INCREASED GENE EXPRESSION OF CATECHOLAMINE-SYNTHESIZING ENZYMES IN ADRENAL GLANDS CONTRIBUTES TO HIGH CIRCULATING CATECHOLAMINES IN PIGS WITH TACHYCARDIA-INDUCED CARDIOMYOPATHY
INTRODUCTION
Neurohormonal activation is fundamental in the complex pathophysiology of heart failure (HF) (1, 2). The prominent feature of neurohormonal activation in HF is the augmented sympathetic drive, which is reflected in increased levels of catecholamines adrenaline (ADR) and noradrenaline (NOR) in peripheral blood (3). Although high levels of circulating catecholamines have been reported in numerous studies performed both in patients with HF (3, 4) and animals with experimental HF (5), the origin of these hormones in the circulation is equivocal (6).
Circulating catecholamines derive mainly from the sympathetic nerve endings (releasing NOR) and from the adrenal medulla (releasing ADR and NOR) (7). ADR and NOR are synthesized from tyrosine by three enzymes: tyrosine hydroxylase (TH), aromatic L-amino acid decarboxylase (AAAD) and dopamine-β-hydroxylase (DBH). The final step in the synthesis of ADR is catalyzed by phenylethanolamine N-methyltransferase (PNMT) (7) (Fig. 1A). In rats, during stress, the increase in plasma adrenalin is derived almost completely from the adrenal medulla, whereas most plasma noradrenalin (about 70%) is derived from sympathetic nerves (8).
Although many studies describe changes of plasma catecholamine levels under different stress conditions, this knowledge is mainly based on data from rodents (7). Due to the fact that there are significant differences in anatomy and physiology between rodents and large mammals (5), we measured the mRNA expression of key enzymes synthesising catecholamines in adrenal homogenates obtained from pigs at subsequent stages of systolic HF and in healthy animals. The adrenal mRNA expression of these enzymes was then related to the plasma levels of circulating catecholamines (ADR and NOR).
MATERIALS AND METHODS
Animals
In order to investigate the changes in the expression of key enzymes synthesizing catecholamines in adrenal homogenates during the natural history of HF, we used an experimental porcine model of chronic systolic non-ischaemic HF, i.e. tachycardia-induced cardiomyopathy (TIC), which was previously established by our group (9). The study was performed on 24 adult homogenous male sibling Polish Great White pigs. All animals received animal care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23, revised in 1996). All experiments were performed in compliance with the guidelines of the Bioethical Committee of the Wroclaw University of Environmental and Life Sciences for experimenting on animals.
Experimental procedures
Briefly, single-chamber pacemakers (SENSIA SESR01, Medtronic, Poland) were implanted in all 24 pigs, with a bipolar screw-in pacing transvenous lead (CAPSUREFIX NOVUS 58 cm, Medtronic, Poland) positioned at the apex of the right ventricle (RV) of the myocardium. The pacemakers were programmed for sequential RV pacing at 170 bpm in 18 randomly chosen animals, whereas 6 pigs served as sham-operated controls. Clinical examinations and echocardiography assessments confirmed that the RV-paced animals developed symptomatic systolic HF and were presented for euthanasia at subsequent predefined stages of HF. The development of HF was accompanied by a progressive left ventricle systolic and diastolic dysfunction (9, 10), as well as an increase in circulating BNP, ADR and NOR (9, 11).
Tissue sections from porcine adrenals were taken during autopsy and immediately frozen in liquid nitrogen. The sections for histology were fixed in 4% paraformaldehyde buffered solution and embedded in paraffine for hematoxylin-eosin, modified Hopsu and Makinen’s (noradrenaline chromaffin cells) (12) and phenylethanolamine N-methyltransferase (PMNT) (adrenalin chromaffin cells) staining.
Histological analysis was performed using the Nis-Elements AR software (Nikon Instruments Inc; Poland). Adrenalin storing cells were stained immunohistochemically with a rabbit anti-PNMT polyclonal serum (Bioss Antibodies, Poland) using the Dako Cytomatation LSAB + System-HRP (Dako, Poland). The sections were counterstained using Mayer’s haematoxylin (Sigma-Aldrich, Poland).
Total RNA was prepared from 30-mg adrenal samples using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Poland) according to the manufacturer’s instructions. The RNA was reverse-transcribed using a SuperScript III First-Strand Synthesis System (Invitrogen, Poland).
