DISRUPTED BRAIN THYROID HORMONE HOMEOSTASIS AND ALTERED THYROID HORMONE-DEPENDENT BRAIN GENE EXPRESSION IN AUTISM SPECTRUM DISORDERS
INTRODUCTION
Accumulating evidence suggests that autism spectrum disorders (ASD) result from interplay between environmental and genetic components. Many of the environmental toxicants such as phthalates, alkylphenolic compounds, polychlorinated biphenyls (PCBs), dibenzodioxins, organochlorine pesticides, bisphenol A, and metals, lead, cadmium and mercury compounds are potent disruptors of endocrine system including thyroid hormone (TH) (1). Some of the same compounds have been implicated in autistic pathology (2). As TH is essential for cellular metabolism, growth and differentiation, and thus critical for brain development, TH deficiency during embryonic or early postnatal periods would likely lead to developmental abnormalities, including autistic pathology. Yet, several clinical studies to date have shown no evidence of thyroid dysfunction or TH blood levels deficiency in autism (3-5). Could the environmental toxicants impact brain TH metabolism directly, independently of the systemic TH levels?
Our recent animal study of perinatal mercury exposure in rats supports the possibility that the environmental toxicants can affect brain deiodinases and thus affect brain TH status even in absence of systemic hormonal deregulation (2). In the brain, approximately 70-80% of T3, the biologically active form of TH, originates from the local intracerebral thyroxine (T4) to 3',3,5-triiodothyronine (T3, TH) (2) deiodination (6, 7) catalyzed by the type 2 iodothyronine deiodinase (D2) (8); local T3 levels are also regulated by the type 3 deiodinase (D3), which catalyzes the deiodination of T4 and T3 into inactive metabolites, reverse 3',3,5-triiodothyronine (rT3) and 3,3'-deiodothyronine (3,3'-T2) (6-9). Thus, local brain T3 can be directly regulated at the deiodinase levels. Perinatal exposure of Sprague-Dawley (SD) and the spontaneously hypertensive rats (SHR) rats to organic mercury (thimerosal, TM) resulted in a significant increase in cerebellar levels of the oxidative stress marker 3-nitrotyrosine (3-NT), a decrease in the activity of cerebellar D2, and altered expression of TH-responsive genes (10). Among the genes altered in the mercury-exposed rat brain tissue were notably genes negatively regulated by TH, such as suppressor-of-white-apricot-1 (SWAP-1) (11) which was increased in mercury-exposed pups (10), while positively regulated T3-target genes, such as Purkinje cell protein 2 (Pcp2) and Forkhead box protein P4 (FoxP4), showed a trend towards decreased expression (10). Furthermore, gene expression showed sex-dependent differences between the toxic impacts of TM in males and females. Importantly, activation of brain genes negatively regulated by TH in mercury-exposed rat pups supports our hypothesis of local brain hypothyroidism being induced by environmental toxins.
The present study was designed to examine human postmortem brain tissue for evidence of local brain TH deficiency in ASD, by measuring activity of brain tissue deiodinases D2 and D3, and tissue levels of T3. The feasibility of assessing TH status in the postmortem human brain was based on our animal studies showing decreased D2 activity in TM-treated rat neonates (2), and a single report of deiodinase being detected in human postmortem hypothalamus and pituitary (12), although deiodinase D2 and D3 enzymatic activities have not been previously measured in the human autistic brain. However, recently, type 3 iodothyronine deiodinase (DIO3) gene copy number variants (CNVs) have been identified in high-risk ASD families, suggesting structural variation in D3 (13). And, in the rat model of fetal alcohol spectrum disorder (FASD), increase in hippocampal DIO3 gene was associated with social and behavioral deficits reminiscent of autism (14).
Furthermore, based on the observation that the decrease in cerebellar D2 activity in TM-treated rat neonates was associated with a selective up-regulation of negatively-regulated T3-responsive genes (10), in the present study we compared TH-regulated gene expression in control and ASD brain samples. This comparison included expression analysis of several TH dependent genes negatively regulated by TH such as transthyretin family (TTRa, TTRb, and TTRc), DIO2, SWAP and the cold-inducible RNA binding protein (Cirbp) gene and genes positively regulated by TH and relevant to brain development such as DIO3 and brain derived neurotrophic factor (BDNF). The expression of these genes was measured in several brain regions derived from male and female control and ASD cases.
Additionally, the study aimed to confirm increased 3-NT and brain mercury levels in ASD reported by us previously (13). This confirmation deemed important due to the animal studies of TM exposure suggesting relationship between mercury exposure and altered brain TH status (2). The levels of 3NT were measured in several brain regions of control and ASD cases, while the mercury analysis requiring much larger sample size was confined to the brain stem and the cerebellum.
MATERIALS AND METHODS
Subjects
Animals; postmortem analysis in rat brains
Using a rat model of human postmortem process (15), we compared brain levels of 3-NT, D2, D3 and T3 in tissue derived from rats exposed to increasing postmortem interval (PMI) at room temperature (RT). Sprague-Dawley (SD) rat dams with pups, purchased from Charles River Breeding Laboratories (Germantown, NY), were used for the analysis; the experimentation protocols were approved by the Institutional Animal and Use Committee at Harvard Medical School. Weanling SD rats were euthanized by CO2 asphyxiation. For the purpose of establishing the postmortem effect, the bodies of euthanized pups were kept at RT for 1 h, 2 h, 4 h, 6 h, 8 h, and 10 h, before the heads were separated and the cerebellum, brain stem, and other cortical and subcortical tissues were dissected out, frozen on dry ice, and stored at -80°C for further analysis; for the 0 time, the dissection followed immediately the euthanasia.
