DIFFERENTIAL EXPRESSION OF GENES INVOLVED IN THE CALCIUM HOMEOSTASIS IN MASTICATORY MUSCLES OF MDX MICE
INTRODUCTION
A hereditary severe muscles disease, Duchenne muscular dystrophy (DMD), and its animal model, the mdx mice (X-linked muscular dystrophy), lead to progressive fibrosis and weakness of muscular tissue. This chronic ailment is caused by mutation in the gene dystrophin, a cytoskeletal protein which contributes to the stability of muscle cell membranes and is essential for the long-term function of skeletal muscle (1). The DMD mainly results in respiratory failure and in dilated cardiac myopathy (2). However, the outcome of DMD is also reflected in orofacial system where affected masticatory muscles often have effects on the occlusion (3). Dysfunction of the masticatory muscles usually leads to various diseases of the stomatognathic system, such as pain in the ear region, impairment of the course of abduction and adduction of the mandible, lack of symmetry of its lateral movements and excessive tension of stomatognathic system’s muscles (4). In various studies a high prevalence of malocclusions and orofacial dysfunctions was found in DMD patients, including posterior cross bites, anterior and lateral open bites and skeletal Class III malocclusion with dental compensation (5-9).
Muscle activity and function appear to be related to ionic concentrations in the muscle (10). Cardiac and vascular contraction is initiated by calcium via influx in cardiac and smooth muscle from extracellular fluid (11). This fact could also explain why there is a mechano-sensitive response of the vascular system during mechanical stimulation of the masseter muscle (12). The muscle plasticity is closely linked to and highly dependent on the Ca2+ handling system. The regulation of Ca2+ homeostasis beside the Ca2+ pumps of the plasma membranes such the plasma membrane Ca2+-ATPase (PMCA), the Na+/Ca2+ exchanger (NCX1), or the Ca2+ pump of sarco(endo)plasmic reticulum (SERCA), it also involves a large array of Ca2+ binding proteins, for instance troponin and parvalbumin (13), and other proteins like sarcolipin (SLN) and phospholamban (PLN) which are confined to the ER or SR and inhibit the SERCA activity by lowering the apparent Ca2+ affinity of the pump (14, 15). One other protein located in the SR, the ryanodine receptor (RyR) releases Ca2+ in the cytostol (16).
The absence of dystrophin in muscles can result in abnormally regulation of sarcolemmal channels responsible for calcium handling (2). Numerous studies on dystrophic muscles have shown that the dystrophin-deficient muscle fibres are overloaded with calcium and the increased protein degradation results from the elevated Ca2+ levels (1). Moreover, there is unquestionable evidence that voltage-independent calcium leak channels and stretch-activated calcium channels show an increased activity in dystrophic muscle cells (17). Another potential reason giving rise to calcium accumulation is an incorrect function of L-type Ca2+ channels (18). Defects in the Ca2+ regulating proteins might also result in abnormal chronic and acute Ca2+ concentrations which can lead to various forms of impaired muscle function (16).
In a previous study was shown, that extraocular muscles (EOMs) of both, DMD patients and dystrophic mice, are not clinical and histological affected by muscular dystrophy (19). The EOMs are protected against calcium overload by an enhanced expression of genes involved in the Ca2+ homeostasis (20). Recently, it was demonstrated that different dystrophic masticatory muscles develop inter-muscular differences in muscle histology and gene expression pattern in response to dystrophy. The dystrophic masseter and temporalis shows muscle histology comparable to all other skeletal muscles as found in mdx mice, whereas dystrophic tongue muscles seem to develop a milder phenotype (21-24). Following the hypothesis that an altered Ca2+ homeostasis seems to underlie the mdx masticatory muscle pathology, the aim of the current study was to investigate changes of the expression level of proteins involved in Ca2+ handling, in order to explain the inter-muscular differences. The investigation of pathological events in masticatory muscle atrophy is urgently needed to understand the disease more profoundly and develop therapeutic strategies for DMD patients.