We analyzed the mRNA expression in the adrenals of the following genes: TH (AY044828.1), AAAD (EF091886), DBH (DQ139273.1) and PNMT (DQ917626). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ABO38240) gene was chosen as a reference. Primer sequences were designed using a Molecular Beacon Software (Bio-Rad, Poland): TH:
forward-ACAGAACGGCGAGGTGAAGG,
reverse-CGAAGTAGACGGGCTGGTAGG; AAAD:
forward-TTCAGGATGTACGGAGTCAAGG,
reverse-GCGAGCAGATGGCGAACC; DBH:
forward-CACATACAACACGGAAGACC,
reverse-TGCTCGCTGTCAAACCTG; PNMT:
forward-CGAGGGCAAGGGTGAATCC,
reverse-CCAGGCAGAAGGCAGAGAC; GAPDH:
forward-TCACTGCCACCCAGAAGA,
reverse-TACCAGGAAATGAGCTTGAC.
The relative transcript levels were analyzed by quantitative real-time PCR using the iQ5 Optical System (Bio-Rad) with the EvaGreen SuperMix (Bio-Rad). The reactions were performed under the following conditions: an initial denaturation at 94°C for 30 s, 35 cycles at 94°C for 10 s and at 58°C for 15 s. All measurements were performed in duplicate. The Pfaffl method was used to determine the relative expression (13). mRNA expression was presented in arbitrary units (AU), where the adrenal sample from one of the control pigs was chosen as the calibrator, and its mean mRNA expression was considered as 1.
Statistical analysis
Continuous variables were presented as arithmetic means ± standard errors of a mean (S.E.M.). All molecular assessments were performed in duplicate. Spearman’s rank correlation coefficients were used for all correlation analyses. P<0.05 was considered as statistically significant. Statistical analyses were performed using the Polish version of Statistica 9.1 (StatSoft, Poland).
RESULTS
The progression of HF was associated with the chromaffin cell enlargement (R=0.58, P<0.05). Strong hyperaemia in the medulla of animals with HF was observed. Capillaries contained a large amount of erythrocytes. In some cases, the presence of hemosyderin was noted (Fig. 1B and 1C). While most of the chromaffin cells in the control animals contained chromophilic granules, the HF animals showed signs of a prolonged release of secretory granules (Fig. 1). The amount of both adrenalin and noradrenalin storing cells did not change along with the HF progression.
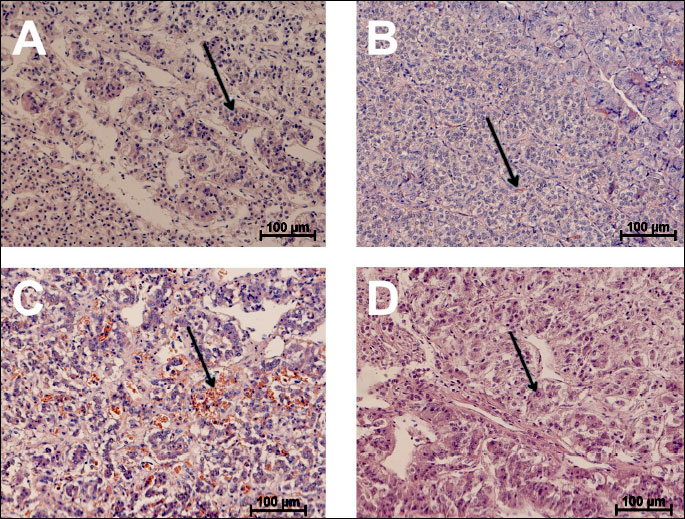
Gene expression of key enzymes synthesizing catecholamines in adrenal homogenates was investigated during the natural history of HF in male pigs with mild (6 ± 2 weeks of pacing, n=8), moderate (11 ± 3 weeks of pacing, n=6), severe HF (18 ± 4 weeks of pacing, n=4) and 6 sham-operated controls.
Pigs with severe HF demonstrated an increased expression of TH and DBH (but neither AAAD nor PNMT) compared to animals with milder HF and healthy controls (all P<0.05) (Fig. 2B).
The increased adrenal mRNA expression of TH and DBH (Fig. 2A) was found in pigs with the most severe HF compared to animals with milder HF and healthy controls, as evidenced by a poor clinical status, reduced LVEF (all P<0.001) and high plasma BNP (all P<0.01).
