AQUAGLYCEROPORINS IN THE KIDNEY:
PRESENT STATE OF KNOWLEDGE AND PROSPECTS
West Pomeranian University of Technology, Szczecin, Poland
INTRODUCTION
The discovery of the first aquaporin (AQP1) in the membrane of red blood cells by Peter Agre and colleagues in the eighties, awarded the Nobel Prize in 2003, has allowed to explain the then unknown mechanism of rapid water flow across cell membranes (1). Understanding this fundamental process underlying each life shed new light on the mechanisms regulating the body’s water balance and made it possible to clarify a number of pathophysiological changes in water transport across biological membranes of cells of many organs. To date, more than 300 different aquaporins have been discovered, and their presence has been confirmed in all phylogenetic kingdoms. In mammals thirteen isoforms of this protein have been identified (AQP0–AQP12), which are found in different cell types of the body (2).
Aquaporins are integral, hydrophobic, transmembrane proteins with a molecular weight from 27 kDa (AQP8) to 37 kDa (AQP7), which polypeptide chains do not exceed 300 amino acids (3). Structural results of several aquaporins have established that this protein channels share a common structural features. The functional AQP unit is a homotetramer. The each single monomer of this protein has six highly hydrophobic transmembrane (TM) domains of alpha-helix structure, which both carboxylic and amine termini are located in the cytosol (4, 5). The membrane domains are connected by five intracellular (ICL) and extracellural (ECL) loops: A, B, C, D and E (6). Two of them, loop B and E, contain a highly conserved motif of three amino acids asparagine - proline - alanine (NPA). These two loops pass through the cell membrane in opposite directions relative to each other and form a characteristic pore of selective permeability. Hemipores - other term denoting loops B and E, are surrounded by transmembrane domains, forming clockwise scaffold, its shape resembling an hourglass. Nearly 2 – 3 × 109 water molecules per second is transported through a single aquaporin channel formed in this manner (7, 8). The flow of water through the channel may occur in either direction, depending on the osmotic pressure on both sides of the cell membrane (4, 9, 10). Membranes of AQP-expressing cells contain several thousand, or more, AQPs per µm2. It is worth mentioning, that ion channels per µm2 is ten or fewer less (11).
Since the discovery of the first aquaporin, a number of studies carried out in subsequent years have proved that aquaporins can transport not only water molecules but also other small molecules, i.e., glycerol, urea and ammonia. Therefore, two main groups of aquaporins are distinguished: (i) classical aquaporins, permeable only to water molecules (AQP0, AQP1, AQP2, AQP4, AQP5) and (ii) aquaglyceroporins, permeable for other small molecules (AQP3, AQP7, AQP9, AQP10) (12, 13). In addition, a third group has been recently isolated, the so-called unorthodox aquaporins (AQP11 and AQP12), which share low homology with other proteins from this family (2, 9). According to the most recent literature, AQP6 and AQP8 are classified as unorthodox auquaporins; however, due to their ability to transport other small molecules, the present review will discuss them along with the rest of aquaglyceroporins located in the kidneys (14).
It is generally known that kidney play an important role in regulating the water-electrolyte balance, and maintaining the acid-base balance of the body (15, 16). Given this fact, of 13 known isoforms of aquaporins, up to nine isoforms of these proteins are localized in tubular epithelial cells (Fig. 1). AQP1 is localized in the proximal tubule epithelial cells, the thin descending limb of loop of Henle and descending straight arterioles and constitute nearly 1% of all renal cortex proteins. In human kidney, AQP1 is localized also in the epithelium of the glomerular capillaries, mesangial cells and peritubular capillaries (17). AQP2, AQP3 and AQP4 are localized in the epithelial cells of the collecting duct (CD) and connecting tubule (CNT) (2, 18). AQP7 expression was observed in the apical membrane, while AQP8 in the cytosol of proximal tubule cells and collecting ducts. AQP6 is localized in the membrane of intracellular vesicles of the dark cells type A in the connecting tubule and collecting ducts, while AQP5 in the apical membrane and cytosol of the dark cells type B (17, 19, 20). In addition, the expression of AQP5 was observed in cortical epithelial cells of the distal and connecting tubules (19). The presence of AQP11 was detected in the membrane of the endoplasmic reticulum of renal proximal tubules (17, 21).
