THE ROLE OF HYDROGEN SULFIDE IN PATHOLOGIES OF THE VITAL ORGANS AND ITS CLINICAL APPLICATION
INTRODUCTION
Hydrogen sulfide (H2S) is a colourless gas with a strong odor of rotten eggs (1). It can be oxidised into sulfur, sulfur oxide as well as sulfates, best known for its toxic effects which range from eye irritation to causing rapid unconsciousness and cardiac arrest (2). However, it has been discovered that H2S is also produced in living organisms and possesses roles beyond its toxic properties as an environmental pollutant.
Similarities have been drawn between H2S and the other gaseous transmitters, such as nitric oxide (NO), carbon monoxide (CO) and ammonium, where they have been demonstrated to be important gaseous mediators in inflammation and sepsis (3-5). They serve as important autocrine and paracrine messengers. NO and CO with the addition of hydrogen cyanide, have been reported to inhibit mitochondrial cytochrome oxidase (6). It was found that H2S could both induce and inhibit NO production, depending on its concentration (7, 8). The interaction between these gaseous transmitters is beyond the scope of this paper and has been explored in recent reviews (5, 9, 10).
Several studies have indicated the roles of H2S in regulating vascular tone and its possible associations with inflammation and disease progression. However, research of H2S is still at its infancy, and the understanding of the mechanisms behind its role still limited. Nevertheless, many researchers have proposed the use of H2S donors and inhibitors as possible therapeutic agents in different disease conditions.
Due to the breadth of the studies available concerning the pathophysiological effect of H2S, it is not possible to include and critically appraise all the information available.
In this article, we will summarise the pathophysiological effects of the H2S on the central nervous system, cardiovascular system, lungs, liver and kidneys, focusing on conditions where H2S demonstrates therapeutic potential.
HYDROGEN SULFIDE SYNTHESIS (Fig. 1)
The production of H2S involves the conversion of L-cysteine to H2S via the action of two enzymes, cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) (11). H2S can also be produced from cysteine through a third enzyme, 3-mercaptopyruvate sulphurtransferase (3-MST). This particular pathway involves another enzyme, cysteine aminotransferase, which generates 3-mercaptopyruvate from cysteine and α-ketoglutarate. 3-MST then produces H2S from 3-mercaptopyruvate. 3-mercaptopyruvate contributes sulfur to the active-site cysteine residue of 3-MST to form persulfide, which releases H2S in the presence of dithiothreitol (12). However, dithiothreitol is not physiologically freely available. A recent study by Yoshinori et al. demonstrated the effects of 2 physiological reducing disulfides, thioredoxin and dihydrolipoic acid in releasing H2S from persulfide at the active site of 3-MST (13).
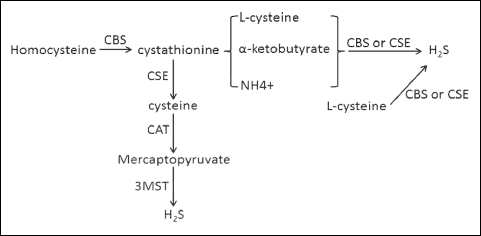 |
Fig. 1. Pathway of hydrogen sulfide synthesis. 3-MST, 3-mercaptopyruvate sulphurtransferase; CAT, catalase; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; H2S, hydrogen sulfide. |
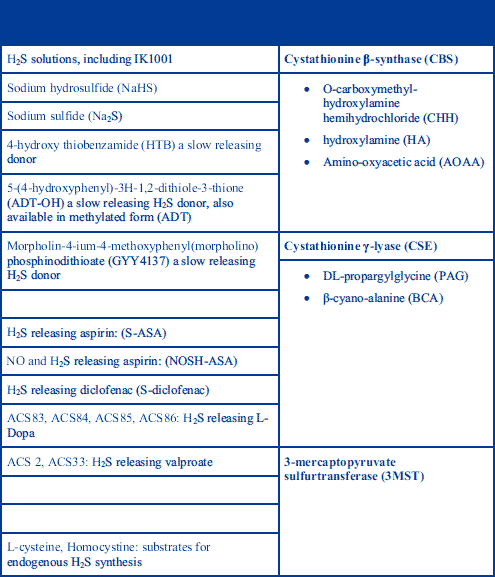
Hydrolisation of H2S yields hydrosulfide and sulfide ions in the following reactions: H2S ⇔ H+ + HS- ⇔ 2H+ + S2–. At pH 7.4 in an aqueous solution, approximately one third of H2S remains undissociated and is permeable to plasma membrane (1). The reports of different concentration levels of H2S in mammals are dependent on the methods used to determine its concentration. These methods include spectophotometric measurement of methylene blue formed from the reaction of N-dimethyl-p-phenylenediamine with sulfide, or alternatively, sulfide anion measurement with an ion-selective electrode. Olson et al. (14) discussed these methods in detail and their respective drawbacks. In mammals, it has been proposed that H2S has a circulating concentration of between 1 and 300 µM (15-17) in blood, and concentrations in the 50–160 µM range (18) in the brain, although the true concentrations are likely to be lower than the values mentioned above. H2S is also present in the lumen of human large intestine at millimolar concentrations, but the level of unbound sulfide is in the micromolar range due to the binding of sulfide to faecal components (19). Even after systemic administration of H2S donors (Table 1) at doses that produce pharmacological effects, plasma H2S concentrations rarely rise above the normal range, or only for brief periods of time (20).
GENERAL FUNCTIONS OF HYDROGEN SULFIDE
Physiologically, H2S is an endogenous gaseous transmitter and an important signaling molecule involved in inflammation (21-24). H2S is able to stimulate and inhibit the mitochondrial electron transport, depending on its concentrations. It has been reported to act as both substrate, at low concentration (<5 µM)(25); and inhibitor, at high concentration (>20 µM) (6) for the cytochrome oxidase system. H2S has also been demonstrated to regulate L and T type calcium channels and open KATP channels.(26-29). H2S also possesses anti-oxidative properties, increasing superoxide dismutase (SOD) activity, glutathione (GSH) turnover, decreasing reactive oxygen species (ROS) production; and anti-apoptotic properties by inhibiting nuclear factor kappa beta (NF-κB) pathways, mitogen-activated protein kinase p38 (MAPK p38), C-Jun N-terminal kinase (JNK), Bcl-2 associated X protein (BAX) and caspase 3, as well as up regulating Bcl (30, 31). Interestingly studies have also demonstrated that H2S can mediate its cytoprotective excitation of the sensory nerve supplying the tissue (29).
Previous studies exploring the roles of H2S have used its donors and inhibitors of its producing enzymes to investigate the effects of H2S as well as its inhibition. For instance, DL-propargyl glycine (PAG) is an irreversible inhibitor of CSE and, when administered to rodents, produces an almost complete inhibition of the activity of this enzyme (32). The examples of H2S donor, H2S enzymes and their inhibitors are listed in Table 1.