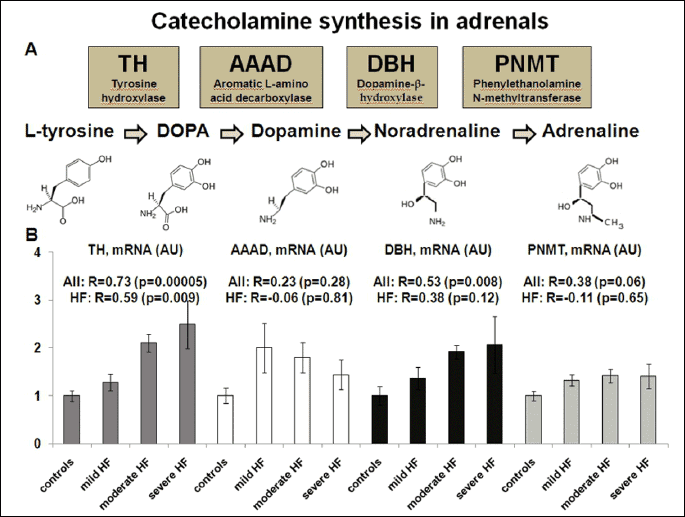
In univariable models, there were positive relationships between the increased adrenal mRNA expression of TH and DBH, and high circulating ADR and NOR levels (TH-ADR: R=0.54, P=0.02; TH-NOR: R=0.72, P<0001; DBH-ADR: R=0.54, P=0.02; DBH-NOR: R=0.47, P=0.04). The relationships between the adrenal mRNA expression of TH and DBH, and circulating NOR (but not ADR) remained statistically significant, also when adjusted for LVEF and plasma BNP (TH-NOR: β=0.71, P<0.001; DBH-NOR: β=0.52, P=0.03).
DISCUSSION
Based on the current state of knowledge, the origin of augmented sympathetic nervous activity during the progression of HF (13) remains unclear. To the best of our knowledge, this report is the first to show a link between the gene expression of catecholamine-synthesising enzymes in the adrenals and circulating catecholamines in large animals with HF. It is also worth noting that the previous knowledge on the autonomic status in HF was derived mainly from animals involved in short-term experiments lasting hours, whereas long-term studies were scarce (14). An experimental porcine model of tachycardia-induced cardiomyopathy used in our study is based on a relatively slow, long-term RV pacing and meets these expectations, allowing an insight directly into the subsequent changes occurring in the gene expression in different tissues, including the adrenals.
Importantly, we found a positive relationship between an increased mRNA expression of TH and DBH in the adrenals and high circulating ADR and NOR levels. However, such an association did not occur in the case of the remaining two enzymes involved in the synthesis of catecholamines (AAAD, PNMT). TH is the major rate-limiting enzyme in the catecholamine biosynthetic pathway, and changes in its expression constitute the main mechanism of the catecholaminergic system response to stress. Repeated or chronic exposure to stress was also found to increase the expression of DBH (7). A strong correlation of TH expression in the adrenals and NOR in plasma suggests that porcine adrenals are the main source of this catecholamine during the development of HF. This result contrasts with the well established knowledge (based on the rodent data) that when under stress, the sympathetic nerve terminals release NOR (7). The relative proportion of ADR and NOR-producing chromaffin cells in the adrenals was shown to be species specific (15). In the rat, A-cells (ADR-producing cells) constitute about 80%, and NA-cells (NOR-producing cells) about 20% of the parenchyma of the adrenal medulla, whereas in pigs the A- and NA-cells are equally distributed (16). This difference could also partially explain why we did not observe an increase in the expression of PMNT in pigs, while it occurred in rodents (17). What is more, regulation of other hypothalamo-pituitary-adrenocortical axis dependent (9) pathway has been already shown to be different in porcine and rodent adrenocortical cells (18).
Despite PMNT being considered a second “rate-limiting” enzyme for ADR synthesis in rodents (7), no changes in it on a RNA and protein level were noted in our pig model. Therefore, it can be deducted that in big mammals, PMNT may not be involved in the regulation of catecholamine synthesis. Although it is generally accepted that splenic contraction is mediated locally via humoral stimulation of splenic adrenoreceptors, Bakovic et al. has demonstrated that the spleen can represent a constitutive part of the sympathetic nervous system under stress (19).
