The present work was undertaken to investigate whether some most commonly prescribed antidepressant agents could directly influence vascular tone in an in vitro model of isolated rat aorta. Amitriptyline and tranylcypromine were included in the study as agents often causing hypotension and they were contrasted with fluoxetine, an agent that normally does not affect blood pressure, and venlafaxine, an agent associated with induction or worsening of hypertension (8). Direct effects of amitriptyline and fluoxetine on arterial smooth muscle have already been reported (9-12). Venlafaxine and tranylcypromine have not been investigated for possible effects on arterial contractility in vitro.
This study was performed in accordance with the local Instructions for Animal Care of the Greifswald University and was approved by the state Commission for Animal Protection in Schwerin, Germany. Experiments were performed on aortas taken from Lewis 1A rats (in total 50 animals), weighing 250–400 g. For the experiment, rats were anesthetized by intraperitoneal application of thiopental (100 mg/kg) and by sectioning of abdominal aorta, quickly exsanguinated. The thoracic aortas were then isolated, immersed in Krebs-Henseleit solution (KH; in mM: 113 NaCl, 4.8 KCl, 1.3 MgCl2x6H2O, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 5.7 glucose) and cleaned from the surrounding tissue. In some preparations endothelium was removed mechanically by gently rubbing the interior of the aortal preparation with a moistened cotton-wrapped metal stick. Aortal ring preparations (about 2–3 mm long) were mounted in the customary manner between two stainless steel hooks, where the lower hook served as a fixed point and the upper hook was connected to the isometric force transducer (Entran, ELJ-S045C-35G, purchased from EMKA Technologies, Paris, France). The signal was amplified (STA 2808, EMKA Technologies, Paris, France) and displayed on paper recorder (Rikadenki multipen recorder, R-50 series, Hugo Sachs Elektronik, March-Hugstetten, Germany). The quickly mounted preparation was then immersed in the organ bath (20 ml) filled with KH solution. The KH solution (at 37°C, pH 7.4, gassed with 95% O2/5% CO2) was exchanged every 20 min. The force transducer was attached to micromanipulator which permitted displacement of the upper hook along a strict vertical axis and the adjustment of the muscle length. After a period of stabilization (60 min) the vascular muscle was stretched to its optimal length, which was established, in the preliminary experiments, to correspond to a counter-weight of 2 g (data not shown). The integrity of the endothelium in aortas was tested, at the end of the experiment, by pre-contracting the preparation with phenylephrine (0.1 µM) and a subsequent application of acetylcholine (5 µM) that liberates NO from the endothelium producing relaxation. At least 40% relaxation was taken to indicate intact endothelium. The denudation by rubbing resulted in loss of function of the endothelium, as indicated by the absence of dilation to acetylcholine (data not shown).
The following agents, that are in clinical use as antidepressants were examined: amitriptyline, a secondary amine tricyclic antidepressant mainly inhibiting noradrenaline uptake; tranylcypromine, an irreversible inhibitor of monoamonoxidase and inhibitor of prostanoid synthesis; fluoxetine, a selective serotonin reuptake inhibitor; venlafaxine, an inhibitor of noradrenaline and serotonin reuptake.
Experimental protocol
At first, a series of test for the effects of the antidepressants amitriptyline, fluoxetine, tranylcypromine or venlafaxine on induced tension were examined. Aortal rings (with and without endothelium) were incubated with the antidepressant agents, each 0.5 µM for 30 minutes (this concentration reaches approximately blood level of the agents in patients when used as therapy). Then, tension was elicited by cumulative concentrations of phenylephrine (0.5 nM–0.5 mM). Preparations without incubation with antidepressant agents served as controls.
In the second series of the experiments (with and without endothelium), the effects of the antidepressants on agonist elicited pre-contraction, were examined. The preparations were pre-contracted either with phenylephrine (0.1 µM), KCl (20 or 40 mM) or prostaglandin F2
In the third set of experiments endothelium intact preparations were pre-incubated either with NO generation inhibitor N(G)-nitro-L-arginine methyl ester (L-NAME, 500 µM), an inhibitor of guanylyl cyclase 1H-(1,2,4)oxodiazolo-(4,3-a)quinoxalin-1one (ODQ, 1 µM) or an inhibitor of adenylyl cyclase, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ 22536, 100 µM), for 30 minutes. Then they were pre-contracted with phenylephrine (0.1 µM) and, when the contraction reached a plateau, cumulative concentrations of the antidepressants were applied.
