. Life Sci 2001; 69: 1455-1464.
- Gjurasin M, Miklic P, Zupancic B, et al. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept 2010; 160: 33-41.
- Velisek L, Veliskova J, Chudomel O, et al. Metabolic environment in substantia nigra reticulata is critical for the expression and control of hypoglycemia-induced seizures. J Neurosci 2008; 28: 9349-9362.
- Barisic I, Seiwerth S, Sikiric P, Sindic A. BPC 157 inhibits K+ conductance in WT HEK293 cells J Physiol Pharmacol 2009; 60(Suppl. 2): 10.
- Buttar HS, Chow AY, Downie RH. Glutathione alterations in rat liver after acute and subacute oral administration of paracetamol. Clin Exp Pharmacol Physiol 1977; 4: 1-6.
- Konturek SJ, Brzozowski T. Gastrointestinal and liver physiology. Preface. J Physiol Pharmacol 2008; 59(Suppl 2): 3-5.
- Brzozowski T, Konturek PC, Pajdo R, et al. Physiological mediators in nonsteroidal anti-inflammatory drugs (NSAIDs)-induced impairment of gastric mucosal defense and adaptation. Focus on nitric oxide and lipoxins. J Physiol Pharmacol 2008; 59(Suppl 2): 89-102.
- Konturek SJ, Brzozowski T, Konturek PC, et al. Brain-gut and appetite regulating hormones in the control of gastric secretion and mucosal protection. J Physiol Pharmacol 2008; 59(Suppl 2): 7-31.
- Sikiric P, Seiwerth S, Brcic L, et al. Revised Robert's cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications. Curr Pharm Des 2010; in press.