In conclusion, our results indicate that the increased adrenal mRNA expression of TH and DBH in animals with HF significantly contributes to the circulating pool of catecholamines, regardless of HF severity. In contrast to rodents, the adrenals seems to be the main source of noradrenalin in pigs during the development of HF. Heart failure seems to induce the chromaffin cell hypertrophy to match the increased catecholamine production. It has become clear in recent years that the adrenal medulla and sympathetic nervous system can be regulated differently (20). Therefore, from a therapeutic perspective, more effort should be put into inhibiting the activation of the adrenals.
Acknowledgments: This publication is part of the “WROVASC - Integrated Cardiovascular Centre” project co-financed by the European Regional Development Fund within the 2007-2013 Innovative Economy Operational Program realized in the Regional Specialist Hospital, Research and Development Centre in Wroclaw.
Conflict of interest: None declared.
REFERENCES
- Chatterjee K. Pathophysiology of systolic and diastolic heart failure. Med Clin North Am 2012; 96: 891-899.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure - pathophysiological links. Cardiovasc Res 2006; 70: 434-445.
- Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311: 819-823.
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986; 73: 615-621.
- Dixon JA, Spinale FG. Large animal models of heart failure: A critical link in the translation of basic science to clinical practice. Circ Heart Fail 2009; 2: 262-271.
- Leenen FH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res 2007; 101: 221-223.
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetics approaches. Physiol Rev 2009; 89: 535-606.
- Kvetnansky R, Weise VK, Thoa NB, Kopin IJ. Effects of chronic guanethidine treatment and adrenal medullectomy on plasma levels of catecholamines and corticosterone in forcibly immobilized rats. J Pharmacol Exp Ther 1979; 209: 287-291.
- Kiczak L, Tomaszek A, Bania J, et al. Expression and complex formation of MMP9, MMP2, NGAL and TIMP1 in porcine myocardium but not in skeletal muscles in male pigs with systolic heart failure. Biomed Res Int 2013; 2013: 283856. doi: 10.1155/2013/283856.
- Kiczak L, Tomaszek A, Bania J, et al. Matrix metalloproteinase 9/neutrophil gelatinase associated lipocalin/tissue inhibitor of metalloproteinasess type 1 complexes are localized within cardiomyocytes and serve as a reservoir of active metalloproteinase in porcine female myocardium. J Physiol Pharmacol. 2014; 65: 365-375.
- Tomaszek A, Kiczak L, Bania J, et al. Changes in parasympathetic system in medulla oblongata in male pigs in the course of tachycardia-induced cardiomyopathy. Auton Neurosci 2013; 177: 253-259.
- Khan MB, Lee BR, Kamitani T. A simple and sensitive method for the demonstration of norepinephrine-storing adrenomedullary chromaffin cells. Histochem Cell Biol 2012; 138: 155-165.
- Pfaffl MW. A new mathematical model for quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: 2000-2007.
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 2010; 90: 513-557.
- Aunis D, Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand 1999; 167: 89-97.
- Verhofstad AA, Coupland RE, Colenbrander B. Immunohistochemical and biochemical analysis of the development of the noradrenaline- and adrenaline-storing cells in the adrenal medulla of the rat and pig. Arch Histol Cytol 1989; 52 (Suppl): 351-360.
- Schneider J, Lother A, Hein L, Gilsbach R. Chronic cardiac pressure overload induces adrenal medulla hypertrophy and increased catecholamine synthesis. Basic Res Cardiol 2011; 106: 591-602.
- Kaminska B, Czerwinska J, Wojciechowicz B, Nynca A, Ciereszko R. Genistein and daidzein affect in vitro steroidogenesis but not gene expression of steroidogenic enzymes in adrenals of pigs. J Physiol Pharmacol 2014; 65: 127-133.
- Bakovic D, Pivac N, Zubin Maslov P, Breskovic T, Damonja G, Dujic Z. Spleen volume changes during adrenergic stimulation with low doses of epinephrine. J Physiol Pharmacol. 2013; 64: 649-655.
- Sabban EL, Nankova BB, Serova LI, Kvetnansky R, Liu X. Molecular regulation of gene expression of catecholamine biosynthetic enzymes by stress: sympathetic ganglia versus adrenal medulla. Ann NY Acad Sci 2004; 1018: 370-377.
A c c e p t e d : February 9, 2015