In the last set of experiments preparations with endothelium were incubated for 30min with the potassium channel blocking agents charybdotoxine (0.05 µM, calcium dependent K+ channel), glibenclamide (10 µM, ATP-dependent K channel), tetraethylammoniumchloride (TEA, 10 mM, non-specific K+ channel) or 4-aminopyridine (4-AP, 100 µM, voltage operated K+ channel).
Some endothelium intact preparations were pre-contracted with 40 mM KCl, then incubated for 30 minutes with propranolol (10 µM, ß-adrenergic blocker) or prazosine (10 µM,
Substances
Amitriptyline hydrochloride, fluoxetine hydrochloride, tranylcypromine, venlafaxine hydrochloride, phenylephrine hydrochloride, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine SQ 22536), N(G)-nitro-L-arginine methyl ester (L-NAME), 1H-(1,2,4)oxodiazolo(4,3-23a)quinoxalin-1-one (ODQ), propranolol hydrochloride, prazosine hydrochloride, 4-aminopyridine, charybdotoxin, tetraethylammonium (all were dissolved in distilled water), or glibenclamide (which was dissolved in dimethylsulfoxide-DMSO) were purchased from Sigma-Aldrich, Deisenhofen, Germany; Thiopental (Trapanal®) was purchased from BYK, Konstanz, Germany. The maximal concentrations of the solvent DMSO that were used were inferior to 0.01%. When using high concentrations of KCl, NaCl concentration of the Krebs-Henseleit solution was reduced to achieve equimolarity.
Data analysis
Data are presented as means ±S.E.M. The concentrations of agonist that produced half-maximal effect (EC50) were determined by means of non-linear regression using the Hill-Langmuir equation. The EC50 was related to basic tension (before incubation) and only calculated from the positive values and given as positive numbers on the –log10M, on the p scale as the pEC50. Obtained data were compared using analysis of variance (one-way ANOVA) followed by Dunnet post-hoc test. Probabilities of less than 0.05 were considered statistically significant. Statistical analysis was performed using the software package Prism 4 (Graph Pad Software, Inc., San Diego, CA).
Effects of incubation with antidepressants on phenylephrine induced contractions (Table 1)
Treatment with amitriptyline, fluoxetine shifted the concentration response curve of phenylephrine to the right in preparations compared to controls, incubation with tranylcypromine revealed a left shift (P<0.001). In preparations with endothelium higher concentrations of phenylephrine were required to contract the arteries than in endothelium free arteries (P<0.05).
| Table 1. The effects of endothelium removal on half maximal effects (pEC50) of phenylephrine in rat aorta after 30 minutes incubation with antidepressant agents. |
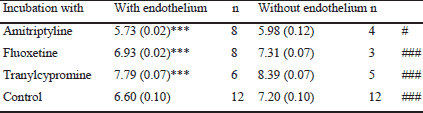 |
| After measuring isometric tension pEC50 values were calculated (mean ±S.E.M; -log10; mol/L) of n-experiments as indicated obtained in preparations with and without endothelium. Data were taken from control and pre-incubated preparations (with either amitryptyline, fluoxetine, tranylcypromine; each 0.5 µM for 30min), witch was followed by stimulation with cumulative concentrations of phenylephrine ((0.5 nM - 0.5 mM). P values derived from one-way ANOVA followed by Dunnet post-hoc-test. Incubation vs. Control: ***P<0.001; preparations with vs. without endothelium: #P<0.05; ###P<0.001. |
Effects of antidepressants on KCl pre-contracted arterial rings
In KCl (20 mM) pre-contracted preparations, fluoxetine and amitriptyline induced complete relaxation which was concentration dependent (Fig. 1). Removal of endothelium did not have an effect. Cumulative concentrations of tranylcypromine hardly relaxed endothelium intact preparations, whereas venlafaxine did not have any effect to the endothelium intact nor endothelium denuded vessels. The pEC50 values could therefore not be calculated.