Human postmortem brain samples
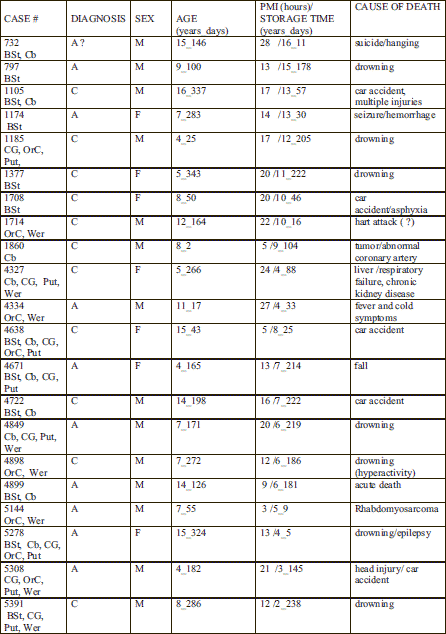
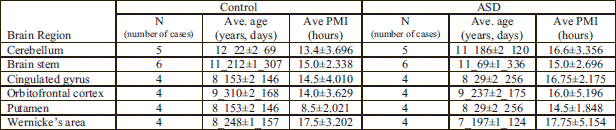
Human postmortem frozen regional brain samples were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland; donor and the donated brain tissue profiles are presented in Table 1. A total of 11 control and 10 ASD cases were examined. For cerebellar (CB) analysis a total of 5 control and 5 ASD cases were used, for the brain stem (BST) analysis, a total of 6 control and 6 ASD cases were used as indicated in Table 1 column 1. For the cingulated gyrus (CG), the orbitofrontal cortex (ORC), the putamen (PT), and the Wernicke's (Wer) analysis, a total of 4 control and 4 ASD cases were used as shown in Table 1 column 1. An average age of control and ASD cases and the PMI of the regional brain samples are shown in Table 2. Controls ranged between 5–16 years of age, while ASD ranged from 4–15 years of age; the PMI of controls ranged between 5 and 24 hours and ASD between 3 and 28 hours. There were no significant differences in PMI or age between control and ASD donors.
Procedures
Analysis of cerebellar 3-NT levels
The 3-NT levels were measured in the animal or human brain homogenates prepared from frozen tissue according to the procedure previously described (16). Briefly, the brain specimens were homogenized in a phosphate buffer containing detergents and protease inhibitors. The supernatants were collected by centrifugation at 16,000×g for 30 min at 4°C. 3-NT in the supernatants was measured using aliquots derived from ~25 mg tissue and a commercially available 3-NT enzyme-linked immunosorbent assay (ELISA) kit (Percipio Biosciences, Inc.; Foster City, CA). The ELISA plates were read at 450 nM. Data on 3-NT levels are expressed in pmol per gram of tissue (pmol g-1).
Analysis of brain D2 activity
D2 activity in rat or human brain tissue was measured by quantifying 125I-release from a 125I labeled T4 tracer (5,700 mCi/mg; Perkin Elmer Life Sciences, Boston, MA) as described previously (17). For the assays, 50 µg of protein were incubated for 4 hours. at 37°C with 1 nM T4, 1 mM propylthiouracil (PTU), and 20 mM dithiothreitol (DTT). Background levels of deiodination were determined under identical conditions using 100 nM unlabeled T4.
Analysis of brain D3 activity
D3 activity in rat or human brain tissue was measured by a modification of previously published procedure (17). For the assays, 20–50 µg of cerebellar homogenate was assayed with a final concentration of 10 mm DTT, 1 nM T3, 100 cpm 125I-T3 tracer, and 1 mM propylthiouracil for 16 hours. at 37°C. Background levels of non-specific deiodination were determined by assaying samples under similar conditions with 100 nM T3.
Analysis of brain tissue T3
Total T3 concentration in rat or human brain tissue was determined by radioimmunoassay as described previously (18, 19). Tissue samples (12-15 mg) were homogenized in 500 µL of ice cold 100% ethanol with 100 µM PTU. The homogenates were spiked with 1000 cpm 125I-T3 to calculate the % recovery. The homogenates were further incubated at 4°C for 10 min and centrifuged at 12,000 RPM for 15 min at 4°C and the supernatant was dried overnight in the rotovac dryer. The residue was re-suspended in 50 µL/1mg tissue glycine acetate buffer (GAB: 0.2 M glycine acetate, 0.13M acetate with pH: 8.6, 0.02M BSA) and checked for % recovery. The RIA was performed as described (18), and the T3 antibody was a generous gift from Dr. P. Reed Larsen (Stock D 2/4).
Analysis of human brainstem and cerebellar Hg levels
All cerebellar and brain stem samples shown in Table 1 were analyzed for mercury content. Prior to the analysis, tissue was powdered in liquid nitrogen and then used for all the additional analysis i.e. 3-NT, D2, D3, and T3. Approximately 0.5 grams of powdered brain tissue was used for mercury analysis by mass spectrometry as described previously (16).