Increase in H2S level has been associated with reduced inflammation in various organ systems, such as in inflammatory bowel disease, particle induced airway irritation and neuroinflammation (33-35). It has demonstrated the ability to reduce oedema formation and leukocyte adherence to the vascular endothelium, and to inhibit pro-inflammatory cytokine synthesis (20). Some studies even demonstrate the organ protective effects of H2S against lipopolysaccharides (LPS) exposure (36, 37). However numerous studies have found that in sepsis, H2S administration significantly worsens the inflammation (38, 39). This is associated with significantly increased histological deterioration in various organs and worse disease outcome (40, 41). Inhibition of H2S production significantly reduces pathology severity and overall survival, which would implicate theraputic benefit of H2S production inhibition in sepsis (42, 43). The effects of H2S in endotoxaemia and sepsis related inflammation have been discussed in great detail in other reviews (20, 22, 24, 38, 39, 42, 44). As such we will not attempt to discuss this topic in detail.
The molecular targets of H2S are summarised in Fig. 2.
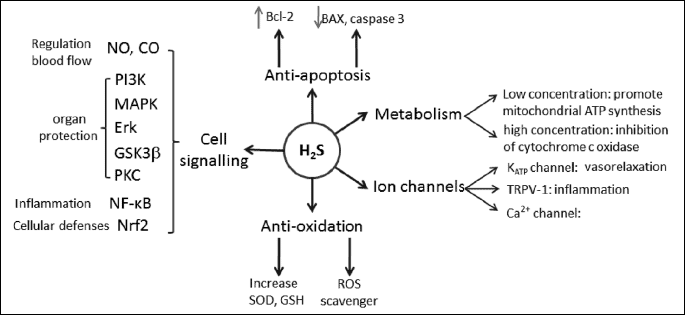
BAX, Bcl-2 associated X protein; CO, carbon monoxide; ERK, extracellular-signal-regulated kinase; GSH, glutathione; GSK3b, glycogen synthase kinase 3 beta; H2S, hydrogen sulfide; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; NF-κB, nuclear factor kappa beta; Nrf2, NF-E2 p45-related factor 2; ROS, reactive oxygen species; SOD, superoxide dismutase; TRPV-1, transient receptor potential cation channel subfamily V member 1.
CARDIOVASCULAR SYSTEM
Hydrogen sulfide is synthesised in the cardiovascular system by CSE and 3-MST, but not by CBS which are found in various blood vessels including the aorta and portal vein (45-47).
Hydrogen sulfide is involved in a number of physiological and pathological processes in the cardiovascular system (28, 48). At low concentrations, exogenous H2S acts as a vasodilator and antihypertensive. Zhao et al. showed that NaHS given at 2.8 µM/kg is enough to cause a measurable reduction in the systolic blood pressure and significant vasodilation (49), while the knockout of the CSE gene increases blood pressure (50). A number of studies have looked into the possible benefit of H2S in hypertension and found that H2S donor administration significantly reduced blood pressure as well as the associated renal injury (51-53). At higher doses, H2S significantly reduced the rate and amplitude of cardiomyocyte action potential, while increasing the rate of repolarisation and significantly reduces pacemaker firing. These studies found significantly reduced potassium and calcium currents across cardiomyocytes, and suggested that the electrophysiological changes are probably due to H2S interaction with the KATP and L type Ca2+ channels (54-56).
At low doses, H2S also promotes endothelial proliferation and angiogenesis. Cai et al. showed that with 10–20 µM NaHS, endothelial cells showed increased proliferation, migration, branching and endothelial tube formation (57). The therapeutic potential of this property has been demonstrated in diabetic wound healing and in heart failure. Liu et al. showed that in diabetic mice administration of H2S donor increased the rate of angiogenesis and the rate of wound closure, and administration of PAG resulted in the opposite (47). Givvimani et al. demonstrated that H2S administration in heart failure mouse model increases angiogenesis and results in better overall ventricular function. Possible mechanisms include Akt phosphorylation, survivin, angiopoietin-1 (ANG1), vascular endothelial growth factor (VEGF) expression and inhibition of angiostatin, endostatin and matrix metalloproteinases (MMP) (58).
However, the pro-proliferative effect is completely abolished with NaHS doses of 100 uM or higher. Indeed, at a higher concentration, H2S exerts an anti-proliferative and pro-apoptotic effect on cardiomyocytes, vascular smooth muscles and endothelial tissue. In CSE over-expressing tissue, proliferation rate and viability are significantly lower, and apoptotic rate is significantly higher (59). Similar effects have been found with regard to aortic smooth muscle cells, vascular endothelium and atrial cardiomyocytes that were exposed to high levels of NaHS (54, 60, 61). These findings were associated with higher activation of extracellular-signal-regulated kinase (ERK), and caspase 3 activities, increased p38 MAPK phosphorylation and reduced cyclin D activity. These perhaps reflect on the ability of H2S to inhibit cardiac remodeling in response to pathology.
It has been suggested that H2S over-expression may play a role in diseases with undesirable angiogenesis, and lowering H2S level may prove therapeutic. Ran et al. showed that patients with proliferative diabetic retinopathy have significantly higher H2S levels than those that have non-proliferative retinopathy (62). Szabo et al. showed that colon cancer cells over-produce H2S, and inhibiting CBS activity decreases angiogenesis and tumor growth (63).
Hydrogen sulfide is also thought to exert protective effects on ischaemia related insults. NaHS administrated at a moderate level of 20–50 µM, before hemorrhagic shock, is enough to significantly improve histological appearance of the heart and haemodynamic stability (64-66). In ischaemic-reperfusion injury, pre-treatment with H2S either immediately or 20 hours before the injury results in less cell damage and apoptosis, histological change and better contractility (67-69). This is associated with reduced BAX and caspase 9 activation, reduced cytokine release and reduced reactive oxygen species (ROS) generation. In addition to that, it is thought that the anti-apoptotic effect is due to the upstream effect of H2S on protein kinase C (PKC) and ATP-sensitive potassium channels (KATP), which causes the phosphorylation of ERK1/2, as well as the phosphorylation of glycogen synthase kinase 3 beta (GSK3β) and the H2S-NO synthase interaction (69). H2S is also thought to contribute to the ischaemic preconditioning (IP) phenomenon - Huang et al. showed that H2S level rises significantly with IP and ischaemic postcoditioning (IPO), while inhibition of H2S synthesis with PAG in heart tissue abolishes the effect of IP and IPO (70). It is suggested that H2S exerts the protective effect via ERK 1/2 activation (68).
Hydrogen sulfide has also demonstrated anti-atherosclerotic properties. Mani et al. showed that CSE knockout mice developed more marked dyslipidemia, atherosclerotic change, increased oxidative stress and adhesion molecule expression, this was reversed by administering NaHS supplement (71). NaSH administration reduces the size of atherosclerotic plaque formation, whereas PAG increases formation (72). S-aspirin has also demonstrated anti-atherosclerotic benefit (73). In a rabbit model of atherosclerosis, restenosis after percutaneous angioplasty was significantly lower with NaHS administration and higher with PAG administration (74). This is the result of decreased oxidative low density lipoproteins (LDL) modification (75), reduced tumor necrosis factor alpha (TNF-α) and intercellular adhesion molecule (ICAM) expression by macrophage (76) and decreased foam cell formation (77).