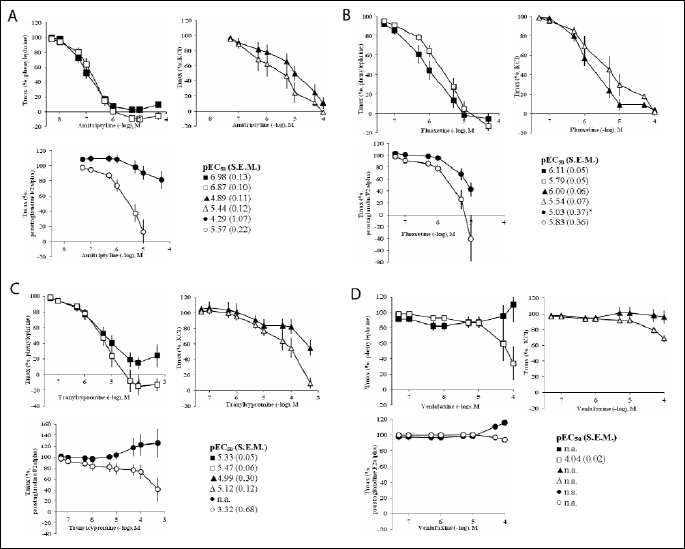 |
| Fig.
1. Effect of cumulative doses (0.05 µM–500 µM) of
(A) amitriptyline, (B) fluoxetine, (C) tranylcypromine and (D) venlafaxine
on rat aortal tension with (black symbols) or after removal of the endothelium
(white symbols) elicited by |
Effects on phenylephrine pre-contracted arterial rings
In phenylephrine (0.1 µM) pre-contracted preparations, cumulative concentrations amitriptyline, fluoxetine and tranylcypromine (in descending order of potency) induced complete relaxation of the arteries in a concentration dependent manner (Fig. 1). There were no differences between endothelium intact and endothelium free arteries. High doses of venlafaxine (>50 µM) induced further contraction of 110±23.2% from Tmax in preparations with endothelium (the pEC50 value could therefore not be calculated), whereas endothelium free aortal rings were slightly dilated.
Effects on prostaglandin F2
In prostaglandin F2
Incubation experiments: effects of nitric oxide-cGMP or cAMP blockade
The eNOS inhibitor L-NAME had already an influence on the basic arterial tone and led to a contraction of maximal 12% from Tmax that was reached in pre-contraction by phenylephrine. ODQ and SQ 22536 had a minor effect (maximal ±7% of Tmax) on the basic tone (Fig. 2). Incubation with L-NAME in phenyleprine (0.1 µM) pre-contracted preparations led to a right-shift of concentration-response curves for amitriptyline, fluoxetine and tranylcypromine (P<0.05). Also after pre-incubation with ODQ significant higher concentrations of fluoxetine and tranylcypromine were necessary to relax rat aorta (P<0.01). Incubation with L-NAME or ODQ had no effect on the dose response curve of venlafaxine, and the pEC50 values could not be calculated. Incubation with SQ 22536 did not change dose response curves of any antidepressant examined.
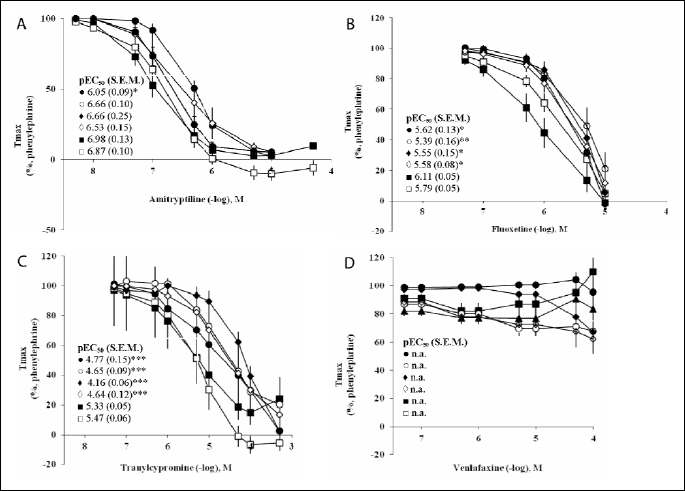 |
| Fig.
2. Effect of cumulative doses (0.05 µM–500 µM) of
(A) amitriptyline, (B) fluoxetine, (C) tranylcypromine and (D) venlafaxine
on rat aortal tension. Pre-tension elicited with phenylephrine (0.1 µM)
in controls ( |
Incubation experiments: effects of potassium channel blockers
TEA had a contracting effect (at the maximum 60% from Tmax reached by phenylephrine), similarly as charybdotoxin (maximal 11% of Tmax) or glibenclamide and 4-aminopyridin (max. ±7% of Tmax) (Fig. 2). Pre-incubation with 4-aminopyridin and TEA lead to a right shift of the relaxation response curves of fluoxetine and tranylcypromine with significant differences in the obtained pEC50 values (P<0.05). Incubation with the K+ channel blocking agents had no effect on the dose response curve of venlafaxine. Incubation with charybdotoxin or glibenclamide did not change the concentration response curves of any of the antidepressant investigated (data not shown).