Analysis of TH-dependent gene expression
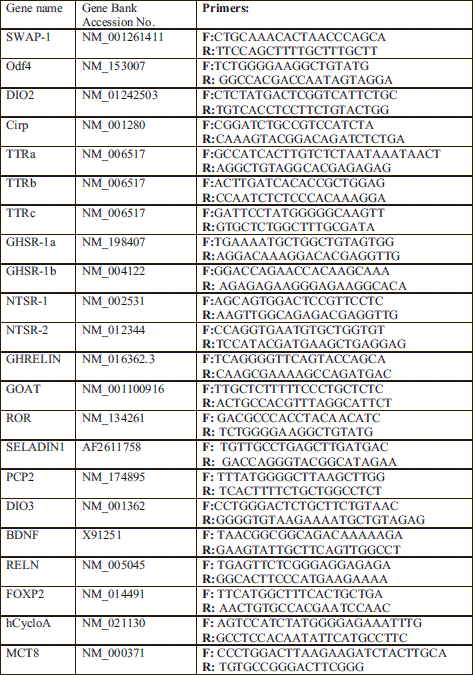
Brain mRNA was isolated using Trizol (InVitrogen, Carlsbad CA) following the manufacturer's instructions. Quantitative real-time PCR (qRT PCR) was used to measure gene expression levels and was performed as described previously (10). Using qRT PCR we have analyzed the expression of the following TH-dependent genes: SWAP-1 (11), outer dense fiber of sperm tails 4 (Odf4) (11), type 2 iodothyronine deiodinase (DIO2), Cirbp, TTR gene family TTRa, TTRb, and TTRc, growth hormone secretagogue receptor type 1 (GHSR-1a) and type 1b (GHSR-1b) (20), neurotensin receptor 1a (NTSR-1a) and 1b (NTSR-1b) (20), ghrelin and the ghrelin-O-acyltransferase (GOAT) enzyme (20), orphan nuclear hormone receptor (ROR), and seladin. We also analyzed genes critically involved in cerebellar development: PCP2 (21), DIO3, BDNF, reelin RELN, and FoxP2. Human cyclophilin A (CycloA) gene was used as a housekeeper gene for normalization. The primers for each of the genes are presented in Table 3.
Statistical analysis
The rat PMI data presented here is derived from two to three male and two to three female pups per time point. A two-way ANOVA was used to determine the sex-specific PMI effect on T3 levels; the effect was sex specific for both the cortical sample F(4,20)=0.1074, p<0.05 (p=0.0462) and for the brainstem F(4,19)=0.1029, p=0.1761. In case of the cortical samples, the statistical significance of sex-specific PMI effect was further confirmed by the Bonferroni test. Human data were analyzed by a one-way ANOVA. All values are reported as a mean ± the standard error of the mean (S.E.M). For all statistical tests, p<0.05 level of confidence was considered significant; 0.05 Remarkably, as previously reported in rat model (15), a PMI up to 10 hours at RT in male pups (Fig. 1A) or up 6 hours in female pups (Fig. 1B) does not significantly affect cerebellar 3-NT levels; the increase in 3-NT in males (Fig. 1A), between 2 and 6 hours was not significant. We have previously observed an increase in 3-NT levels between 4 and 8 hours at RT, with no further changes up to 24 hours (15). Other brain regions appeared to be more sensitive to the PMI length and storage temperature longer than 4 hours (data not shown). Considering the relative mass of animals, that determines the rate of body cooling, the PMI changes in 3-NT are likely to be relevant only under extremely long PMIs (>48 hours) in humans. Simultaneous analysis of rat and human samples showed comparable tissue levels of this oxidative stress marker allowing for the analysis of 3-NT in the human postmortem brain samples. Results of 3-NT analysis in postmortem regional brain samples derived from the control and ASD cases are presented in Fig. 2A (males) and Fig. 2B (females). In the control cases the lowest level of 3-NT was observed in male orbitofrontal cortex, and the highest in the female orbitofrontal cortex; in the ASD cases the lowest level was observed in cingulated gyrus of both males and females and the highest in the male putamen. The levels of 3-NT were significantly (p<0.05) increased in the orbitofrontal cortex (559%) and trended (p<0.1) towards increase in the cingulated gyrus (154%), the putamen (140%) and the Wernicke's area (277%) in the male ASD samples. There was also a trend (p<0.1) towards increase in 3-NT in the brainstem (71%) of ASD female cases. The stability of D2 activity during the postmortem interval was analyzed in rat cerebellum separately in males (Fig. 1C) and females (Fig. 1D). Results presented in Fig. 1 represent the average of two separate experiments in males and one in females. D2 activity appears to decrease with PMI (Fig. 1C and 1D). However, a statistical analysis showed a significant decrease in D2 activity only in males at 4 hours as compared to time 0. Simultaneous analysis of human brain samples showed no measurable D2 activity in human cerebellar and brain stem samples derived from cases with postmortem time ranging between 5–28 hours precluding further D2 analysis in the human postmortem brain samples. Our results suggest that future studies of D2 in human brain tissue can only be reliably performed using samples with a very short PMI. Analysis of rat brain tissue showed measurable D3 activity in rat cerebellum from males (Fig. 1E) and the females (Fig. 1F) that appeared to be fairly stable. However, when human postmortem brain tissue was assayed simultaneously we could not detect any D3 activity. At this point it is unclear why D3 was not detected in the human postmortem tissue, but it appears factors other than postmortem deactivation such a freezer storage time may come into play. Further analysis of D3 activity in the human postmortem brain tissue was