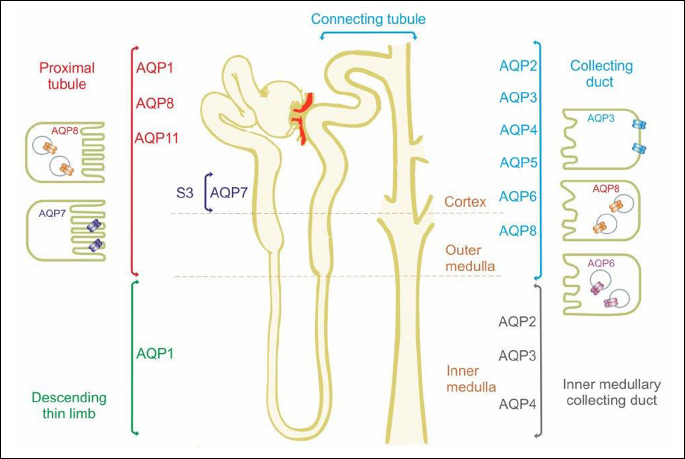
The role of classical aquaporins in the renal regulation of water balance and excretion of concentrated urine is reasonably well characterized, particularly with respect to AQP1 and AQP2. Unfortunately, the role of other aquaporins in the kidneys with „additional“ transport capacities remains a mystery to this day. Therefore, the aim of this review was to collect all available information in the literature on renal AQPs (AQP3, AQP6, AQP7 and AQP8) located in the kidneys and discuss the potential role of these proteins in the renal regulation of the homeostasis. The summary presented in this review on our current knowledge and prospect on renal aquaglyceroporin will hopefully stimulate future research in both basic and clinical fields.
AQUAGLYCEROPORINS IN THE KIDNEY
Transport via AQP3 in the renal collecting ducts
AQP3 cloned initially from rat kidney facilitates water, glycerol and ammonia transport (22-25). Ammonia permeability of this protein is being widely discussed at the moment. Apart from the kidney, AQP3 is also expressed in erythrocytes and identified as a blood cell type antigen, which leads to the identification of AQP3 null humans (26). The protein has also been found in the skin, lungs, cornea, oesophagus, stomach, liver, colon, articular cartilage, intervertebral disc and sperm (5, 27). In the skin, AQP3 is located in the basal layer of proliferating keratinocytes, where it enables glycerol transport and is therefore not only an important factor of moisture retention of the skin but also maintain an appropriate level of cellular glycerol for cell energy and metabolic needs (28-30). In the lungs, AQP3 is expressed in the epithelium lining, where along with AQP4 most probably enables the passage of water into the capillaries of the airways (5, 31). In adipocytes AQP3 is located in plasma membrane and towards lipid droplets and contributes to glycerol efflux from fat depots (32). In the stratified epithelium of the stomach, AQP3 provides water to cells facing harsh condition, while in the stratified epithelium of distal colon, the protein enables water absorption from intestine and colonic fluid transport. In the stratified epithelium of the oesophagus, AQP3 plays a role in the maintenance of intracellular osmolality and cell volume regulation (CVR) to water deprived cells (33, 34). In the musculoskeletal system AQP3 is involved in cell swelling during mechanistic load (35). AQP3 also plays an important role in regeneration and tumor progression (36). The impact of AQP3 in cell proliferation was also observed in the skin, colon and cornea (37, 38). According to Verkman (11), it is the transport glycerol by AQP3 which seems to be a key factor in this process. Namely, under AQP3 deficiency and the related impaired lipid biosynthesis, reduced glycerol metabolism and ATP, impaired MAPK signaling, reduced cell proliferation is observed.
In the kidney AQP3 is localized in the basal membrane of principal cells in the connecting tubule and collecting duct, where together with AQP4 it forms the main outlet of water from these cells (20) (Table 1, Fig. 1). AQP3 is expressed along entire CD in the cortex, outer, and inner medulla, with a maximum in the outer half of the inner medulla, and almost no expression at the tip of the papilla (39, 40). This protein has also been found in the basilar cell layer of the ureter and bladder urothelium (41). Contrary to AQP2, whose expression and location is linked with vasopressin (AVP) stimulation, there is no evidence for short-term regulation of AQP3 by AVP (14, 17, 18). Undoubtedly, this is related to the fact that in the cytoplasm there was no significant amount of AQP3, which could be possibly transported and then fused with basolateral membrane. However, during water deprivation and prolonged increased level of AVP, increased AQP3 protein and mRNA levels both have been found in the cortex and medulla (39, 42). In the production of concentrated urine, AQP3 plays an important role. AQP3-knockout mice have an increased urine volume, lower urine osmolality and reduced osmotic water permeability of the basolateral membrane of the cortical CD (44). However, under water deprivation or after administration of DDAVP (1-desamino-8-D-arginine-vasopressin), a slight increase in urine osmolality is visible in AQP3 knockouts, which is most probably related to an increased expression of AQP2 or AQP4. These factors cause that symptoms in mice suffering from nephrogenic diabetes insipidus resulting from AQP3 deletion are less severe (10, 43).