Effects of blockade of alpha-or beta-adrenoceptors
Prazosine had no effect on concentration response curves of the antidepressants (results not shown). Propranolol incubation caused a left-shift for the concentration response curves of venlafaxine (control with endothelium 3.80±0.06, propranolol incubation 4.14±0.05, n=8; P<0.001). Propranolol did not change the dose-response curves of amitriptyline, fluoxetine or tranylcypromine.
In this study the principle mechanisms of smooth muscle contraction, namely the pharmaco- and the electromechanical coupling (13, 14), are represented by the different pre-contraction conditions caused by phenylephrine, PGF2
The present in vitro experiments demonstrated that various classes of antidepressant agents had prominent direct vasoactive and predominantly vasodilatation properties on isometric tension in the elastic arteries of the rat and interact with the pharmacomechanical and the electromechanical mechanisms of contraction in vascular smooth muscle cells, as well. In addition, incubation with low concentrations of the antidepressants inhibited contracting adrenergic responses which are related to the NO-cGMP-pathway.
Our findings regarding fluoxetine are partially in agreement with previous findings. Ungvari et al. (15) also found no endothelium dependence of fluoxetine relaxing effects in their study performed on cerebellar arterioles. Nevertheless, in the present study low concentrations of fluoxetine inhibit vasoconstriction responses to adrenergic stimuli in a partially endothelium manner. Furthermore, inhibition of NO or cGMP production resulted in a decrease of the preparations’ sensitivity towards fluoxetine. It can be concluded that the NO-cGMP pathway is an important vasorelaxing mechanism of fluoxetine in the rat aorta. However, Ungvari et al. (15) described a fluoxetine induced inhibition of BAY K 8644 activated voltage gated calcium channels in cerebral vessels. It has to be verified whether fluoxetine interacts with calcium channels in aortal smooth muscle since these have been detected in rat aortal smooth muscle (16).
In the present finding, KCl pre-contracted arteries could be rapidly dilated by cumulative concentrations of fluoxetine, indicating an interaction with the electromechanical coupling of this drug. The fact that inhibition of voltage dependent potassium channel reduces sensitivity of preparations to fluoxetine supports this finding.
It has been reported that venlafaxine is associated with induction or worsening of hypertension (8). It releases noradrenaline, serotonin and dopamine in the central nervous system (17). Furthermore, it has been shown that venlafaxine can potentate noradrenaline-evoked venoconstriction of the dorsal hand vein (18) and may lead to a modest increase in pulmonary arterial pressure in the isolated perfused rat lung (19). Therefore, our finding that venlafaxine tends to contract endothelium intact rat aorta needs further clarification. Release of endothelium derived contracting substances, i.e. endothelin-1, angiotensin II or prostaglandins, could be involved. The finding that propranolol incubation facilitates venlafaxine induced vasorelaxation may hint an interplay between venlafaxine action and ß-adrenoceptors. However, since propranolol has been reported to act as an inhibitor to other cellular proteins, like protein kinase C (20), other intracellular mechanisms could be influenced by venlafaxine. It has been shown that venlafaxine interacts with the ATP dependent calcium uptake into the endoplasmatic reticulum, at least in neurons (21).
The vasoactive effects of amitriptyline have been extensively studied as it represents one typical tricyclic antidepressant. Our findings that amitriptyline relaxes aortal ring preparations rapidly after adrenergic elicited tension are in line with these previous reports and represent its
However, in the present study low concentrations of amitriptyline reveal an additional inhibition of adrenergic effects which is connected to the integrity of the endothelium. The finding that pre-treatment with the eNOS inhibitor L-NAME delays the relaxing effects of amitriptyline sustains this result. Tuncok et al. reported that L-NAME treatment of rats ameliorated amitriptyline effect on blood pressure arguing for an involvement of nitric oxide production (24). Furthermore, amitriptyline is an antagonist at postsynaptic cholinergic receptors and its anticholinergic effects in human smooth muscle cells has been described (25). Kalkan and co-workers (26) have extensively studied amitriptyline and its effects on adenosine receptors in rat isolated aorta. An additional vasorelaxing component due to interaction with these receptors should be taken into consideration.