thus not feasible. The PMI effect on the brain tissue T3-content was analyzed in rat cortical (Fig. 1G, - males; Fig. 1H, - females), brain stem (Fig. 1I, - males; Fig. 1J, - females), and cerebellar (Fig. 1K - male) samples. The results presented in Fig. 1 indicate that brain T3-levels and stability are dependent on the sex and on the brain region. T3 appears to be more stable during PMI in tissue derived from males (Fig. 1G and Fig. 1I) than from females (Fig. 1H and i) and more stable during PMI in the cortical regions (Fig. 1G and Fig. 1H) than in the brainstem (Fig. 1I - males; Fig. 1J - females) and cerebellum (Fig. 1K). In male cortex no significant decrease in T3 could be observed during PMI of 8 hour, while in female cortex a significant decrease of ~30% could be observed by 1 hour with an additional decrease at 4 hours. In male brainstem a significant decrease in T3 of ~50% was detected at 2 hours while in the female brainstem a significant decrease could be detected at 1 hour. T3 was also analyzed in male cerebellum where a significant decrease in T3 ~40% could be observed at 1 hour. Results of T3 analysis in postmortem regional brain samples from the control and ASD cases are presented in Fig. 3. Due to a shortage of regional brain samples, this analysis was performed on a limited number of male samples only. The level of T3 was significantly (p<0.05) decreased (~80%) in the orbitofrontal cortex in ASD; it was also decreased in the putamen (~60%0 and the Wernicke's area (~75%) of ASD brains, but the decrease was not statistically significant. However, no change in T3 level was observed in the cerebellum. Total Hg levels were determined in human postmortem cerebellar and brain stem samples derived from both male and female ASD cases. The results of this analysis, presented in Fig. 4 as the male and female combined data, indicate no significant difference in Hg levels between control and ASD cases in either the brainstem or the cerebellar samples. The present study compared gene expression in several brain regions derived from male and the female control and ASD cases. Data of this comparison are presented in Fig. 5; these data are expressed as change in the cycle threshold between the house-keeping gene, cyclophilin A, and the gene of interest (delta CT), so higher values correspond to a lower gene expression with an increase in the delta corresponding to a decrease in gene expression. The most significant changes were observed in TTR family of genes negatively regulated by TH (23). The expression of all three members of the family TTRa (p<0.05), TTRb (p<0.05) and TTRc (p<0.05) was decreased in the putamen of male ASD cases, while TTRa was increased (p<0.05) in the orbitofrontal cortex of male ASD cases, and TTRb was also increased (p<0.0001) in the putamen of the female ASD cases. Expression of additional negatively regulated gene, DIO2, was increased in the putamen (p<0.05) and there was a trend towards increase in cingulated gyrus (p=0.08) of the female ASD cases, expression of the Cirbp gene that is also negatively regulated by T3 (21) was decreased (p<0.05) in the putamen of ASD male cases. The expression of SWAP-1 gene, also under negative TH regulation (24) and critical during development (11), was increased (p<0.05) in the cerebellum of ASD male cases. The expression of yet another negatively regulated gene, GSTa (25), was increased in cingulated gyrus of female ASD cases and showed a trend towards a decrease in the cerebellum (p=0.080) in ASD males, but increase in the cerebellum (p=0.09) female ASD cases. Comparison of genes positively regulated by T3 and relevant to brain development is also presented in Fig. 5. Interestingly, expression of DIO3 was increased (p<0.05) in the putamen and showed a trend towards an increase in the brainstem of ASD female cases. The expression of BDNF was decreased in the putamen (p<0.05) of male ASD cases and showed a trend towards an increase in the cerebellum (p=0.07) of ASD female cases. Additionally, the expression of RELN was decreased (p<0.05) in the putamen of ASD male cases, TH regulated seladin (26) showed a trend towards a decrease (p=0.06) in the brainstem of the ASD males, and TH- regulated NSTR1 gene showed a trend towards an increase (p=0.08) in the brainstem of ASD females. Due to the shortage of brain material, the MCT8 expression was measured in the putamen derived from male cases only. The data showed a trend (p<0.1) towards decreased MCT8 expression in the putamen of ASD male cases (Fig. 5). ASD are grouped as pervasive developmental neuropsychiatric disorders, whose etiology has been eluding researches since they have been first described 64 years ago (27). Although their causation is assumed to have a strong genetic component, most