MATERIAL AND METHODS
Animals
Mice of the inbred strains C57Bl/10ScSn (control) and C57/Bl10ScSn-Dmdmdx/J (mdx) were obtained from the Department of Orthodontics from the University Medicine of Greifswald. Age-matched pairs of mdx and control animals (each n = 9–13; 100 d) of either sex were killed using isofluran inhalation. All procedures were approved by a governmental committee on animal welfare on the State Government (LALLF M-V/TSD/7221.3-2.3-001/09). The muscle tissue samples were prepared from the superficial part of masseter, the middle of the temporal muscle and the superior longitudinal tongue muscle and shock-frozen in liquid nitrogen for quantitative reverse transcription PCR and Western blot analysis.
RNA extraction and reverse transcription
Total RNA was isolated using guanidinium-isothiocyanate (RNeasy Fibrous Mini Kit, Qiagen, Hilden, Germany) and RNA concentration was determined by UV absorbance measurements. An amount of 200 ng total RNA was transcribed reversely using random hexamer primers and the TaqMan Reverse Transcription Reagents (PE Applied Biosystems, Weiterstadt, Germany) as described previously (21, 22, 24-37).
TaqMan RT-PCR
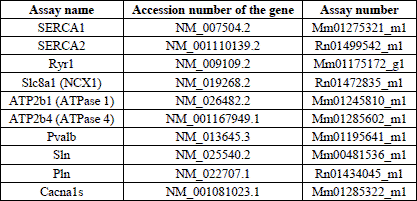
The method was performed as described previously (21, 22, 24, 25) using a real-time PCR cycler (StepOne Plus, Applied Biosystems). Gene specific primers and probes were purchased from PE Applied Biosystems (Table 1) with each probe having been synthesized with a fluorescent 5´-reporter dye (FAM: 6-carboxy-fluorescein) and a 3´-quencher dye (TAMRA: 6-carboxy-tetramethyl-rhodamine). All values are given in relation to the mRNA of 18S rRNA. A “no-template control” using water was performed parallelly in all experiments. Each series of experiments was performed twice.
Western blot analysis
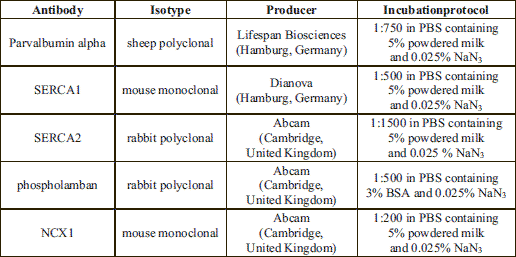
Muscle tissue samples were mechanical homogenized during thawing in lysis buffer (5% glycerol, 0.1% Triton X-100) supplemented with a protease inhibitor cocktail (Sigma, Taufkirchen, Germany) using the SpeedMill P12 homogenizer (Analytikjena, Jena, Germany). The total protein extracts (30 µg) were separated on SDS gels, transferred to nitrocellulose membranes (Schleicher & Schuell) using a tank blotting system (Gibco-BRL, Germany) and incubated with the primary antibody (Table 2) over night at 4°C. Subsequently, HRP-conjugated goat anti-rabbit or goat anti-mouse immunoglobulins (DAKO, Hamburg, Germany) were used at a dilution of 1:5000. Visualisation and detection of bound antibodies were carried out using an enhanced chemiluminescence system (Perbio Science). To assess equal loading of the gel, every membrane was stripped with RestoreTM Plus Western blot stripping buffer (Perbio Science) and incubated with a monoclonal anti-α-actinin antibody (clone AT6/172, Upstate, dilution 1:1000; two hours at room temperature) or monoclonal anti-actin antibody (clone C4, Millipore, dilution 1:1000; two hours at room temperature). Quantitative analyses of protein bands from parvalbumin, SERCA1, SERCA2, phospholamban, NCX1, actin and a-actinin in masticatory muscles were carried out using the GelScan 5.2 software (Serva, Germany). Mod (mean optical density) ±S.D. are given in all cases for n=3 independent Western blot analyses (muscle probes from three different mice of each mice strain). All values are shown in relation to values of actin or α-actinin, respectively.
Statistical analysis
For gene expression the obtained values were compared using Student’s unpaired t-test. If the normality test failed the Mann-Whithney-U rank sum test was used. In contrast, for protein expression analyses the obtained values were compared using paired t-test. If normality test failed the Wilcoxon signed Rank test was used. P<0.05 was considered as statistically significant. Statistical analysis was performed using the SigmaStat version 3.5 software (Systat Software, San Jose, USA). All gene expression data are given as means ±S.E.M., whereas protein expression data are given as means ±S.D.