A number of studies looked into the use of H2S as a marker of cardiovascular disease in humans. Jiang et al. demonstrated that patients with angina have significantly lower levels of plasma H2S compared to a healthy population, and unstable angina patients have even lower H2S levels than stable angina patients (78). In addition to this, multiple vessel pathology, complete occlusion, co-existing hypertension and smoking are all factors associated with lower H2S levels. Kovacic et al. demonstrated that in patients with congestive heart failure, plasma H2S level is closely correlated with the staging of the disease, and its lower level is also a good predictor of mortality and hospitalisation (79).
RESPIRATORY SYSTEM
In the respiratory system, CSE and CBS are expressed in the airway and vascular smooth muscle cells (2).
Hydrogen sulfide administration in airway tissue is associated with anti-proliferative effects. Administration of H2S donor inhibits the proliferation and migration of both smooth muscle cells and fibroblasts, and inhibits the transformation of fibroblasts into myofibroblasts. This is thought to be mediated by the inhibition of ERK expression and phosphorylation, and reduced IL-8 production (80, 81). H2S prevents TGF-β induced epithelial mesenchymal transition, a process which is thought to be implicated in the development of pulmonary fibrosis (82). This finding may have applicability in counteracting drug-induced fibrosis, for example, treatment with H2S would limit bleomycin induced fibrosis through the reduction of inflammatory cell infiltration and the inhibition of NF-κB (81).
Hydrogen sulfide also has pro-apoptotic effects on the airway cells, H2S exposure at levels of around 70 µM is associated with reduced cell viability; increased apoptotic pathway activation and relevant histological changes (83). One possible application of this is in cancer. Indeed, lung adenocarcinoma cells exposed to ACS2 and ACS33 underwent apoptosis, seeming to have a synergistic effect with the conventional chemotherapeutic agent such as cisplatin (84).
Hydrogen sulfide can act as a bronchodilator, as it causes significant airway smooth muscle relaxation and increases airway luminal area. H2S inhibits InsP3-evoked Ca2+ release, through the reduction of disulphide bonds in InsP3 receptors (85). It also activates the calcium activated BK potassium channel, which hyperpolarizes the smooth muscle cell. Guanylyl cyclase and protein kinase G pathway on the other hand does not mediate the effect of H2S (86). The bronchodilatory along with the anti-proliferative properties make H2S a possible therapeutic option for asthma and other airway hyper-responsive conditions. Indeed, it has been found that serum H2S in children with asthma is significantly lower than healthy control, and that H2S level positively correlates with lung function in asthmatics (87). Animal studies also support the role of H2S in asthma. Studies with CSE knockout mice and PAG administration demonstrates that H2S deficiency is associated with higher airway responsiveness, increased airway resistance, goblet cell hyperplasia, increased cellular infiltrates and increased cytokines such as IL4, 5 and 13. H2S exposure reverses the effect of CSE knockout, reduces airway hyper-responsiveness, limits inflammation, airway remodeling and collagen deposition (88-90).
Hydrogen sulfide has also been shown to play a role in the pathology and treatment of chronic obstructive pulmonary disease (COPD). In COPD patients, the serum H2S level is negatively correlated with the disease staging, with late stage COPD patients having significantly lower H2S levels and H2S levels showing a positive correlation with the forced expiratory volume in the first second (FEV1) value of the patient (91). A recent study also found that the H2S sputum-to-serum ratio is significantly higher in COPD patients, especially those with acute exacerbation (92). In animal models of tobacco smoke induced COPD, NaHS significantly reduced airway remodeling and emphysematous change, and demonstrated reduced cytokine release and cell infiltrate (93).
The effect of H2S on pulmonary vasculature is somewhat controversial, as H2S has been shownin vitro to cause significant reduction in pre-constricted human pulmonary artery pressure, and inhibition of CSE with PAG increases the pulmonary arterial pressure (94). On the other hand, H2S administration caused transient vasoconstriction in relaxed pulmonary vasculature, and hypoxia caused an increase H2S generation. It has been suggested that H2S is involved in oxygen sensing in pulmonary vasculature and the hypoxic pulmonary vasoconstriction (HPV) phenomenon (95). This is demonstrated where H2S precursors potentiate HPV, while H2S inhibitors reduce HPV magnitude (96). However, this effect is not seen in chronic hypoxia where H2S administration is associated with clear vasodilatory and anti-hypertensive effects (97). Patients with pulmonary hypertension have significantly lower level of serum H2S than those in healthy controls (98). Similarly, patients with congenital heart disease who develop pulmonary hypertension have significantly lower H2S level (98). This is further supported by animal studies, which show that in animal models of pulmonary hypertension, exposure to sulfur dioxide significantly decreases the pulmonary artery pressure and blood vessel muscularisation (99).
As discussed in the cardiovascular system section, H2S protects tissue from haemodynamic disturbances. NaHS administration decreases lung injury in haemorrhagic shock and lead to increased survival (64). This is achieved through inhibiting apoptosis pathways and reducing radical oxygen species formation (100). In addition to this, H2S also protects against lung injury in ischaemic re-perfusion and in traumatic limb injury, through similar mechanisms (101, 102).
CENTRAL NERVOUS SYSTEM
In the brain, H2S is thought to be produced mainly in the astrocytes using the enzyme CBS, and reaches relatively high concentrations of up to 160 µM (103).
As previously reviewed by Hu et al. and Zhou et al., endogenous H2S is a neuromodulator. It potentiates N-methyl-D-aspartate receptor (NMDA) mediated currents and modulates long term potentiation; stimulate uptake of synaptic glutamate by astrocytes; potentiates the effect of GABA and regulates intracellular calcium levels (104, 105). H2S has also been shown have T type calcium channel mediated effects in peripheral nerves, which includes altering protein expression in the spinal cord. (29).
In CNS pathology H2S is thought to have anti-apoptotic, anti-inflammatory and anti-oxidative benefits. Whiteman et al. found that H2S could directly scavenge reactive oxygen species as effectively as reduced glutathione, that H2S significantly increased human neuroblastoma cell survival and protein oxidation after exposure to superoxide (35, 106). H2S administration is also associated with significantly increased superoxide dismutase activity and tissue GSH level (107, 108). Lu et al. showed that this may be achieved by potentiation of the mitochondrial uncoupling protein 2 (UCP2), which reduces the generation of ROS in the mitochondria (109). Administration of H2S prevents apoptosis by inhibiting mediators such as caspase, BAX and up regulating Bcl, it also down regulates cytokines such as TNF-α and interleukin-6 (IL-6) (110). This neuroprotective effect has been shown in acute neuronal injuries secondary to other modalities of injury including LPS, glutamate and trauma where H2S donor administration significantly increases cell viability (37, 108, 111, 112).