of the known genetic risks have been associated with copy number variants (CNVs); genome-wide scan has failed to discover "the critical autism Loci" (28), and ASD concordance for monozygotic twins is less than 90 percent (29). Thus nongenetic, environmental triggers of ASD pathology are gaining recognition as likely causal factors. Many of the environmental toxicants are also potent disruptors of endocrine system including TH, a key hormone involved in the regulation of brain development (30). TH deficiency during CNS development results in a spectrum of neuropsychiatric disorders including both neurological and cognitive deficiencies (31). Yet analyses of TH plasma levels failed to show major abnormalities in autism; thus TH involvement in autistic pathology has been ruled out. What has been overlooked in dismissing a TH-autism relationship is the fact that majority of active TH hormone in the brain (T3) does not come from circulation, but is converted locally by brain deiodinases. Animal data on the effect of perinatal mercury exposure in rat neonates suggest that the environmental toxicants can indeed trigger oxidative stress, affect brain deiodinases and thus disrupt brain homeostasis of TH even in absence of systemic hormonal deregulation; such exposure results in a significant decrease in the activity of cerebellar type 2 deiodinase (D2), responsible for local intra-brain conversion of thyroxine to the active hormone, 3',3,5-triiodothyronine (T3, TH; 2) and altered expression of TH-responsive genes (10). In the present study we examined human postmortem brain tissue for changes consistent with altered TH homeostasis in autistic brain (32), as well as with the oxidative-stress-mediated model of autistic pathology (16). Consistent with this model, we have previously reported an increase in the oxidative stress marker, 3NT, in ASD (16). Analysis of human postmortem brain samples presented here confirmed our previous data suggesting increased oxidative stress in the brains of ASD donors. Interestingly, we have observed regional brain differences in oxidative stress. We have previously observed similar differences on the examination of a control and ASD brains, with the Wernicke's area being most affected (33). In the present study the brain region with the highest level of oxidative stress was putamen. The results reflect a trend and would require a greater number of cases to reach a statistical significance. Interestingly, the putamen has been recently shown to exhibit growth abnormalities in autism (34). It can be hypothesized that the abnormalities in the putamen, a brain region that is involved in the regulatory role in movement, and reinforcement and implicit learning, may have important implications in autistic pathology. However, data on oxidative stress in autism should be viewed with caution as both seizure and epilepsy are frequent comorbidities and may be associated with increase in oxidative stress (35). Interestingly, animal data suggest that chronic exposure to low dose of mercury results in activation of renin-angiotensin system that could contribute to the endothelial dysfunctions (36) in both epilepsy and autism (37). Additionally, the agonal states, especially of aspyxial form (hanging or drowning) are associated with oxidative stress; although in the present study, there were equal number of asphyxial deaths in the control and ASD groups and consequently we can assume that the contribution of agonal state is similar in the two groups. To address the brain TH status we assayed D2 enzyme activity. To date no studies of the deiodinase enzymatic activity in the autistic brain have been reported although D2 activity has been measured in human postmortem samples in one study in the hypothalamus and pituitary (12). Our initial simultaneous analysis of D2 in rat and human tissue showed measurable D2 activity in the frozen rat samples yet no activity in the human postmortem brain samples. Subsequent analysis of rat brain samples for PMI stability showed that D2 activity is a subject to a rapid PMI deactivation. Furthermore, the PMI inactivation curve appears to be different for males and in females, with greater loss of activity in females. Thus, the lack of the detectable D2 activity in our human postmortem samples could be in part due to rapid deiodinase inactivation in these regions of the brain during a lengthy PMI, or a loss of activity during years of frozen storage. It has been reported (38) that deiodinase activity measured in rat tissue homogenates begins to decrease after six months of storage at -57°C. It can be thus speculated that deiodinase activity also decreases with time in human brain samples with low activity that have been stored for many years in brain banks". PMI