RESULTS
TaqMan RT-PCR
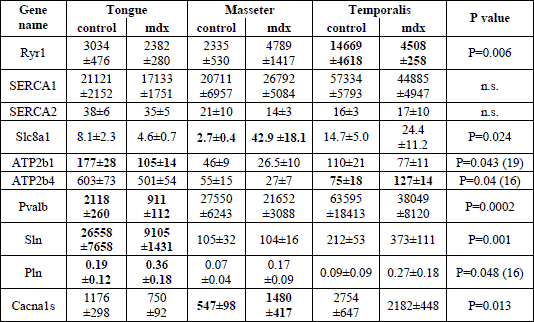
Quantitative TaqMan RT-PCR performed on samples of masticatory muscles revealed the presence of all tested genes in masseter, temporalis and tongue muscular tissue. The gene expression of NCX1 and Cav1.1 was increased 15.8 fold and 2.7 fold in mdx masseter muscular tissue when compared to the control, respectively (Table 3). In contrast, the mRNA amount of RYR1 was significantly reduced in mdx temporalis muscle (MW ±S.E.M.; control versus mdx: 14669±4618 versus 4508±258, P=0.006), whereas ATP2b4 was significantly increased (75±18 versus 127±14; P=0.04; Table 3). Most differences in the gene expression between control and mdx mice were found in tongue muscles. In mdx tongue a down-regulation of about 40%, 65% and 57% of the ATP2b1, sarcolipin and parvalbumin mRNA expression was found, respectively. Furthermore, the phospholamban mRNA level was significantly increased compared to controls (0.19±0.12 versus 0.36±0.18; P=0.048; Table 3).
Western blot analysis
Western blot analysis allowed us to semi-quantify SERCA1, SERCA2, NCX1, phospholamban and parvalbumin proteins (Fig. 1). Single immunoreactive bands of approximately 110, 115, 100, 6 and 12 kDa, were detected, respectively. These bands agree with the expected molecular weights of these proteins (Fig. 1). The quantitative evaluation of the Western blots did not reveal any differences in the expression of SERCA1 and SERCA2 between mdx and control mice (Fig. 2A, 2B). The parvalbumin western blots showed significantly decreased levels between mdx and control mice in tongue and masseter muscles (mean ±S.D.; control versus mdx: tongue, 2.38±1.1 versus 1.6±0.9, P=0.031; masseter, 3.17±0.85 versus 2.48±1.18, P=0.031). A reduction of 22% and 33% in protein expression in dystrophic muscles was detected, respectively (Fig. 2C). The protein expression of phospholamban was significantly increased only in dystrophic masseter muscle (mean ±S.D.; control versus mdx: masseter, 0.96±0.24 versus 1.35±0.31, P=0.046 with 4 degrees of freedom, Fig. 3A). Furthermore, in dystrophic masseter muscle a significantly higher NCX1 protein expression was detected compared to control muscles (masseter, 0.49±0.05 versus 0.54±0.05, P=0.041), whereas no differences were found in tongue and temporalis (Fig. 3B).