Hydrogen sulfide is thought to play a role in the protection against Alzheimer’s disease. Eto et al. showed that post-mortem H2S level in the brain tissue of Alzheimer’s patients on average half that of the control population (113). Similar results were also found in studies involving animal models of Alzheimer’s disease, where H2S donors significantly reduced amyloid plaque formation, memory impairment and learning impairment (110). This is achieved through preventing apoptosis and alleviating oxidative stress (114, 115). A study by Nagpure suggests that H2S also directly reduces β-amyloid production by down regulating the production of amyloid precursor protein and the activity of β and γ secretase (116).
Similar findings have also been seen in Parkinson’s disease where H2S exposure in a Parkinson’s disease mice model prevented the development of movement disorders and substantia nigra degeneration (117). The therapeutic benefits have also been demonstrated in human cell lines (107). In addition to direct anti-oxidative and anti-apoptotic effects, H2S has also been demonstrated to reduce the expression of TNF-α and IL-6 from glia cells, and reduces neurotoxicity of glia cell secretion (37).
Similar to its effect in myocardium ischaemia, H2S also protects neuronal cells against hypoxia. Gheibi et al. showed that treatment with NaHS significantly reduced infarct size, also significantly reduced brain edema and apoptosis associated with reperfusion injury (118). This can also be attributed to the anti-oxidative and anti-inflammatory effect of H2S, which reduces cytokine release by microglia, inhibits inducible nitric oxide synthase (iNOS) expression, and inhibits phosphorylation of MAPK and ERK (119, 120). H2S has also been demonstrated to promote angiogenesis after hypoxia via up regulation of angiopoietin-1 and 2, VEGF and p-AKT, which may further improve recovery (121).
LIVER
Hydrogen sulfide producing enzymes are expressed in hepatocytes and hepatic stellate cells, but not in the sinusoidal endothelial cells (122). The ability of H2S in regulating blood flow has been implicated in treating portal hypertension, which is characterised by increased hepatic vascular resistance and increased splanchnic blood flow (123). Due to the dynamic and reversible nature of intrahepatic vasculature in cirrhotic liver, the interplay between vasoconstrictors and vasodilators plays an important role in the progression of the condition. Fiorucci et al. demonstrated that infusion of NaHS prevented the increase of hepatic resistance induced by noradrenaline infusion (124), preserving hepatic blood flow, suggesting that H2S acts as a vasodilator and an important endogenous modulator of the hepatic microcirculation.
The relationship between the concentrations of H2S and the severity of portal hypertension was also studied. Wang et al. reported a significantly lower endogenous H2S levels in patients with portal hypertension than those in the healthy controls (125). In the same study, the authors reported lower concentrations of H2S in rabbit liver tissue as well as lower expressions of CSE.
Hydrogen sulfide has also been shown to reduce hepatic fibrosis. It has been shown that administration of NaHS reduces the hepatic stellate cell proliferation as well as collagen I expression (126).
In a study examining the role of CSE in acute liver failure induced by D-GalN and LPS, the authors reported an attenuation of liver injury in mice with congenital deficiency of CSE and inhibition of CSE using PAG. In mice with congenital deficiency of CSE, there are markedly elevated homocysteine and thiosulfate levels, up regulation of Nrf2 and antioxidant proteins, activation of Akt-dependent anti-apoptotic signaling, and inhibition of GalN/LPS-induced c-JNK phosphorylation in the liver (127).
Hydrogen sulfide has also been reported to demonstrate potent anti-hepatocellular carcinoma activity (128). The authors examined the effect of H2S with GYY4137, a H2S donor. The exact mechanisms involved remain to be investigated. They demonstrated the effects of GYY4137 in suppressing cell proliferation in human hepatocellular carcinoma cell lines.
Hydrogen sulfide has also been shown to be beneficial in the events of ischaemic/reperfusion injury. In a hepatic ischaemic/reperfusion model, it was reported that animals treated with H2S showed reduction in serum ALT and AST levels and necrotic lesions at 24 hours after ischaemia/reperfusion. H2S was also reported to reduce the TNF-α level and IL-6 mRNA level increases induced by ischaemic/reperfusion injury and in turn, H2S may suppress cell necrosis, apoptosis and inflammation (129). Its preconditioning is shown to protect rat liver against ischemia/reperfusion injury (130). The proposed mechanism is via the activation of Akt-GSK-3β. The levels of serum ALT and AST were lower compared to the ischaemic/reperfusion group without NaHS treatment, probably through the mechanism of suppressing cytochrome c release and caspase activation.
Hydrogen sulfide has also been shown to reduce liver damage in the case of acitaminophen-induced hepatotoxicity. In a rodent acitaminophen-induced hepatotoxicity model, H2S was reported to cause significant decrease in serum alanine aminotransferase and hepatic malondialdehyde and nitric oxide levels, with a concurrent increase in hepatic glutathione content compared to acetaminophen only group (131). The authors proposed that the therapeutic benefits of H2S are comparable to N-acetylcysteine in alleviating hepatotoxicity caused by acitaminophen.
Interestingly, H2S has been reported to cause vasoconstriction in intrahepatic vasculature during sepsis. Portal infusion of Na2S causes a small but significant decrease in sinusoidal diameter (132). Norris et al. also proposed the unique role of H2S in pathophysiological states by demonstrating that the metabolism of H2S is prioritised over the availability of oxygen during sepsis in rats (15). Understandably, sepsis causes multi-organ dysfunction and the roles of H2S in sepsis have been explored in many reviews (36, 39-41, 133-137).
KIDNEY
Both CSE and CBS are abundantly present in renal cells. Within the kidneys, H2S is produced from L-cysteine via the activity of aforementioned CSE and CBS. In diabetic nephropathy, one of the complications from diabetes mellitus, the production of endogenous H2S is greatly reduced. The reduction of H2S production is greater as the severity of type 2 diabetes mellitus progresses. Treatment with H2S donor has shown to improve the outcome suggesting the protective roles of H2S in kidney conditions. H2S was shown to rescue the mesangial cells by high glucose-induced damage, partly due to the reduced production of ROS (138). One other proposed mechanism was via the increased expression of heme oxygenase-1 (HO-1) in both mesangial and podocyte cells. HO-1 is an antioxidant enzyme and is the rate-limiting enzyme in the conversion of heme to carbon monoxide (CO) and bilirubin (139), which is well known to have direct anti-oxidant properties (140).
Chronic kidney disease is associated with significant reduction in plasma H2S concentration, diminished remnant kidney and liver tissue H2S-producing capacity and down regulation of the H2S-producing enzymes (141). The exact relationship between H2S and CKD is poorly defined. It has been recently reported that the decrease in H2S in CKD is related to the reduced gene expression of CBS and CSE, and decreased protein levels of both CBS and CSE in the liver, kidney, brain of a CKD rat model (142).