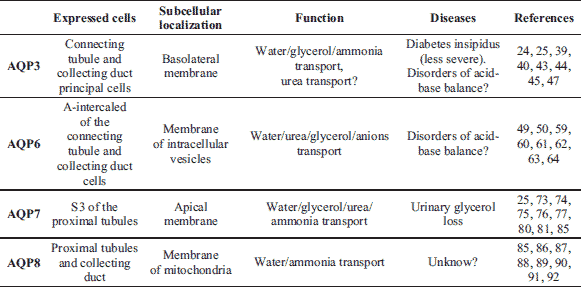
Despite the fact that transport glycerol by AQP3 in other tissues plays a very important role in many of the previously described process, the importance of glycerol transporting function of this protein in the kidney has not been clarified so far. In fact, there is only one paper in the literature in which the authors report hypotriglyceridemia in mice lacking AQP3 there (43). In what way, however, plasma triglyceride levels are reduced, and what is the role of glycerol transport by AQP3 in this process - this still has not been explained.
In adult humans, an average of 25 g of urea is removed with urine daily, and urea transport in the kidney is vital for the urinary concentrating mechanism (3). However, does AQP3 play an important role in these processes? According to some authors, the protein, - besides water and glycerol, - is also permeable to urea. However, the AQP3 ability to transport urea is questionable and is still debated. Early works on AQP3 by Ishibashi et al. (24) suggest that rat AQP3 is urea permeable. The authors observed that expression of AQP3 in Xenopus oocytes increased urea uptake twofold after a 30-minute incubation of oocytes with radio-labelled 165 mM urea. Meinlid et al. (44), on the other hand, found no urea transport using similar oocyte volumetric techniques. It is noteworthy, however, that these authors used much lower concentration of urea (20 mM urea) and recorded changes in permeability after about 1 minute. The results obtained in this way do not, however, seem to preclude the transport of urea by AQP3. According to Kitchen et al. (3), results of both sited papers may indicate that AQP3 urea transport is so slow that at 20 mM it does not induced large enough volume changes to be measured on the timescale of an oocyte swelling experiment or that transport is non-linear. The authors stress, however, that non-linear transport of urea seems unlikely given the linear nature of water and glycerol transport by AQP3. Interesting data on potential urea permeability of AQP3 result from the research carried out by Zhao et al. (40). This author’s demonstrated that mice with AQP3 deletion and nephrogenic diabetes insipidus are able to concentrate urine after an intraperitoneal urea infusion, but at the expense of decreased excretion of other molecules. The mechanisms of this phenomenon are still not explained, however, the authors suggest that the AQP3 permeability also for urea is probably a decisive factor in this process.
Despite the fact that the literature brings now much evidence for ammonia permeability of AQP3, AQP7, AQP8, AQP9 and AQP10, the role of this additional transport is still unclear. AQP3, like other ammonia-permeable AQPs, has a distinct ar/R region, a major size-limiting filter. This ar/R motif is localized about 7Å toward the extracellular side from the NPA region (25). It is interesting that mammalian membrane proteins of the Rhesus-type (Rh), known mainly as group antigens of the red blood cells, also function as ammonia transporters. It is also interesting that ammonia-permeable aquaporins and the Rh proteins family (RhAG, RhBG and RhCG) are co-localized in the tissues involved in ammonia transport (45), including kidneys, where AQP3 is co-localized with RhBG and RhBC (46, 47). Localization of these proteins in the basolateral membrane of the CD cells may suggest their mutual coordination during ammonia transport. According to Litman et al. (25), AQP3 could play a direct role in the final steps of acid secretion via NH4+, which may also be concluded from studies carried out by Nowik et al. (48) and Ma et al. (43). These experiments indicated that in a well-established metabolic acidosis rodent model induced by NH4Cl in drinking water, AQP3 was significantly upregulated (48). On the other hand, AQP3 knockout mice, in addition to the loss of ability to concentrate urine, showed tubular acidosis (43).