Vasoactive properties of tranylcypromine have not been studied in detail, so far. In our study, it shows vasorelaxing properties only to a smaller extent, as compared to the other substances tested. It augments adrenergic effects on arterial smooth muscle contraction, presumably due to its mechanism of action as inhibitor of the monoaminooxidase resulting in an increasing concentration of noradrenaline. Tranylcypromine seems to interfere with the prostaglandin metabolism as it further contracts prostaglandin F2
Recently, Bujak-Gizycka and co-workers (28) described the ability of rat aortic tissue to generate proangiotensinogen-12 as substrate for a renin-independent generation of angiotensinogen I and II. The latter play also a crucial role in local regulation of blood pressure homeostasis. Interference of the antidepressants with proangiotensinogen-12 and actions of the renin-angiotensinogen-system require further investigations.
We used relatively high concentration of L-NAME which, although often used (29-31), may have yet effects to other enzymes and signaling pathways. Therefore the interpretation of these results must be taken with some reserve.
This study supports the hypothesis that antidepressant induced side effects on blood pressure are at least in part by their direct effect on blood vessels, since amitriptyline, fluoxetine and tranylcypromine showed vasorelaxing properties and venlafaxine further contracted aortal rings. These differences could be relevant when deciding whether to discontinue antidepressants or not before anaesthesia (32).
This study was performed on rat aorta, which is a conducting elastic blood vessel and not a resistance vessel and therefore not responsible for the modulation of blood pressure. It has to be proofed whether the antidepressant agents act similarly in small arteries and arterioles and it would be of clinical relevance to examine the effects of these drugs in human resistance arteries. Although the antidepressants relax vascular smooth muscle independently of the integrity of endothelium, used in low concentrations they change the contraction responses to adrenergic agents in an endothelium dependent manner. The NO-cGMP pathway plays a crucial role in mediating these effects.
Acknowledgements: Silvia Ribback and Dragan Pavlovic contributed equally to this work. The authors express their thanks to Prof. Dr. med. Frank Dombrowski (Institut fur Pathologie, 24 Universitatsmedizin Greifswald) for his critical correction of the manuscript.
Conflict of interests: None declared.
- Pacher P, Ungvari Z, Kecskemeti V, Furst S. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem 1998; 5: 381-390.
- Edwards RP, Miller RD, Roizen MF, et al. Cardiac responses to imipramine and pancuronium during anesthesia with halothane or enflurane. Anesthesiology 1979; 50: 421-425.
- Glisson SN, Fajardo L, El-Etr AA. Amitriptyline therapy increases electrocardiographic changes during reversal of neuromuscular blockade. Anesth Analg 1978; 57: 77-83.
- Sprung J, Schoenwald PK, Levy P, Krajewski LP. Treating intraoperative hypotension in a patient on long-term tricyclic antidepressants: a case of aborted aortic surgery. Anesthesiology 1997; 86: 990-992.
- Huang Y. Inhibition of contractions by tricyclic antidepressants and xylamine in rat vas deferens. Eur J Pharmacol 1997; 327: 41-47.
- Huang Y, Lau CW. Inhibitory effect of amitriptyline on contraction of the rat isolated trachea. Pharmacology 1997; 54: 312-318.
- Velasco A, Arruza A, Maroto M, Carvajal A, Fernandez del Busto E, Garcia del Pozo J. Effect of venlafaxine hydrochloride in different preparations of isolated guinea-pig and rat organ tissues. J Auton Pharmacol 1999; 19: 109-113.
- Feighner JP. Cardiovascular safety in depressed patients: focus on venlafaxine. J Clin Psychiatry 1995; 56: 574-579.
- Ocharan ME, Asbun J, Valencia I, Castillo C, Castillo EF. A study of the interactions of tricyclic antidepressants with alpha 1-adrenoceptor subtypes in rat aorta and caudal arteries. Proc West Pharmacol Soc 1998; 41: 141-143.
- Pacher P, Ungvari Z, Kecskemeti V, Friedmann T, Furst S. Serotonin reuptake inhibitors fluoxetine and citalopram relax intestinal smooth muscle. Can J Physiol Pharmacol 2001; 79: 580-584.