In hyperhomocysteinaemia found in end-stage renal failure, the level of endogenous H2S production is reduced. Sen et al. demonstrated the use of H2S donors to prevent hyperhomocysteinemia related renal injury (143). They reported that in mice with hyperhomocysteinaemia, there is an increase in superoxide production, but this is suppressed by H2S donors. Supplementation with H2S donors also causes an increase in intracellular SOD and CAT levels, which are believed to be inhibiting the oxidative stress related injury.
Compared with normal rats, obstructive injury decreased the plasma H2S level. CBS was dramatically reduced in the ureteral obstructed kidney, but another enzyme CSE was increased. The authors reported that NaHS inhibited renal fibrosis by reducing the production of collagen, extracellular matrix, and the expression of α-smooth muscle actin (144). This study suggests a therapeutic role of H2S in preventing chronic kidney failure.
In a pneumonia associated kidney injury, it was reported that NaHS reversed the fall in glomerular filtration rate and renal function. The proposed mechanism was that H2S led to decreased protein leakage, thereby preserving the endothelial barriers (36). Similarly, H2S has shown to be protective in renal ischaemic/reperfusion injury. Hunter et al. reported that H2S can reduce creatinine levels in a swine model of renal ischaemic/reperfusion model as well as achieve a shorter time in reaching levels of creatinine of less than 250 µM compared to the control (145). Another study reported that H2S decreases blood pressure and oxidative stress and improves renal haemodynamic and secretory function in spontaneously hypertensive rats, which are used as a model for human essential hypertension (53).
Interestingly, H2S plays an opposite role in nephrotoxicity. In animal models of gentamicin associated tubular necrosis, PAG is found to reduce creatinine levels. The authors proposed that the reduction of renal injury is partly due to the decreased H2S formation (146). In another report on a rat model of cisplatin and adriamycin nephrotoxicity, the PAG reduces the renal injury (147, 148). The exact roles of H2S in kidney diseases are still being discovered today and the mechanisms involved warrant further study.
SUMMARY
In summary, in healthy tissue, H2S generally has pro-apoptotic and anti-proliferative effects. However, it is also involved in a number of organ specific functions such as thermoregulation, modulating myocardial activity and broncho-dilation. This is further complicated by the concentration-dependent nature of the H2S action, and the fact that H2S synthesis itself is affected by the H2S level and the interaction between different gaseous mediators. Much like NO before, H2S has been successfully added to existing drug molecules without compromising their efficacy, and it is likely that H2S releasing modification of current drugs will be the main mode of H2S therapy in the future.
In ischaemia induced injurious settings, studies into central nervous system, heart, lungs, liver and kidney all show that administration of H2S donor reduces the extent of the ischaemic damage and improves organ function through a combination of reducing oxygen consumption and promoting cell survival. This could be valuable as a therapeutic tool as there are presently limited treatments available in established tissue ischaemia. Additionally, H2S also promotes angiogenesis, which should limit the extent of the secondary tissue injury and further promote healing from ischaemia. Theoretically, H2S donor could be a viable adjuvant treatment in myocardium ischaemia, cerebral vascular accidents and other organ ischaemia.
Conversely, as H2S promotes angiogenesis, drugs which reduce H2S level may be of use in conditions associated with undesired angiogenesis, examples of which include cancers and diabetic retinal neovascularisation.
Hydrogen sulphide s is also a vasodilator and negative inotrope which has been demonstrated to alleviate hypertension as well as its associated organ damage. While it might not add much to the myriad of anti-hypertensive drugs already available, its benefit in pulmonary and portal hypertension are likely to be of clinical interest as there are currently very limited treatment available for those.
Hydrogen sulphide generally has a protective effect in acute inflammation and oxidative stress from causes such as allergy and toxins. H2S administration has been shown to be beneficial in lungs, brains and livers against a large range of different offending agents. In the kidneys, inhibiting H2S seems to protect from nephrotoxicity. Clinically, patients with chemical poisoning may benefit from H2S administration of H2S in addition to other supportive treatment.
At higher levels, H2S does have anti-proliferative and apoptotic effects. H2S has been shown to cause cell death of both lung and liver cancer. However, as it is not known if that level of H2S exposure is safe for healthy cells, more studies need to be conducted before H2S can be considered as a possible anti-cancer chemical.
In chronic organ pathology, low H2S level has been observed in a number of different diseases of the brain, heart, lungs, liver and kidney. In the cardiovascular system, lungs, liver and kidney, H2S inhibits fibrosis and pathological remodeling. In neuro-degenerative conditions, H2S promotes cell survival by reducing oxidative stress and prevents apoptosis. However, a common problem between all such studies is the lack of consideration of the difference in the timeline; it is possible that H2S acutely alleviates the cell injury in those disease models, as it does in other acute insults, but will not have much benefit in the long term. This is arguably the most difficult aspect of H2S therapeutic potential to investigate, however, if successful, may result in disease modifying or curative treatment for a large number of diseases.
In conclusion, H2S donors have consistently shown to be beneficial in acute ischaemia, and may have an important role in acute organ injury from toxins and in chronic organ pathology. Considering the success in animal models andin vitro work, trials on patients are warranted to further explore the potential of H2S modulators in clinical medicine as well as to further elucidate the role of H2S in human pathophysiology.
Abbreviations: 3-MST: 3-mercaptopyruvate sulphurtransferase; ANG1: angiopoietin-1; AOAA: aminooxyacetic acid; BAX: Bcl-2 associated X protein; BKCa: calcium activated BK potassium channel; CAT: catalase; CBS: cystathionine β-synthase; CKD: chronic kidney disease; CLP: cecal ligation and puncture; CO: carbon monoxide; COPD: chronic obstructive pulmonary disease; COX-2: cyclooxygenase 2; CSE: cystathionine γ-lyase; D-GalN: D-galactosamine; ERK: extracellular-signal-regulated kinase; FEV1: forced expiratory volume in first second; GSH: glutathione; GSK3β: glycogen synthase kinase 3 beta; H2S: hydrogen sulphide; HO-1: heme oxygenase-1; HPV: hypoxic pulmonary vasoconstriction; ICAM: intercellular adhesion molecule; IL: interleukin; InsP3: inositol-1,4,5-trisphosphate; IP: ischaemic pre-conditioning; IPO: ischaemic post-conditioning; JNK: C-Jun N-terminal kinase; KATP: ATP-sensitive potassium channels; LDH: lactate dehydrogenase; LDL: low density lipoprotein; LPS: lipopolysaccharide; MAPK: mitogen-activated protein kinase; MCP: monocyte chemoattractant protein; MIP2: macrophage inhibitory protein 2; MIPa: macrophage inhibitory protein-a; MMP: matrix metalloproteinases; NF-κB: nuclear factor kappa beta; NMDA: N-methyl-D-aspartate receptor; NO: nitric oxide; Nrf2: NF-E2 p45-related factor 2; PAG: DL-propargyl glycine; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; ROS: reactive oxygen species; SOD: superoxide dismutase; SP: substance P; TGF-β: transforming growth factor-β; TNF-α: tumour necrosis factor-α; TRPV-1: transient receptor potential cation channel subfamily V member 1; UCP2: mitochondrial uncoupling protein 2; VEGF: vascular endothelial growth factor.