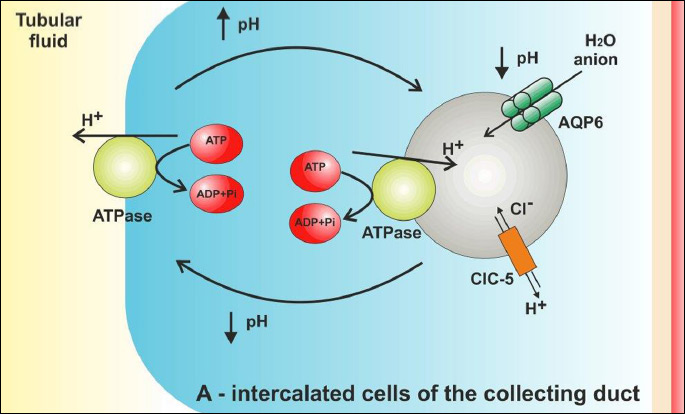
AQP6 in the regulation of pH in intracellular vesicles
Aquaporin 6 is expressed primarily in the membrane of the intracellular vesicles in A-intercalated cells in the CNT and CD (Table 1, Fig. 1) (49). Originally AQP6 was identified as a homolog of AQP0 and AQP2 (50). Recently AQP6 has also been observed in parotid gland acinar cells, in the inner ear, in the cerebellum, at synaptic vesicles and in neural retina (51-56). In addition, in the hepatocytes of human liver mRNA of AQP6 were detected (57). Unlike other aquaporins, AQP6 exhibits low water permeability (49). Moreover, this protein enables transport of urea, glycerol and anionic ions, especially nitrate. (58, 59). The anions permeability of this protein is increased at last 5-fold by exposure to low pH. Permeability changes of AQP6 for anions are also observed in response to Hg2+ activation (49). The expression of AQP6 in acid-secreting intercalated cells of kidney collecting ducts, suggest that AQP6 might be involved in the renal acid-base regulation (60). However, the role of this transport in the renal tubules is still not fully clear. It is widely known, that the intercalated cells are characterized by a rich inclusion of mitochondria, which provide energy for the cells necessary for proper functioning (61). In these cells are also localized intracellular vesicles containing H+ ATPase to transport proton and CIC- 5 chloride channel (62). At the same vesicles is also localized AQP6 (63). In spite of that several studies have demonstrated that the H+ ATPase is shuttled from the cytoplasmic vesicles to the apical plasma membrane in response to acid-base changes, in cells membrane of the intercalated cells were not found presence of AQP6 (63-67). Lack of AQP6 in the apical plasma membrane, indicating that this protein must function exclusively at the intracellular sites. Additionally, according to Beitz et al. (60), constitutive expression of AQP6 in the apical plasma is toxic for the cells and the prolonged expression of this protein in plasma membrane lead to cell death. The results and observations of all the cited authors explicitly indicate that AQP6 functionally interact with H+ ATPase in the vesicles of A-intercalated cells to regulated intra-vesicle pH (Fig. 2). The mechanism of this process, however, has not been explained yet. According to Ikeda et al. (63), AQP6 may enable anion transport into the intracellular vesicles, which is necessary to maintain electroneutrality across the membranes. These authors suggest that AQP6 may also be involved in the regulation of H+ ATPase, since - as it was demonstrated - nitrate in renal collecting duct inhibits this pomp (68). According to Promeneur et al. (62), changes in AQP6 permeability resulting from lowered pH may also contribute to vesicle swelling and membrane fusion during exocytosis. It should be stressed that rapid activation of AQP6 is accompanied by a selective chloride conductance (49). This was confirmed by Gunther et al. (69), who demonstrate that the CIC-5 chloride channel is important for endocytosis, probably by providing an electrical shunt for the H+ ATPase.
Transport via AQP7 in proximal straight tubule
Aquaporin 7 is an aquaglyceroporin that is abundantly expressed in the kidney, adipocytes, and testis (24, 42, 57, 70). Although AQP7 was found also in ovarian granulose cells, capillaries of adipose tissue, brain, cardiac and striated muscle (71-73). AQP7 is permeable mainly for water and glycerol. Placing this protein in oocyte membranes increased their osmotic water permeability tenfold, whereas transport of glycerol increased fivefold (42). Participation of AQP7 in the flow of glycerol is particularly important in the metabolism of adipose tissue (74). Recent studies demonstrated, that reduced plasma membrane glycerol permeability, resulting in increased in fat mass and adipocyte hypertrophy (75, 76). The role of AQP7 in the testis has not been fully explained so far. Experiments carried out in recent years show that this protein is expressed in tail of spermatids and spermatozoa, where it is most probably involved in the maintenance of sperm motility (77, 78). In ovarian granulose cells AQP7 enables transcellular water flow in folliculogenesis. Expression of AQP7 in cardiac and striated muscle is still unclear, although, according to Skowronski et al. (72), this protein could serve as an entry and/or exit pathway for glycerol or other solutes/metabolites.
In the kidney AQP7 is localized in the apical brush border of the S3 segment of the proximal tubule (Table 1, Fig. 1) (79, 80). In this part of the nephron, AQP1, which is the major route for water flow in the proximal tubule, is also abundantly expressed. In experiments on AQP1- and AQP7- knockout mice, as well as on AQP1-AQP7 double mice, it was demonstrated that the amount of water reabsorbed through AQP7 in the proximal straight tubules is much lower compared with the amount of water reabsorbed through AQP1 (80). In AQP7 knockout mice osmotic water permeability in apical plasma membrane of the proximal tubules is slightly reduced and these mice do not exhibit an impaired urinary concentrating ability. On the other hand, renal glycerol excretion significantly increased in AQP7 knockout mice (80). Glyceroluria observed in AQP7 knockout mice clearly indicates that this protein plays a major role in the glycerol-reabsorbing pathway in the kidney (73, 80). An important role of AQP7 in the tubular glycerol transport seems to have been confirmed by previous studies, which revealed that it is in proximal convoluted and straight tubules where renal glycerol metabolism is located (81, 82). According to Sohara et al. (73), after the reabsorption of glycerol through AQP7, its phosphorylation by glycerol kinase takes place in the epithelial cells of the proximal tubule, to produce L-glycerol 3-phosphate (G-3-P) in this way.