inactivation has also been observed in the case of GSH metabolic enzymes with inactivation being different in distinct brain regions (39). D3 activity was also previously measured in the hypothalamus and pituitary in human postmortem samples in one study (12). However, simultaneous analysis of rat and human brain tissue in our study showed measurable activity in the rat brain but no D3 activity in the human postmortem brain tissue. Subsequent analysis of rat brain tissue for PMI inactivation showed relative stability of D3. Thus, the lack of detectable D3 activity in the postmortem human brain tissue may be due to other yet unidentified factors such as prolonged storage time. Problems encountered with measuring the enzyme activity in postmortem tissue are frequent although not universal. Interestingly, we have previously addressed the stability of the oxidative stress marker, 3-NT, in the rat brain. In the present study we have reexamined the stability of 3-NT in the rat cerebellar samples derived from the same male and female weanling rats used for D2 and D3 PMI studies. The results presented here confirm our previous conclusion that 3-NT is relatively stable during PMI. Due to the lack of detectable activity of both D2 and D3 in the human postmortem brain tissue we turned our attention to the brain tissue T3 levels. Interestingly, in Alzheimer's disease (AD) where there also is no evidence of thyroid dysfunction as assessed by serum TH levels (40) yet there is an evidence for a localized hypothyroidism in the brain. Direct measures of T3 and T4 in the postmortem AD brains indicate no changes in T4, but significantly lower T3 levels in advanced stages of the disease (41). Simultaneous analysis of rat and human brain T3 tissue levels in the present study showed comparable T3 levels, and the subsequent analysis of the PMI effect using rat brain tissue showed relative stability of brain T3 levels in the cortical region but a significant effect of PMI in the brainstem and the cerebellum, and greater PMI effects observed in females than in males. We have attempted to eliminate the potential impact of the PMI on brain T3 levels and concentrated on T3 content in the cortical samples; due to the shortage of brain samples our analysis was limited to male cases only. Consequently, the decrease in T3 levels in ASD male cases suggests that the relative deficiency of T3 may be indeed part of autistic pathology. The variation in T3 levels between brain regions and differences between males and females further suggest that much greater number of brain samples is required to reach a statistical significance and confirm this hypothesis. Based on the data derived from our animal studies, we proposed that the perinatal TM exposure results in the decrease in the local brain D2 levels and local hypothyroid conditions suggesting a possible link between mercury exposure, lower local T3 levels and developmental abnormalities in autism. And indeed we previously reported an increase in cerebellar Hg levels in ASD (16). However, in the present study we observed no differences between Hg levels in the brain stem and cerebellar samples derived from control and ASD donors. One of the factors that may be at play is the age distribution of cases in the two studies with the oldest ASD case being 16 years old in the present study versus 35 years old ASD case in the initial study (16). While the mechanism responsible for the decrease in brain T3 levels in ASD is unclear, the relationship between T3 and Hg should not be that easily dismissed. Our animal studies (2) indicated strain-dependent effect of exposure to organic mercury compounds on D2 activity, supporting the hypothesis that certain individuals are more sensitive to the environmental pollutants. Perinatal TM exposure significantly decreased D2 activity in the cerebellum of SHR rat male neonates and showed a tendency to decreased activity in male and female SD neonates (2). Independently, the effect of postnatal TM exposure was evident in autoimmune disease-sensitive SJ/LJ mice, but not in autoimmunity resistant C57BL/6J mice (42). It is possible that D2, a selenoezyme, is more sensitive to Hg in the individuals with ASD and consequently T3 levels could be decreased even though there are no differences in Hg levels. Future, toxicity comparison studies in the pediatric population could establish the differences in sensitivity to environmental toxins between control and ASD population as suggested by the increased oxidative stress in ASD. Oxidative stress has been found to decrease D2 activity in several cellular models both through transcriptional and post-transcriptional mechanisms (43, 44). Thus, changes in oxidative stress levels reported here could also modulate D2 activity. It