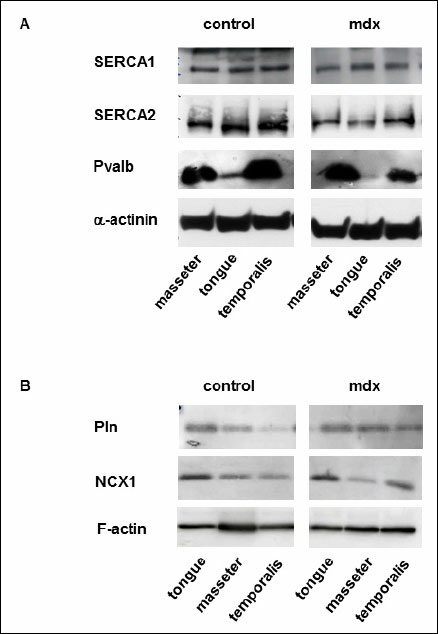 |
Fig. 1. Western blot analyses in masticatory muscles of control and mdx mice. (A): Representative Western blots of SERCA1, SERCA2 and parvalbumin in masseter, temporal muscle as well as tongue from control and mdx mice. A monoclonal antibody was used to detect α-actinin serving as an internal control. (B): Representative Western blots of NCX11 and phospholamban in masseter, temporal muscle as well as tongue. A monoclonal antibody was used to detect F-actin serving as an internal control. |
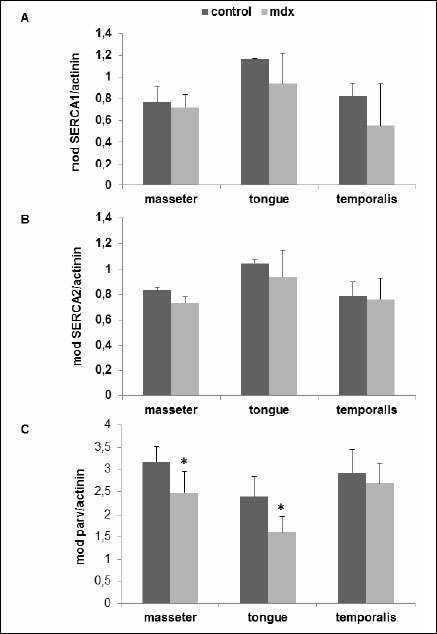 |
Fig. 2. Quantitative analyses of (A) SERCA1, (B) SERCA2 and (C) parvalbumin western blots as shown in Fig. 1. Protein bands attributed to SERCA1, SERCA2 and parvalbumin as well as α-actininwere evaluated using the GelScan 5.2 software (Serva, Germany). The mean optical densities (mod) ±S.D. of control and mdx mice are given in all cases for 3 independent experiments. *P<0.05; significant differences between control and mdx mice; paired t-test. |
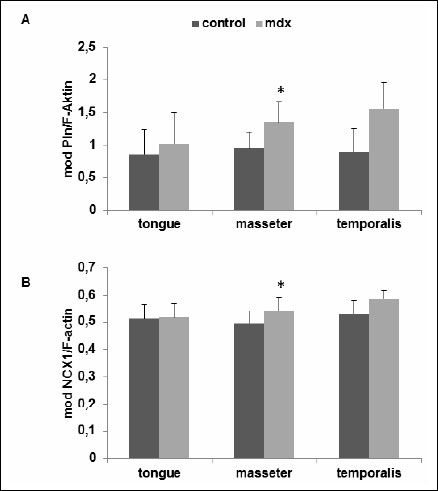 |
Fig. 3. Quantitative analyses of (A) phospholamban and (B) NCX1 western blots as shown in Fig. 1. Protein bands attributed to phospholamban and NCX1as well as F-actin were evaluated using the GelScan 5.2 software (Serva, Germany). The mean optical densities (mod) ±S.D. of control and mdx mice are given in all cases for 3 independent experiments. *P<0.05; significant differences between control and mdx mice; paired t-test. |
DISCUSSION
Mdx skeletal muscles of torso, diaphragm and limbs often present varying patterns of histopathology (28). In a recent study from our laboratory we confirmed such inter-muscular differences in masticatory muscles of 100-days-old mdx mice (23). This work is a follow up study of the above mentioned studies on mdx orofacial muscles because we wanted to know why the orofacial muscles were affected differentially by this disease. Muscle activity and function appear to be related to ionic concentrations in the muscle (10). It is well known that aberrances in the intracellular Ca2+ concentration play an important role in the excitation-contraction-relaxation processes in muscular tissue. Defects in the Ca2+ regulating proteins result in abnormal chronic and acute Ca2+ concentrations, which can lead to various forms of impaired muscle function (16). On the other hand, enhanced Ca2+ buffering has been proposed as a contributory mechanism to explain escape damage in the presence of excess intracellular calcium. Previously, it was shown that the dystrophic phenotype in δ-sarcoglycan-null mice and mdx mice is dramatically improved by skeletal muscle-specific overexpression of SERCA1 (29). SERCA1 overexpression reversed a defect in sarcoplasmic reticulum Ca2+ reuptake that characterizes dystrophic diseases.