Expression of AQP7 in Xenopus oocytes also increases nine fold their permeability to urea (42), although the role of this transport in the kidneys is currently being debated. Generally, it is known that urea reabsorbed from the thick ascending limbs enters the neighboring proximal straight tubules. According to Ishibashi et al. (79) this process is mediated by AQP7, which most probably functions as a passive urea secretory pathway, thus contributing to formation and/or maintenance of the medullary urea concentration gradient. Sohara et al. (80) demonstrated, however, that in AQP7 deficient mice the concentration of urea in plasma and urine and the urea content of papilla did not differ from those in wild-type. No changes in renal urea distribution where found even in AQP7 knockout mice fed a low-protein (4%) diet and during dehydration. Therefore, these authors suggested that AQP7 did not play a significant role in renal excretion and recycling of this compound.
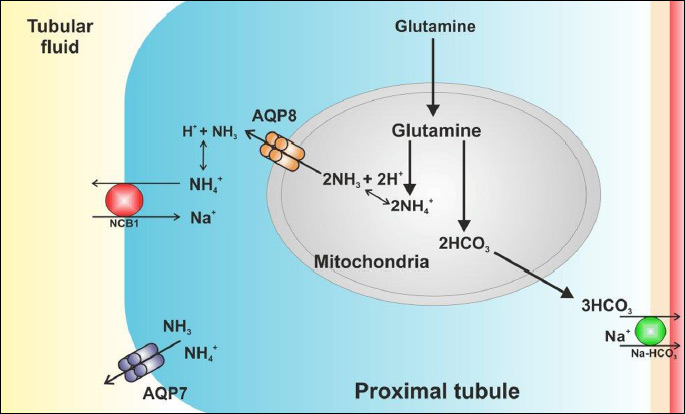
As it has been previously mentioned, AQP7, similarly as AQP3, AQP8, AQP9 and AQP10, is permeable to ammonia (25, 83). The role of this transport, however, remains partly unexplained. Results of the studies on removal of NH3 from the circulation by adipose tissue enabled Esbjornsson et al. (84) to propose a concept that AQP7 most probably participates in this ammonia uptake and thereby provides a mechanism for NH3 detoxification. To this day, the literature lacks reports which would clearly describe the role of AQP7 permeability to ammonia in proximal tubules and whether this transport in the kidney is of any importance whatsoever. According to Geyer et al. (83) AQP7 may be involved in the secretion of NH3 or/and NH4+ (Fig. 3). It is generally known that there is a process of glutamine metabolization in the proximal tubule resulting in production of HCO3– and NH4+, which are then excreted into tubular fluid. Some NH4+ may exit from the proximal tubule cells and enter to the tubular fluid as NH3, where it is then protonated (47). AQP7 may be permeable to both ammonia and ammonium ions. As suggested by Litman et al. (25), aquaporins may transport ammonia in the neutral form, NH3, and the transport may be accompanied by H+ flux (through the same channel or by a separate pathway). Ammonia may also cross the aquaporins in its ionic form, NH4+. Transport of NH4+ as compared to NH3 + H+ occurs rapidly and causes changes of ammonia concentration on both sides of the membrane in a shorter time.
AQP8 and mitochondrial transport
AQP8 is phylogenetically different from other members of this family and has a unique, primary structure resulting in a novel substrate specificity. AQP8 has been reported to be expressed in the gastrointestinal tracks (jejunum and colon), in airways and salivary glands, in liver, in testis and in the kidney (85, 86). AQP8 is mainly localized in the inner mitochondrial membrane, though it was also confirmed in the apical plasma membrane of the pancreatic acinar cells and in apical plasma membrane of the gall bladder epithelial cells (10, 86, 87). AQP8 selectively transports water, ammonia (permeable only to NH3 but not to NH4+) and H2O2 (hydrogen peroxide). Ammonia permeability of AQP8 is nearly twice as high as that of water (87-89). The biological relevance of AQP8 is under dispute. Expression of this protein mainly in the inner mitochondria membrane of several mammalian tissues may indicate, however, a strong role of AQP8 in the mitochondrial transport of ammonia in the urea cycle and in the transport of H2O2 across membranes (45, 87, 88, 90).