- Pacher P, Ungvari Z, Kecskemeti V, Koller A. Serotonin reuptake inhibitor, fluoxetine, dilates isolated skeletal muscle arterioles. Possible role of altered Ca2+ sensitivity. Br J Pharmacol 1999; 127: 740-746.
- Vila JM, Medina P, Segarra G, et al. Relaxant effects of antidepressants on human isolated mesenteric arteries. Br J Clin Pharmacol 1999; 48: 223-229.
- Itoh T. Pharmacomechanical coupling in vascular smooth muscle cells-an overview. Jpn J Pharmacol 1991; 55: 1-9.
- Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther 1968; 159: 129-145.
- Ungvari Z, Pacher P, Kecskemeti V, Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca(2+) channel opener. Stroke 1999; 30: 1949-1954.
- Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res 1999; 84: 210-219.
- Czubak A, Nowakowska E, Golembiowska K, Kus K, Burda K, Metelska J. Effect of venlafaxine and nicotine on the level of neurotransmitters and their metabolites in rat brains. J Physiol Pharmacol 2010; 61: 339-346.
- Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of venlafaxine, desipramine, and paroxetine on noradrenaline- and methoxamine-evoked constriction of the dorsal hand vein. Br J Clin Pharmacol 1999; 48: 345-354.
- Reeve HL, Nelson DP, Archer SL, Weir EK. Effects of fluoxetine, phentermine, and venlafaxine on pulmonary arterial pressure and electrophysiology. Am J Physiol 1999; 276: L213-L219.
- Sozzani S, Agwu DE, McCall CE, et al. Propranolol, a phosphatidate phosphohydrolase inhibitor, also inhibits protein kinase C. J Biol Chem 1992; 267: 20481-20488.
- Couture L, Elie R, Lavoie PA. Effect of antidepressants on ATP-dependent calcium uptake by neuronal endoplasmic reticulum. Can J Physiol Pharmacol 2001; 79: 946-952.
- Huang Y. Inhibitory effect of noradrenaline uptake inhibitors on contractions of rat aortic smooth muscle. Br J Pharmacol 1996; 117: 533-539.
- Nojimoto FD, Mueller A, Hebeler-Barbosa F, et al. The tricyclic antidepressants amitriptyline, nortriptyline and imipramine are weak antagonists of human and rat alpha1b-adrenoceptors. Neuropharmacology 2010; 59: 49-57.
- Tuncok Y, Kalkan S, Murat N, et al. The effect of the nitric oxide synthesis inhibitor L-NAME on amitriptyline-induced hypotension in rats. J Toxicol Clin Toxicol 2002; 40: 121-127.
- Rehavi M, Weiss H, Nissenkorn I, Rubinstein R, Cohen S. A comparative study of the affinities of some tricyclic antidepressants for the muscariniccholinergic receptor in human and guinea-pig bladder, ileum and brain in relation to differential drug potency. Life Sci 1987; 40: 1819-1827.
- Kalkan S, Hocaoglu N, Akgun A, Gidener S, Tuncok Y. Effects of adenosine receptor antagonists on amitriptyline-induced vasodilation in rat isolated aorta. Clin Toxicol (Phila). 2007; 45: 600-604.
- Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci 2006; 256: 268-273.
- Bujak-Gizycka B, Olszanecki R, Suski M, Madek J, Stachowicz A, Korbut R. Angiotensinogen metabolism in rat aorta: robust formation of proangiotensin-12. J Physiol Pharmacol 2010; 61: 679-682.
- Senejoux F, Girard-Thernier C, Berthelot A, Bevalot F, Demougeot C. New insights into the mechanisms of the vasorelaxant effects of apocynin in rat thoracic aorta. Fundam Clin Pharmacol 2012; doi: 10.1111/j.1472-8206.2011.01025.x. (epub ahead of print)
- Kajita M, Murata T, Horiguchi K, Iizuka M, Hori M, Ozaki H. iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats. Am J Physiol Heart Circ Physiol 2011; 300: H1021-H1031.
- Skogvall S, Berglund M, Dalence-Guzman MF, et al. Effects of capsazepine on human small airway responsiveness unravel a novel class of bronchorelaxants. Pulm Pharmacol Ther 2007; 20: 273-280.
- Kudoh A, Katagai H, Takazawa T. Antidepressant treatment for chronic depressed patients should not be discontinued prior to anesthesia. Can J Anaesth 2002; 49: 132-136.