In this study, we found higher gene and protein expression of the Na+/Ca2+ exchanger NCX1 in the dystrophic masseteric muscle. NCX1 is known to have a high capacity for calcium transport (30). Recently, Deval and coworkers (30) could demonstrate that the withdrawal of external sodium ions generate elevated intracellular calcium activity in isolated myotubes. Furthermore, it was shown that the amplitudes of the Na+-free induced [Ca2+]int rises were generally higher in myotubes from DMD patients compared to controls. Imbert et al. (31) found an abnormal elevation of the resting calcium level in DMD myotubes. An upper activity of the exchange mechanism due to an elevated [Ca2+]int in DMD myotubes can be suggested. It is known that NCX1 is regulated by cytoplasmic calcium (32, 33). Mackiewicz and Lewartowski (34) have found that the effect of SR Ca2+ leak from ryanodine receptors is strictly dependent on the activity of NCX1 and SERCA.
Cully and coworkers (35) demonstrated that PMCAs are up-regulated in mdx muscles. Using western blot analyses a 2fold increase in the expression of PMCAs in dystrophic Extensor digitorum longus (EDL) muscles were detected compared to controls. In our study, increased expression levels for PMCA4 were also found in dystrophic temporalis muscular tissue, whereas the amount of PMCA1 mRNA was significantly reduced in tongue muscles of mdx mice. The plasma membrane Ca2+-ATPase is known to be activated due to the release of sarcoplasmic reticulum Ca2+. Interestingly, a reduction in SERCA activity has been observed in dystrophic muscles (36-38). This is in contrast to our findings in orofacial muscles. We did not find any changes in the expression of SERCA1 and SERCA2 in masseteric and temporal muscles as well as in tongue muscular tissue.
An up-regulated mRNA expression of phospholamban, sarcolipin, parvalbumin and Cacna1s was found in extraocular muscles (EOMs) myotubes compared to tibialis muscle myotubes (20). In fully differentiated adult tissue from normal and dystrophic mice the following genes showed significantly higher expression in EOMs compared to tibialis muscle: SERCA2, PMCA1, PMCA4, NCX1, phospholamban and sarcolipin (20, 39). Furthermore, the expression levels of these proteins in EOMs did not differ between control and mdx mice (39). EOMs are clinically and histologically spared in DMD patients and mdx mice and these muscles are more resistant to elevated Ca2+ levels than limb muscles (19).
From all these results obtained in this study compared with those from the literature it can be concluded that masseteric and temporal muscular tissue of 100 day old mdx mice might be more resistant to elevated Ca2+ levels than tongue muscles. Recently, it was shown that mdx masseter and temporal muscles consist of mostly regenerated fibers with central nuclei and show just few inflammatory foci and increased collagen content. The degree of centronucleation, the mean fiber diameter as well as the amount of collagen did not significantly differ between masseter, temporal and soleus muscle of mdx mice (23). In further studies could be also shown that masticatory muscles has many similarities in the expression profile to the soleus muscle (21, 24, 40, 41). Furthermore, the studies on the expression of the calcium-regulating genes showed no significant differences between the masseter, temporalis and soleus (unpublished data). In contrast, dystrophic tongue muscles contain only 11.2% of regenerated muscle fibers and inflammatory foci are hardly detectable (23). At this histological finding is to realize that the muscle fibers of mdx masseter and temporalis have already undergone a cycle of muscle fiber degeneration followed by regeneration. Furthermore, it was speculated that mdx tongue muscle seemed to be almost hit by this disease. It is well known that in 2–9 weeks old mdx mice muscle fibre degeneration is dominating (42). From the fifth week of life, the muscle fiber regeneration prevailed in the mdx mice, so that there was no further muscle wasting and the animals did not show any signs of muscle weakness up to the age of two years (43, 44). Therefore our findings suggest that regenerated fibres in 100 days old mdx masticatory muscles express Ca2+ handling protein characteristic for EOM muscles.
In conclusion, our findings revealed that mdx masticatory muscles showed an unequally altered expression of genes involved in the Ca2+ homeostasis that can support the differences in masticatory muscles response to dystrophin deficiency. Regenerated dystrophic fibres of masticatory muscles expressed Ca2+ handling protein characteristic for EOM muscles and seemed to be protected against calcium overload. In contrast, dystrophic tongue seems a priori secured from muscle degeneration even though in this muscle we found expression levels of Ca2+ handling proteins typical for dystrophic muscles. These characteristics provide insight for the potential role of superior calcium buffering in dystrophic mice muscles and its possibility of muscle regeneration. Further studies should find out, if the expression of genes and proteins involved in Ca2+ handling is age-dependent and wether the expression is associated with muscle degeneration/regeneration.