In the rat kidney, AQP8 is localized almost exclusively in the epithelial cells of the proximal tubules. Expression of AQP8 was observed in cytoplasmic domains, in the apical, basal and central parts of the proximal tubule cells. Weak labeling of AQP8 was also observed in intracellular structures of the collecting duct (85). Molinas et al. (91) demonstrated that in human AQP8 knockdown proximal tubule cells line the rate of ammonia excretion decreased by 31% at pH 7.4 and by 90% at pH 6.9. The results of the cited reports may lead to conclusions that permeability of AQP8 to ammonia might be required for renal ammonia excretion and be involved in the renal adaptive response to acidosis. As it was mentioned in the previous section, the proximal tubule is the primary site of renal ammonia production. The source of ammonia is glutamine, which penetrates through the apical and basolateral membrane to the cytoplasm of proximal tubule cells, and is next transported into the mitochondria, where it is further transformed to glutamate and α-ketoglutarate. In the process of glutamine metabolism, HCO3– and NH4
+ ions (Fig. 3) are produced (92). HCO3– ions are transported across the basolateral membrane mainly via the Na-HCO3 cotransporter into the venous blood (91, 92). Ammonia ions are transported to the renal tubule lumen through Na+ – H+ exchanger (NHE-3) localized in the apical plasma membrane. They may also be getting into the lumen of the tubule through AQP7 localized in the apical plasma membrane in the segment S3 of the proximal tubule. About 20 – 45% of ammonia produced in the proximal tubule segments is transported across the basolateral membrane into the renal veins (46, 47). Transport across the proximal tubule epithelial cell membranes of both HCO3– and ammonia ions is a key element of renal regulation of the systemic acid balance. It is still unknown, however, in what way ammonia ions resulting from glutamine metabolism are transported from mitochondria to the cytoplasm. According to Molinas et al. (91), the process is most probably accompanied by AQP8, which - localized in the inner mitochondrial membrane - enables the flow of NH3 to the cell interior. AQP8 seems to play the key role in diffusional transport of ammonia also in the epithelial cells of the collecting duct. NH3 produced in mitochondria is transported through AQP8 to cytoplasm, and through RhBG and RhCG, localized in the basolateral and apical plasma membrane, it is secreted into urine or transported to blood. Presumably, AQP3 also takes part in the process of NH4+ transport across basolateral of the connecting tubule and the collecting duct. Relatively small production of ammonia in the collecting duct, as compared with the proximal tubule, most probably underlies the fact that a weak expression of AQP8 is observed in the CD epithelial cells (94).
SUMMARY AND CONCLUSIONS
It has been nearly thirty years since the discovery of the first aquaporin and the definition of the role of these proteins in rapid water transport across biological membranes. At the moment, we have substantial base of knowledge on the structure, cellular localization and biological function of mammalian AQPs. Many years of research on the function and location of aquaporins in the renal tubules allowed defining their role in renal excretion of water and their importance in the development of diseases such as nephrogenic diabetes insipidus. However, although there are new reports being constantly published that - besides water, glycerol, ammonia and urea - aquaporins enable transport of other compounds, i.e. carbon dioxide, silicon, mannitol, sorbitol and adenine, the role of these additional transport functions of AQPs is still not fully understood. Aquaglyceroporins localized in the kidney seem to have a particular importance for this additional transport. It is known that the kidney is the main organ to sustain homeostasis of the organism and to regulate the retention or elimination of many different compounds and metabolites. Therefore, it seems that the challenge for modern biomedicine is to determine the role of the additional transport of renal AQPs, as well as to seek new factors controlling their expression. Especially, as strong evidence is that these proteins can play a key role in the renal regulation of nitrogen and acid-base balance. Let us hope that further research conducted using modern and innovative research techniques will allow full explanation of the additional transport functions of renal AQPs, and thus explanation of how these „small“ proteins affect renal function in both physiological and pathophysiological conditions.
Conflict of interests: None declared.
REFERENCES
- Agre P, Preston GM, Smith BL, et al. Aquaporin chip: the archetypal molecular water channel. Am J Physiol Renal Physiol 1993; 265: F463-F476.
- Holmes RP. The role of renal water channels in health and disease. Mol Aspects Med 2012; 33: 547-552.
- Kitchen P, Day RE, Salman MM, Corner MT, Bill RM, Conner AC. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim Biophys Acta 2015; 1850: 2410-2421.
- Nejsum LN. The renal plumbing system: aquaporin water channels. Cell Mol Life Sci 2005; 62: 1692-1706.
- Day RE, Kitchen P, Owen SD, et al. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta 2014; 1840: 1492-1506.
- Gomes G, Agasse A, Thiebaud P, Delrot S, Geros H, Chaumont F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta 2009; 1788: 1213-1228.
- Agre P, King LS, Yasui M, et al. Aquaporin water channels - from atomic structure to clinical medicine. J Physiol 2002; 542: 3-16.
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 2003; 555: 72-78.
- Benga G. Water channel proteins (later called aquaporins) and relatives: past, present, and future. IUMB Life 2009; 61: 112-133.
- Ishibashi K, Hara S, Kondo S. Aquaporin water channel in mammals. Clin Exp Nephrol 2009; 13: 107-117.
- Verkman AS. Aquaporins at a glance. J Cell Sci 2011; 124: 2107-2112.
- Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci 2005; 118: 3225-3232.
- Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquagliceroporin. Annu Rev Physiol 2008; 70: 301-327.
- Park EJ, Kwon TH. A minireview on vasopressin-regulated aquaporin-2 in kidney collecting duct cells. Electrolyte Blood Press 2015; 13: 1-6.
- Mrozikiewicz-Rakowska B, Maroszek P, Nehring P, et al. Genetic and environmental predictors of chronic kidney disease in patients with type 2 diabetes and diabetic foot ulcer: a pilot study. J Physiol Pharmacol 2015; 66: 751-761.
- Sansoe G, Mastrocola R, Aragno M, Parola M. Dynamics of sodium retention in preascitic cirrhotic rats assessed through parathyroid hormone injection. J Physiol Pharmacol 2014; 65: 649-657.
- Kortenoeven LA, Fenton RA. Renal aquaporins and water balance disorders. Biochim Biophys Acta 2014; 1840: 1533-1549.
- Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 2007; 87: 1083-1112.
- Procino G, Mastrofrancesco L, Sallustio F, et al. AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney. Cell Physiol Biochem 2011; 28: 683-692.
- Nielsen S, Frokićr J, Marples D, Kwon T, Agre P, Knepper M. Aquaporins in the kidney. From molecules to medicine. Physiol Rev 2002; 82: 205-244.
- Nielsen S, Kwon T, Frokićr J, Knepper MA. Key roles of renal aquaporins in water-balance and water-balance disorders. News Physiol Sci 2000; 15: 136-143.
- Ma T, Frigeri A, Hasegawa H, Verkman AS. Cloning of a water channel homolog expressed in brain meningeal cells and kidney collecting duct that functions as a stilbene-sensitive glycerol transporter. J Biol Chem 1994; 269: 21845-21849.
- Eschevarria M, Windhager EE, Tate SS, Frindt G. Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci USA 1994; 91: 10997-11001.
- Ishibashi K, Sasaki S, Fushimi K, et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA 1994; 91: 6269-6273.
- Litman T, Sogaard R, Zeuthen T. Ammonia and urea permeability of mammalian aquaporins. Handb Exp Pharmacol 2009; 190: 327-358.
- Roudier N, Ripoche P, Gane P, et al. AQP3 deficiency in humans and the molecular basis of novel blood group system, GIL. J Biol Chem 2002; 227: 45854-45859.
- Patsouris D, Mandard S, Voshol PJ, et al. PPARa governs glycerol metabolism. J Clin Invest 2004; 114: 94-103.
- Sugiyama Y, Ota Y, Hara M, Inoue S. Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim Biophys Acta 2001; 1522: 82-88.
- Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem 2002; 277: 17147-17153.
- Sougrat R, Morand M, Gondran C, et al. Functional expression of AQP3 in human skin epidermis and reconsrtructed epidermis. J Invest Dermatol 2002; 118: 678-685.
- Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol 2007; 159: 324-330.
- Rodriguez A, Catalan V, Gomez-Ambrosi J, et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab 2011; 96: E586-E597.
- Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J Histochem Cytochem 1999; 47: 1275-1286.
- Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. Aquaporins in the digestive system. Med Electron Microsc 2004; 37: 71-80.
- Richardson SM, Knowles R, Marples D, Hoyland JA, Mobasheri A. Aquaporin expression in the human intervertebral disc. J Mol Histol 2008; 39: 303-309.
- Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol 2008; 28: 326-332.
- Levin MH, Verkman AS. Aquaporin-3 dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci 2006; 47: 4365-4372.
- Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med 2008; 86: 523-529.
- Ecelbarger CA, Terris J, Frindt G, et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 1995; 269: F663-F672.
- Zhao D, Bankir L, Qian L, Yang D, Yang B. Urea and urine concentrating ability in mice lacking AQP1 and AQP3. Am J Physiol Renal Physiol 2006; 291: F429-F438.
- Spector DA, Wade JB, Dillow R, Steplock DA, Weinman EJ. Expression, localization, and regulation of aquaporin-1 to -3 in rat urothelia. Am J Physiol 2002; 282: F1034-F1042.
- Ishibashi K, Kuwahara M, Gu Y, et al. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem 1997; 272: 20782-20786.
- Ma T, Song Y, Yang B, et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci USA 2000; 97: 4386-4391.
- Meinild AK, Klaerke DA, Zeuthen T. Bidirectional water fluxes and specificity for small hydrophilic molecules in aquaporins 0 to 5. J Biol Chem 1998; 273: 32446-32451.
- Li C, Wang W. Urea transport mediated by aquaporins water channel proteins. Subcell Biochem 2014; 73: 228-265.
- Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 2007; 69: 317-340.
- Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ 2009; 33: 275-281.
- Nowik M, Kampik NB, Mihailova M, Eladari D, Wagner CA. Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats-species differences and technical considerations. Cell Physiol Biochem 2010; 26: 1059-1072.