Conflict of interests: None declared.
REFERENCES
- Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002; 16: 1792-1798.
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 2012; 92: 791-896.
- Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 1989; 65: 1-21.
- Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol 1998; 76: 1-15.
- Wang R. Gasotransmitters: growing pains and joys. Trends Biochem Sci 2014; 39: 227-232.
- Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 2008; 40: 533-539.
- Predmore BL, Julian D, Cardounel AJ. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front Physiol 2011; 2: 104.
- Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology 2007; 232: 138-146.
- Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacologica Sinica 2007; 28: 1709-1716.
- Tang C, Li X, Du J. Hydrogen sulfide as a new endogenous gaseous transmitter in the cardiovascular system. Curr Vasc Pharmacol 2006; 4: 17-22.
- Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 2011; 51: 169-187.
- Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 2010; 285: 21903-21907.
- Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 2011; 439: 479-485.
- Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 2009; 1787: 856-863.
- Norris EJ, Culberson CR, Narasimhan S, Clemens MG. The liver as a central regulator of hydrogen sulfide. Shock 2011; 36: 242-250.
- Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol 2008; 295: H801-H806.
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008; 295: C849-868.
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996; 16: 1066-1071.
- Blachier F, Davila AM, Mimoun S, et al. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids 2010; 39: 335-347.
- Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci 2007; 28: 501-505.
- Cunha TM, Dal-Secco D, Verri WA, et al. Dual role of hydrogen sulfide in mechanical inflammatory hypernociception. Eur J Pharmacol 2008; 590: 127-135.
- Dal-Secco D, Cunha TM, Freitas A, et al. Hydrogen sulfide augments neutrophil migration through enhancement of adhesion molecule expression and prevention of CXCR2 internalization: role of ATP-sensitive potassium channels. J Immunol 2008; 181: 4287-4298.
- Hui Y, Du J, Tang C, Bin G, Jiang H. Changes in arterial hydrogen sulfide (H2S) content during septic shock and endotoxin shock in rats. J Infect 2003; 47: 155-160.
- Li L, Bhatia M, Zhu YZ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 2005; 19: 1196-1198.
- Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp Biochem Physiol B Biochem Mol Biol 2001; 129: 129-137.
- Modis K, Panopoulos P, Coletta C, Papapetropoulos A, Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol 2013; 86: 1311-1319.
- Munaron L, Avanzato D, Moccia F, Mancardi D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium 2013; 53: 77-84.
- Zoccali C, Catalano C, Rastelli S. Blood pressure control: hydrogen sulfide, a new gasotransmitter, takes stage. Nephrol Dial Transplant 2009; 24: 1394-1396.
- Matsunami M, Kirishi S, Okui T, Kawabata A. Hydrogen sulfide-induced colonic mucosal cytoprotection involves T-type calcium channel-dependent neuronal excitation in rats. J Physiol Pharmacol 2012; 63: 61-68.
- Shen Y, Guo W, Wang Z, Zhang Y, Zhong L, Zhu Y. Protective effects of hydrogen sulfide in hypoxic human umbilical vein endothelial cells: a possible mitochondria-dependent pathway. Int J Mol Sci 2013; 14: 13093-13108.
- Guo R, Lin J, Xu W, et al. Hydrogen sulfide attenuates doxorubicin-induced cardiotoxicity by inhibition of the p38 MAPK pathway in H9c2 cells. Int J Mol Med 2013; 31: 644-650.
- Uren JR, Ragin R, Chaykovsky M. Modulation of cysteine metabolism in mice - effects of propargylglycine and L-cyst(e)ine-degrading enzymes. Biochem Pharmacol 1978; 27: 2807-2814.
- Wallace JL, Flannigan KL, McKnight W, Wang L, Ferraz JG, Tuitt D. Pro-resolution, protective and anti-nociceptive effects of a cannabis extract in the rat gastrointestinal tract. J Physiol Pharmacol 2013; 64: 167-175.
- Wang T, Wang L, Zaidi SR, et al. Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol 2012; 47: 491-496.
- Whiteman M, Cheung NS, Zhu YZ, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 2005; 326: 794-798.
- Aslami H, Pulskens WP, Kuipers MT, et al. Hydrogen sulfide donor NaHS reduces organ injury in a rat model of pneumococcal pneumosepsis, associated with improved bio-energetic status. PLoS One 2013; 8: e63497.
- Lee M, McGeer E, Kodela R, Kashfi K, McGeer PL. NOSH-aspirin (NBS-1120), a novel nitric oxide and hydrogen sulfide releasing hybrid, attenuates neuroinflammation induced by microglial and astrocytic activation: a new candidate for treatment of neurodegenerative disorders. Glia 2013; 61: 1724-1734.
- Mok YY, Atan MS, Yoke Ping C, et al. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol 2004; 143: 881-889.
- Zhang H, Zhi L, Moochhala SM, Moore PK, Bhatia M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J Leukoc Biol 2007; 82: 894-905.
- Chen D, Pan H, Li C, Lan X, Liu B, Yang G. Effects of hydrogen sulfide on a rat model of sepsis-associated encephalopathy. J Huazhong Univ Sci Technolog Med Sci 2011; 31: 632-636.
- Ang SF, Moochhala SM, MacAry PA, Bhatia M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: involvement of substance P and ERK-NF-kappaB signaling. PLoS One 2011; 6: e24535.
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol 2005; 145: 141-144.
- Zhang J, Sio SW, Moochhala S, Bhatia M. Role of hydrogen sulfide in severe burn injury-induced inflammation in mice. Mol Med 2010; 16: 417-424.
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 2006; 20: 2118-2120.
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 2009; 146: 623-626.
- Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids 2011; 41: 113-121.
- Liu F, Chen DD, Sun X, et al. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014; 63: 1763-1778.
- Wagner CA. Hydrogen sulfide: a new gaseous signal molecule and blood pressure regulator. J Nephrol 2009; 22: 173-176.
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 2001; 20: 6008-6016.
- Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008; 322: 587-590.
- Snijder PM, Frenay AS, de Boer RA, et al. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates Angiotensin II-induced hypertensive heart disease in rats. Br J Pharmacol 2014; Jun 24. Epub ahead of print. doi: 10.1111/bph.12825
- Holwerda KM, Burke SD, Faas MM, et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol 2014; 25: 717-725.
- Ahmad FU, Sattar MA, Rathore HA, et al. Hydrogen sulphide and tempol treatments improve the blood pressure and renal excretory responses in spontaneously hypertensive rats. Ren Fail 2014; 36: 598-605.
- Sheng J, Shim W, Wei H, et al. Hydrogen sulphide suppresses human atrial fibroblast proliferation and transformation to myofibroblasts. J Cell Mol Med 2013; 17: 1345-1354.
- Wei H, Zhang G, Qiu S, et al. Hydrogen sulfide suppresses outward rectifier potassium currents in human pluripotent stem cell-derived cardiomyocytes. PLoS One 2012; 7: e50641.