Conflict of interests: None declared.
REFERENCES
- Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J Physiol 1999; 515: 859-868.
- Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol 2007; 292: H846-H855.
- Botteron S, Verdebout CM, Jeannet PY, Kiliaridis S. Orofacial dysfunction in Duchenne muscular dystrophy. Arch Oral Biol 2009; 54: 26-31.
- Pihut M, Majewski P, Wisniewska G, Reron E. Auriculo-vestibular symptoms related to structural and functional disorders of stomatognatic system. J Physiol Pharmacol 2011; 62: 251-256.
- Kiliaridis S, Katsaros C. The effects of myotonic dystrophy and Duchenne muscular dystrophy on the orofacial muscles and dentofacial morphology. Acta Odontol Scand 1998; 56: 369-374.
- Matsumoto S, Morinushi T, Ogura T. Time dependent changes of variables associated with malocclusion in patients with Duchenne muscular dystrophy. J Clin Pediatr Dent 2002; 27: 53-61.
- Matsuyuki T, Kitahara T, Nakashima A. Developmental changes in craniofacial morphology in subjects with Duchenne muscular dystrophy. Eur J Orthod 2006; 28: 42-50.
- Morel-Verdebout C, Botteron S, Kiliaridis S. Dentofacial characteristics of growing patients with Duchenne muscular dystrophy: a morphological study. Eur J Orthod 2007; 29: 500-507.
- Symons AL, Townsend GC, Hughes TE. Dental characteristics of patients with Duchenne muscular dystrophy. ASDC J Dent Child 2002; 69: 277-283, 234.
- Gedrange T, Mai R, Richter G, Wolf P, Lupp A, Harzer W. X-ray microanalysis of elements in the masticatory muscle after paresis of the right masseter. J Dent Res 2005; 84: 1026-1030.
- Burt MG, Mangelsdorf BL, Srivastava D, Petersons CJ. Acute effect of calcium citrate on serum calcium and cardiovascular function. J Bone Miner Res 2013; 28: 412-418.
- Turturici M, Roatta S. Inactivation of mechano-sensitive dilatation upon repetitive mechanical stimulation of the musculo-vascular network in the rabbit. J Physiol Pharmacol 2013; 64: 299-308.
- Martonosi AN, Pikula S. The network of calcium regulation in muscle. Acta Biochim Pol 2003; 50: 1-30.
- Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 2005; 38: 291-302.
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J 2005; 389: 151-159.
- Gommans IM, Vlak MH, de Haan A, van Engelen BG. Calcium regulation and muscle disease. J Muscle Res Cell Motil 2002; 23: 59-63.
- Hopf FW, Turner PR, Denetclaw WF, Reddy P, Steinhardt RA. A critical evaluation of resting intracellular free calcium regulation in dystrophic mdx muscle. Am J Physiol 1996; 271: C1325-1339.
- Morel JL, Rakotoarisoa L, Jeyakumar LH, Fleischer S, Mironneau C, Mironneau J. Decreased expression of ryanodine receptors alters calcium-induced calcium release mechanism in mdx duodenal myocytes. J Biol Chem 2004; 279: 21287-21293.
- Khurana TS, Prendergast RA, Alameddine HS, et al. Absence of extraocular muscle pathology in Duchenne’s muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J Exp Med 1995; 182: 467-475.
- Zeiger U, Mitchell CH, Khurana TS. Superior calcium homeostasis of extraocular muscles. Exp Eye Res 2010; 91: 613-622.
- Kunert-Keil C, Gredes T, Lucke S, et al. Caveolin-1, caveolin-3 and VEGF expression in the masticatory muscles of mdx mice. Folia Histochem Cytobiol 2011; 49: 291-298.
- Spassov A, Gredes T, Gedrange T, et al. Differential expression of myosin heavy chain isoforms in the masticatory muscles of dystrophin-deficient mice. Eur J Orthod 2010; 33: 613-619.
- Spassov A, Gredes T, Gedrange T, Lucke S, Pavlovic D, Kunert-Keil C. Histological changes in masticatory muscles of mdx mice. Arch Oral Biol 2010; 55: 318-324.
- Spassov A, Gredes T, Gedrange T, Lucke S, Pavlovic D, Kunert-Keil C. The expression of myogenic regulatory factors and muscle growth factors in the masticatory muscles of dystrophin-deficient (mdx) mice. Cell Mol Biol Lett 2011; 16: 214-225.