is of interest that TH regulates GSH levels in the developing brain and treatment of astrocyte cultures with TH results in increased GSH levels and improved antioxidant defense, suggesting that TH plays a positive role in maintaining GSH homeostasis and protecting the brain from oxidative stress (45). Thus lower T3 levels in ASD brain may exacerbate the oxidative stress. To examine the possibility that a decrease in T3 levels in ASD brain is associated with downstream changes in the pattern of gene expression we compared the expression of representative genes regulated by T3. T3 regulates key metabolic genes through the group of transcriptional cofactors referred to as corepressors (CoRs) involved in silencing of genes that contain positive T3 response elements, while negatively regulated genes are stimulated by unliganded thyroid hormone receptor (TR) and repressed by T3 (46). Our comparison between controls and ASD involved both negatively and positively regulated by T3. We based our approach on the observation that in mice with a global targeted disruption of the Dio2 gene (D2KO mice) the decrease in T3 content in cerebral cortex, cerebellum, and hypothalamus (19) is associated with preferential up regulation of expression of genes negatively regulated by T3 (24). We therefore assumed that a decrease in T3 levels in ASD brains would result in an activation of genes negatively regulated by TH. However, we found limited changes in the expression of these genes. We did find altered expression of TTR- gene family. TTR genes are negatively regulated by TH and up-regulated in PTU- treated mouse cortex (23). TTR protein is a T4 carrier protein both in plasma and the cerebrospinal fluid; however TTR KO mice do not have decreased T3 content in their brains (47). TTR protein also has neuroprotective properties (48). The expression of all three members of the family TTRa, TTRb, and TTRc was significantly decreased in the putamen in the male ASD cases, while TTRa was, increased in the orbitofrontal cortex of male ASD cases, and TTRb was also increased in the putamen of the female ASD cases. Expression of several other negatively regulated TH-dependent genes was also altered but the changes were both brain region- and sex-dependent. Cirbp was decreased in the putamen of ASD male cases, while SWAP-1 developmentally expressed gene negatively regulated by TH was increased in the cerebellum of ASD male cases. The expression of yet another negatively regulated gene, GSTa (25), was increased in the cingulated gyrus of ASD female cases and DIO2 was also increased in the putamen of ASD female cases. We have also examined the expression of several genes positively regulated by TH and of special relevance to cerebellar development. Interestingly, DIO3 coding for T3 inactivating enzyme and positively regulated by T3 (49) was significantly increased in the putamen of ASD female cases. BDNF was decreased in the putamen (p<0.05) of the male ASD cases. BDNF expression is decreased in the cerebellum of PTU-treated hyperthyroid mice during postnatal development (50) and increased by TH in several regions of the developing brain (51). Interestingly, increased BDNF expression is positively correlated with mood and cognitive functions (52) disrupted in autism (53). RELN, involved in regulation of neuronal migration, was decreased (p<0.05) in the putamen of the ASD males. RELN is positively regulated by TH and down-regulated by methiamazole-induced hypothyroidism (54). It has been implicated in autistic pathology (55). While some of the patterns of gene expression are in concordance with the expected changes in thyroid hormone levels, others are not indicating that region-specific sensitivity may exist, and further, that other factors are also regulating expression of these genes. Since many of the changes of gene expression were found in the putamen, we also analyzed monocarboxylate transporter 8 (MCT8) gene expression in putamen tissue derived from male cases only. MCT8 is involved in TH transport across plasma membranes regulating TH access to the brain (56). MCT8 deficiency is an X-linked disorder (57) resulting in fragile X syndrome and mental retardation (58); an overlap between fragile X and autism has been observed (59). Interestingly, MCT8 gene expression showed a trend towards a decrease in the putamen of male ASD cases. It may be speculated that lower MCT8 gene expression may result in deficiency of MCT8 protein and contribute to the lower T3 levels observed in ASD cortical tissue and ASD pathology. The results presented here suggest that putamen is the brain region that exhibits not only an increase in oxidative stress and a decrease in T3 levels, but also most prominent changes in gene expression in ASD. Interestingly, the