- Xu M, Wu YM, Li Q, Liu S, He RR. Electrophysiological effects of hydrogen sulfide on human atrial fibers. Chin Med J (Engl) 2011; 124: 3455-3459.
- Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 2007; 76: 29-40.
- Givvimani S, Munjal C, Gargoum R, et al. Hydrogen sulfide mitigates transition from compensatory hypertrophy to heart failure. J Appl Physiol (1985) 2011; 110: 1093-1100.
- Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J 2006; 20: 553-555.
- Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 2004; 18: 1782-1784.
- Meng QH, Yang G, Yang W, Jiang B, Wu L, Wang R. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol 2007; 170: 1406-1414.
- Ran R, Du L, Zhang X, et al. Elevated hydrogen sulfide levels in vitreous body and plasma in patients with proliferative diabetic retinopathy. Retina 2014; 34: 2003-2009.
- Szabo C, Coletta C, Chao C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA 2013; 110: 12474-12479.
- Chai W, Wang Y, Lin JY, et al. Exogenous hydrogen sulfide protects against traumatic hemorrhagic shock via attenuation of oxidative stress. J Surg Res 2012; 176: 210-219.
- Gao C, Xu DQ, Gao CJ, et al. An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor kappaB inhibitor kinase/nuclear factor kappab inhibitor/nuclear factor-kappaB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull 2012; 35: 1029-1034.
- Snijder PM, de Boer RA, Bos EM, et al. Gaseous hydrogen sulfide protects against myocardial ischemia-reperfusion injury in mice partially independent from hypometabolism. PLoS One 2013; 8: e63291.
- Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. Am J Physiol Heart Circ Physiol 2010; 298: H1310-H1319.
- Hu Y, Chen X, Pan TT, et al. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch 2008; 455: 607-616.
- Hu LF, Pan TT, Neo KL, Yong QC, Bian JS. Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflugers Arch 2008; 455: 971-978.
- Huang YE, Tang ZH, Xie W, et al. Endogenous hydrogen sulfide mediates the cardioprotection induced by ischemic postconditioning in the early reperfusion phase. Exp Ther Med 2012; 4: 1117-1123.
- Mani S, Li H, Untereiner A, et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013; 127: 2523-2534.
- Wang Y, Zhao X, Jin H, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2009; 29: 173-179.
- Zhang H, Guo C, Zhang A, et al. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur J Pharmacol 2012; 697: 106-116.
- Ma B, Liang G, Zhang F, Chen Y, Zhang H. Effect of hydrogen sulfide on restenosis of peripheral arteries after angioplasty. Mol Med Rep 2012; 5: 1497-1502.
- Laggner H, Muellner MK, Schreier S, et al. Hydrogen sulphide: a novel physiological inhibitor of LDL atherogenic modification by HOCl. Free Radic Res 2007; 41: 741-747.
- Wang XH, Wang F, You SJ, et al. Dysregulation of cystathionine gamma-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell Signal 2013; 25: 2255-2262.
- Zhao ZZ, Wang Z, Li GH, et al. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011; 236: 169-176.
- Jiang HL, Wu HC, Li ZL, Geng B, Tang CS. Changes of the new gaseous transmitter H2S in patients with coronary heart disease. Di Yi Jun Yi Da Xue Xue Bao 2005; 25: 951-954.
- Kovacic D, Glavnik N, Marinsek M, et al. Total plasma sulfide in congestive heart failure. J Card Fail 2012; 18: 541-548.
- Perry MM, Hui CK, Whiteman M, et al. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am J Respir Cell Mol Biol 2011; 45: 746-752.
- Fang LP, Lin Q, Tang CS, Liu XM. Hydrogen sulfide suppresses migration, proliferation and myofibroblast transdifferentiation of human lung fibroblasts. Pulm Pharmacol Ther 2009; 22: 554-561.
- Fang LP, Lin Q, Tang CS, Liu XM. Hydrogen sulfide attenuates epithelial-mesenchymal transition of human alveolar epithelial cells. Pharmacol Res 2010; 61: 298-305.
- Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J 2007; 21: 247-255.
- Tesei A, Brigliadori G, Carloni S, et al. Organosulfur derivatives of the HDAC inhibitor valproic acid sensitize human lung cancer cell lines to apoptosis and to cisplatin cytotoxicity. J Cell Physiol 2012; 227: 3389-3396.
- Castro-Piedras I, Perez-Zoghbi JF. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol 2013; 591: 5999-6015.
- Huang J, Luo YL, Hao Y, et al. Cellular mechanism underlying hydrogen sulfide induced mouse tracheal smooth muscle relaxation: role of BK. Eur J Pharmacol 2014; 741: 55-63.
- Tian M, Wang Y, Lu YQ, Yan M, Jiang YH, Zhao DY. Correlation between serum H2S and pulmonary function in children with bronchial asthma. Mol Med Rep 2012; 6: 335-338.
- Zhang G, Wang P, Yang G, Cao Q, Wang R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol 2013; 182: 1188-1195.
- Chen YH, Wu R, Geng B, et al. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 2009; 45: 117-123.
- Chen YH, Wang PP, Wang XM, et al. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 2011; 53: 334-341.
- Chen YH, Yao WZ, Geng B, et al. Endogenous hydrogen sulfide in patients with COPD. Chest 2005; 128: 3205-3211.
- Saito J, Mackay AJ, Rossios C, et al. Sputum-to-serum hydrogen sulfide ratio in COPD. Thorax 2014; 69: 903-909.
- Han W, Dong Z, Dimitropoulou C, Su Y. Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxid Redox Signal 2011; 15: 2121-2134.
- Ariyaratnam P, Loubani M, Morice AH. Hydrogen sulphide vasodilates human pulmonary arteries: A possible role in pulmonary hypertension? Microvasc Res 2013; 90: 135-137.
- Olson KR, Whitfield NL, Bearden SE, et al. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol 2010; 298: R51-R60.
- Madden JA, Ahlf SB, Dantuma MW, Olson KR, Roerig DL. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J Appl Physiol (1985) 2012; 112: 411-418.
- Wei HL, Zhang CY, Jin HF, Tang CS, Du JB. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol Sin 2008; 29: 670-679.
- Sun L, Sun S, Li Y, et al. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: the role of homocysteine and hydrogen sulfide. Chin Med J (Engl) 2014; 127: 893-899.
- Luo L, Liu D, Tang C, et al. Sulfur dioxide upregulates the inhibited endogenous hydrogen sulfide pathway in rats with pulmonary hypertension induced by high pulmonary blood flow. Biochem Biophys Res Commun 2013; 433: 519-525.
- Xu DQ, GaoC, Niu W, et al. Sodium hydrosulfide alleviates lung inflammation and cell apoptosis following resuscitated hemorrhagic shock in rats. Acta Pharmacol Sin 2013; 34: 1515-1525.
- Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci 2008; 82: 1196-1202.