- Gredes T, Kunert-Keil C, Dominiak M, Gedrange T, Wrobel-Kwiatkowska M, Szopa J. The influence of biocomposites containing genetically modified flax fibers on gene expression in rat skeletal muscle. Biomed Tech (Berl) 2010; 55: 323-329.
- Gedrange T, Buttner C, Schneider M, Oppitz R, Harzer W. Myosin heavy chain protein and gene expression in the masseter muscle of adult patients with distal or mesial malocclusion. J Appl Genet 2005; 46: 227-236.
- Gredes T, Spassov A, Mai R, et al. Changes in insulin like growth factors, myostatin and vascular endothelial growth factor in rat musculus latissimus dorsi by poly-3-hydroxybutyrate implants. J Physiol Pharmacol 2009; 60 (Suppl. 3): 77-81.
- Boland B, Himpens B, Denef JF, Gillis JM. Site-dependent pathological differences in smooth muscles and skeletal muscles of the adult mdx mouse. Muscle Nerve 1995; 18: 649-657.
- Goonasekera SA, Lam CK, Millay DP, et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 2011; 121: 1044-1052.
- Deval E, Levitsky DO, Marchand E, Cantereau A, Raymond G, Cognard C. Na+/Ca2+ exchange in human myotubes: intracellular calcium rises in response to external sodium depletion are enhanced in DMD. Neuromuscul Disord 2002; 12: 665-673.
- Imbert N, Cognard C, Duport G, Guillou C, Raymond G. Abnormal calcium homeostasis in Duchenne muscular dystrophy myotubes contracting in vitro. Cell Calcium 1995; 18: 177-186.
- Levitsky DO, Fraysse B, Leoty C, Nicoll DA, Philipson KD. Cooperative interaction between Ca2+ binding sites in the hydrophilic loop of the Na+-Ca2+ exchanger. Mol Cell Biochem 1996; 160-161: 27-32.
- Levitsky DO, Nicoll DA, Philipson KD. Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+ exchanger. J Biol Chem 1994; 269: 22847-22852.
- Mackiewicz U, Lewartowski B. The effect of sarcoplasmic reticulum Ca2+ leak on contractile activity of guinea pig heart myocytes depends in activity of sarcoplasmic reticulum Ca2+-ATPase and Na+/Ca2+ exchanger. J Physiol Pharmacol 2008; 59: 287-300.
- Cully TR, Edwards JN, Friedrich O, Stephenson DG, Murphy RM, Launikonis BS. Changes in plasma membrane Ca-ATPase and stromal interacting molecule 1 expression levels for Ca2+ signaling in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol 2012; 303: C567-C576.
- Divet A, Huchet-Cadiou C. Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch 2002; 444: 634-643.
- Divet A, Lompre AM, Huchet-Cadiou C. Effect of cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca-ATPase, on skeletal muscles from normal and mdx mice. Acta Physiol Scand 2005; 184: 173-186.
- Kargacin ME, Kargacin GJ. The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim Biophys Acta 1996; 1290: 4-8.
- Zeiger U. Physiological, Molecular and Biochemical Charactreization of Rodent Extraocular Muscles: Implication for Duchenne Muscular Dystrophy. Greifswald, University of Greifswald, 2011. http://ub-ed.ub.uni-greifswald.de/opus/volltexte/2011/1092/
- Spassov A, Gredes T, Lehmann C, et al. Myogenic differentiation factor 1 and myogenin expression not elevated in regenerated masticatory muscles of dystrophic (mdx) mice. J Orofac Orthop 2011; 72: 469-475.
- Spassov A, Gredes T, Pavlovic D, et al. Talin, vinculin and nestin expression in orofacial muscles of dystrophin deficient mdx mice. Arch Immunol Ther Exp (Warsz) 2012; 60: 137-143.
- Tanabe Y, Esaki K, Nomura T. Skeletal muscle pathology in X chromosome-linked muscular dystrophy (mdx) mouse. Acta Neuropathol. 1986; 69: 91-95.
- Carnwath JW, Shotton DM. Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci 1987; 80: 39-54.
- Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6-28 months old. J Physiol 2001; 535: 591-600.
A c c e p t e d : February 11, 2014