putamen's main function is to regulate movements and influence reinforcement and implicit learning, processes that rely on interaction with the environment; abnormal sensory reactions are part of autistic pathology (60). Thus, present study further implicates this brain region in autistic pathology. While the overall changes in TH-dependent gene expression in ASD brain are not striking, importantly these genes are related to the developmental processes or have been implicated in autistic pathology. Furthermore, TH-dependent gene expression is specific of developmental processes and differs between males and females. Gene expression in the human brain of 6 year old boy may be very different than that found in 12 year old girl. Under these circumstances, changes in gene expression observed in ASD brain are significant; rather than dismiss present findings one should consider their implication as guidelines for future studies. Decreased brain TH levels and changes in gene expression in ASD brains, suggested by the present study, are likely to impact the developing brain and have clinical implications (61). It has been previously observed that deficiency of T3 during early postnatal periods impacts basic stages of development i.e. neurogenesis (62), cell migration of (63), and synaptogenesis (64) that could contribute to downstream functional and structural damages observed in ASD brains. At this point, because the instability of D2 in the postmortem tissue and lack of detectable D3 activity we can only speculate on the molecular mechanisms involved in decreased TH in ASD brains. However, present data suggest that the role of TH in ASD pathology should not be dismissed prematurely and certainly requires further study, especially since correction of TH deficiency may offer new therapies. The present study, examined human postmortem brains for changes consistent with the hypothesis of local brain TH deficiency in ASD. Our results showed, for the first time, brain region-specific decrease in TH levels in the cortical regions of ASD male cases. Data reported here, although derived from a limited sample size, suggest the possibility of brain region-specific disruption of TH homeostasis in autistic brain. Furthermore, brain region-specific changes in TH-dependent gene expression reported here suggest disruption of gene expression that could possibly impact the developing brain and contribute to the autistic pathology. While the postmortem instability of brain deiodinases precluded further molecular studies, the role of TH in ASD pathology and TH-based new therapies warrant future studies. Acknowledgments: This research was supported by a grant from SafeMinds to Dr. Sajdel-Sulkowska and NIDDK-DK76117 grant to Dr. Zavacki. Human tissue was obtained from NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. Authors thank Dr. Alessandro Marsili for help with brain T3 analysis. Conflict of interests: None declared.RESULTS
Postmortem interval effect on 3-NT levels in rat brain tissue; 3-NT levels in the postmortem human brain samples
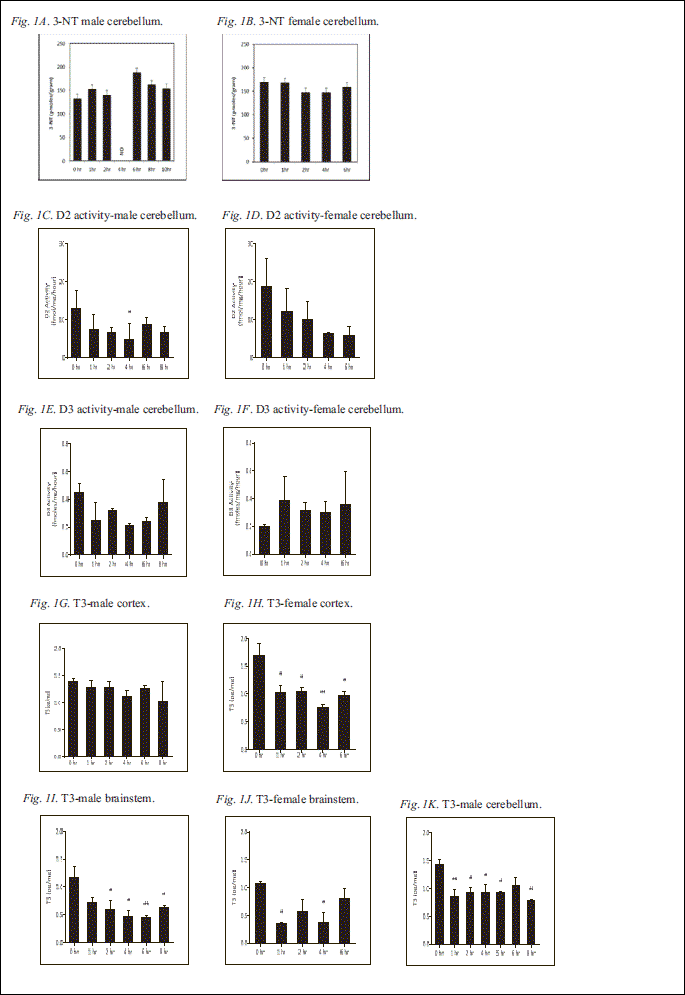
Postmortem interval effect on D2 activity in rat brain tissue and lack of measurable D2 activity postmortem human brain samples
Postmortem interval effect on D3 activity in rat brain tissue and lack of measurable D3 in human brain samples
Postmortem interval effect on T3 levels in rat brain tissue; T3 levels in the postmortem human brain samples
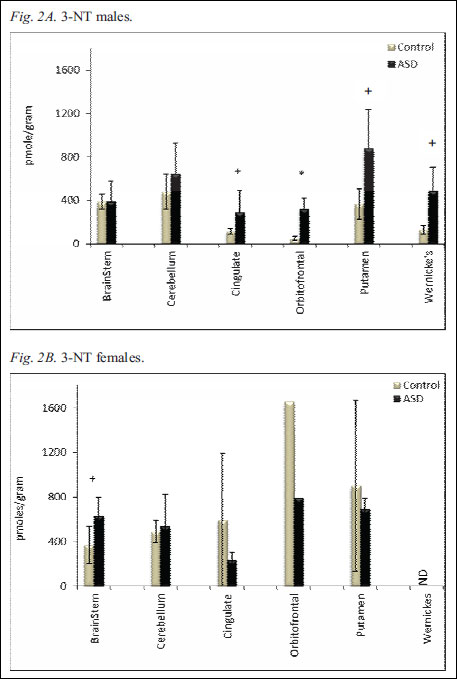
Fig. 2. Regional 3-NT levels in human postmortem brains of control and autistic male (Fig. 2A) and female (Fig. 2B) cases. The levels of brain 3-NT were measured with ELISA and recorded as absorbance at 450 nm. Data are converted to picomole per gram of tissue (pmol g-1) and presented as mean ± S.E.M. The * indicates statistical significance (p<0.05), and + indicates a trend (p<0.1).
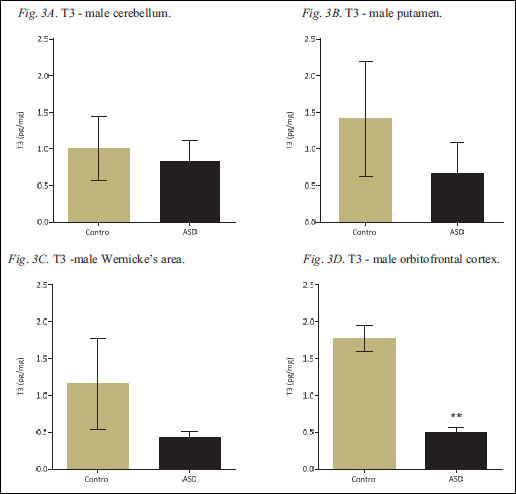
Fig. 3. Regional T3 levels in human postmortem brain samples derived from control and ASD cases. T3-content was measured in the male cerebellum (Fig. 3A), putamen (Fig. 3B), Wernicke's area (Fig. 3C) and orbitofrontal cortex (Fig. 3D), by RIA and expressed as picograms per miligram of tissue (pg mg-1); all values are presented as mean ± S.E.M. The ** indicates statistical significance (p<0.01).
Hg levels in the human postmortem brain tissue
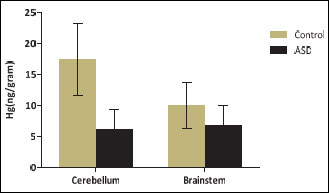
Fig. 4. Hg levels in human postmortem brainstem and cerebellar tissue of control and ASD cases. Brain Hg was measured by mass spectrometry. Data are converted to nanogram per gram of tissue (ng g–1) and presented as mean ± S.E.M.
Gene expression analysis
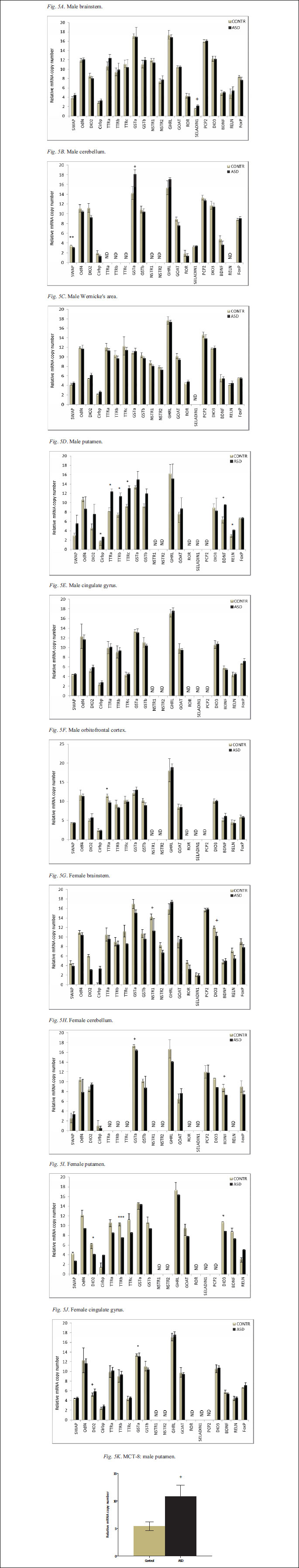
DISCUSSION
Conclusions
REFERENCES
A c c e p t e d : March 5, 2014