- Ning J, Mo L, Zhao H, et al. Sodium hydrosulphide alleviates remote lung injury following limb traumatic injury in rats. PLoS One 2013; 8: e59100.
- Wang JF, Li Y, Song JN, Pang HG. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int 2014; 64: 37-47.
- Zhou CF, Tang XQ. Hydrogen sulfide and nervous system regulation. Chin Med J (Engl) 2011; 124: 3576-3582.
- Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal 2011; 15: 405-419.
- Whiteman M, Armstrong JS, Chu SH, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 2004; 90: 765-768.
- Xie L, Hu LF, Teo XQ, et al. Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson’s disease rat model. PLoS One 2013; 8: e60200.
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 2004; 18: 1165-1167.
- Lu M, Zhao FF, Tang JJ, et al. The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxid Redox Signal 2012; 17: 849-859.
- Giuliani D, Ottani A, Zaffe D, et al. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol Learn Mem 2013; 104: 82-91.
- Li X, Mao XB, Hei RY, et al. Protective role of hydrogen sulfide against noise-induced cochlear damage: a chronic intracochlear infusion model. PLoS One 2011; 6: e26728.
- Kesherwani V, Nelson KS, Agrawal SK. Effect of sodium hydrosulphide after acute compression injury of spinal cord. Brain Res 2013; 1527: 222-229.
- Eto K, Asada T, Arima K, Makifuchi T, Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun 2002; 293: 1485-1488.
- Tang XQ, Yang CT, Chen J, et al. Effect of hydrogen sulphide on beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol Physiol 2008; 35: 180-186.
- Wei HJ, Xu JH, Li MH, et al. Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrκB pathway. Acta Pharmacol Sin 2014; 35: 707-715.
- Nagpure BV, Bian JS. Hydrogen sulfide inhibits A2A adenosine receptor agonist induced β-amyloid production in SH-SY5Y neuroblastoma cells via a cAMP dependent pathway. PLoS One 2014; 9: e88508.
- Kida K, Yamada M, Tokuda K, et al. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxid Redox Signal 2011; 15: 343-352.
- Gheibi S, Aboutaleb N, Khaksari M, et al. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci 2014; 54: 264-270.
- Lan A, Xu W, Zhang H, et al. Inhibition of ROS-activated p38MAPK pathway is involved in the protective effect of H2S against chemical hypoxia-induced inflammation in PC12 cells. Neurochem Res 2013; 38: 1454-1466.
- Zhang Q, Yuan L, Liu D, et al. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res 2014; 84: 32-44.
- Jang H, Oh MY, Kim YJ, et al. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res 2014; 92: 1520-1528.
- Guettler C, Kubes P. Hydrogen sulfide, another simple gas with complex biology. Am J Physiol Gastrointest Liver Physiol 2013; 304: G1066-G1069.
- Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology 2006; 131: 259-271.
- Fiorucci S, Antonelli E, Mencarelli A, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 2005; 42: 539-548.
- Wang C, Han J, Xiao L, Jin CE, Li DJ, Yang Z. Role of hydrogen sulfide in portal hypertension and esophagogastric junction vascular disease. World J Gastroenterol 2014; 20: 1079-1087.
- Fan HN, Wang HJ, Yang-Dan CR, et al. Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep 2013; 7: 247-253.
- Shirozu K, Tokuda K, Marutani E, Lefer D, Wang R, Ichinose F. Cystathionine gamma-lyase deficiency protects mice from galactosamine/lipopolysaccharide-induced acute liver failure. Antioxid Redox Signal 2014; 20: 204-216.
- Lu S, Gao Y, Huang X, Wang X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int J Oncol 2014; 44: 1259-1267.
- Bos EM, Snijder PM, Jekel H, et al. Beneficial effects of gaseous hydrogen sulfide in hepatic ischemia/reperfusion injury. Transpl Int 2012; 25: 897-908.
- Zhang Q, Fu H, Zhang H, et al. Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating Akt-GSK-3beta signaling and inhibiting mitochondrial permeability transition. PLoS One 2013; 8: e74422.
- Morsy MA, Ibrahim SA, Abdelwahab SA, Zedan MZ, Elbitar HI. Curative effects of hydrogen sulfide against acetaminophen-induced hepatotoxicity in mice. Life Sci 2010; 87: 692-698.
- Norris EJ, Feilen N, Nguyen NH, et al. Hydrogen sulfide modulates sinusoidal constriction and contributes to hepatic microcirculatory dysfunction during endotoxemia. Am J Physiol Gastrointest Liver Physiol 2013; 304: G1070-G1078.
- Ang SF, Sio SW, Moochhala SM, MacAry PA, Bhatia M. Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J Immunol 2011; 187: 4778-4787.
- Bodkin JV, Fernandes ES. TRPV1 and SP: key elements for sepsis outcome? Br J Pharmacol 2013; 170: 1279-1292.
- Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol 2005: 146: 498-505.
- Spiller F, Orrico MI, Nascimento DC, et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am J Respir Crit Care Med 2010; 182: 360-368.
- Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2007; 292: L960-L971.
- Yuan P, Xue H, Zhou L, et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant 2011; 26: 2119-2126.
- D’Araio E, Shaw N, Millward A, Demaine A, Whiteman M, Hodgkinson A. Hydrogen sulfide induces heme oxygenase-1 in human kidney cells. Acta Diabetologica 2014; 51: 155-157.
- Jansen T, Daiber A. Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front Pharmacol 2012; 3: 30.
- Aminzadeh MA, Vaziri ND. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transplant 2012; 27: 498-504.
- Perna AF, Ingrosso D. Low hydrogen sulphide and chronic kidney disease: a dangerous liaison. Nephrol Dial Transplant 2012; 27: 486-493.
- Sen U, Basu P, Abe OA, et al. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol 2009; 297: F410-F419.
- Song K, Wang F, Li Q, et al. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int 2013; 85:1318-1329.
- Hunter JP, Hosgood SA, Patel M, Rose R, Read K, Nicholson ML. Effects of hydrogen sulphide in an experimental model of renal ischaemia-reperfusion injury. Br J Surg 2012; 99: 1665-1671.
- Francescato HD, Chierice JR, Marin EC, et al. Effect of endogenous hydrogen sulfide inhibition on structural and functional renal disturbances induced by gentamicin. Braz J Med Biol Res 2012; 45: 244-249.
- Della Coletta Francescato H, Cunha FQ, Costa RS, et al. Inhibition of hydrogen sulphide formation reduces cisplatin-induced renal damage. Nephrol Dial Transplant 2011; 26: 479-488.
- Francescato HD, Marin EC, Cunha F de Q, Costa RS, Silva CG, Coimbra TM. Role of endogenous hydrogen sulfide on renal damage induced by adriamycin injection. Arch Toxicol 2011; 85: 1597-1606.
A c c e p t e d : December 15, 2015
Dr Kaizhi Lu, Department of Anesthesiology, Southwest Hospital, Third Military Medical University, 30 Gaotanyan Road, Chongqing, China. e-mail: lukaizhi@163.net