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporins. Nature 1999; 402: 184-187.
- Ma T, Yang B, Kuo WL, Verkman AS. cDNA cloning and gene structure of a novel water channel expressed exclusively in human kidney: evidence for a gene cluster of aquaporins atchromosome locus 12q13. Genomics 1996; 35: 543-550.
- Jeremic A, Cho WJ, Jena BP. Involvement of water channels in synaptic vesicle swelling. Exp Biol Med 2005; 230: 674-680.
- Lopez IA, Ishiyama G, Lee M, Baloh RW, Ishiyama A. Immunohistochemical localization of aquaporins in the human inner ear. Cell Tissue Res 2007; 328: 453-460.
- Nagase H, Agren J, Saito A, et al. Molecular cloning and characterization of mouse aquaporin 6. Biochem Biophys Res Commun 2007; 352: 12-16.
- Matsuki-Fukushima M, Fujita-Yoshigaki J, Murakami M, Katsumata-Kato O, Yokoyama M, Sugiya H. Involvement of AQP6 in the Mercury-sensitive osmotic lysis of rat parotid secretory granules. J Membr Biol 2012; 246: 209-2014.
- Taguchi D, Takeda T, Kakigi A, Okada T, Nishioka R, Kitano H. Expression and immunolocalization of aquaporin-6 (Aqp6) in the rat inner ear. Acta Otolaryngol 2008; 128: 832-840.
- Iandiev I, Dukic-Stefanovic S, Hollborn M, et al. Immunolocalization of aquaporin-6 in the rat retina. Neurosci Lett 2011; 490: 130-134.
- Gregoire F, Lucidi V, Zerrad-Saadi A, et al. Analysis of aquaporin expression in liver with a focus on hepatocytes. Histochem Cell Biol 2015; 144: 347-363.
- Hazama A, Kozono D, Guggino WB, Agre P, Yasui M. Ion permeation of AQP6 water channel protein. J Biol Chem 2002; 277: 29224-29230.
- Holm LM, Klaerke DA, Zeuthen T. Aquaporin 6 is permeable to glycerol and urea. Pflugers Arch 2004; 448: 181-186.
- Beitz E, Liu K, Ikeda M, Guggino WB, Agre P, Yasui M. Determinants of AQP6 trafficking to intracellular sites versus the plasma membrane in transfected mammalian cells. Biol Cell 2006; 98: 101-109.
- Ohshiro K, Yaoita E, Yoshida Y, et al. Expression and immunolocalization of AQP6 in intracaled cells of the rat kidney collecting duct. Arch Histol Cytol 2001; 64: 329-338.
- Promeneur D, Kwon TH, Yasui M, et al. Regulation of AQP6 mRNA and protein expression in rats in response to altered acid-base or water balance. Am J Physiol Renal Physiol 2000; 279: F1014-F1026.
- Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasumi M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells requirement of pore-lining residue threonine. J Biol Chem 2002; 277: 39873-39879.
- Madsen KM, Tisher CC. Response of intercalated cells of rat outer medullary collecting duct to chronic metabolic acidosis. Lab Invest 1984; 51: 268-276.
- Verlander JW, Madsen KM, Tisher CC. Effect of acute respiratory acidosis on two populations of intercalated cells in rat cortical collecting duct. Amer J Physiol 1987; 253: F1142-F1156.
- Brown D. Membrane recycling and epithelial cell function. Amer J Physiol 1989; 256: F1-F12.
- Rabaud NE, Song L, Wang Y, Agre P, Yasui M, Carbrey A. Aquporin 6 binds calmodulin in a calcium dependent manner. Biochem Biophys Res Commun 2009; 383: 54-57.
- Arai H, Pink S, Forgac M. Interaction on anions and ATP with the coated vesicle proton pomp. Biochemistry 1989; 28: 3075-3082.
- Gunther W, Luchow A, Cluzeaud F, Vandewalle A, Jentsch TJ. CIC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci USA 1989; 95: 8075-8080.
- Kishida K, Kuriyama H, Funahashi T, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 2000; 275: 20896-20902.
- McConnell NA, Yunus RS, Gross SA, Bost KL, Clemens MG, Hughes FM. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002; 143: 2905-2912.
- Skowronski MT, Lebeck J, Rojek A, et al. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am J Physiol 2007; 292: F956-F965.
- Sohara E, Uchida S, Sasaki S. Function of aquaporin-7 in the kidney and the male reproductive system. Handb Exp Pharmacol 2009; 190: 219-231.
- Kuriyama H, Kawamoto S, Ishida N, et al. Molecular cloning and expression of a novel human aquaporin from adipose tissue with glycerol permeability. Biochem Biophys Res Commun 1997; 241: 53-58.
- Hara-Chikuma M, Sohara E, Rai T, Ikawa M, et al. Progressive adipocyte hypertrophy in aquaporin-7 deficient mice: adipocyte glycerol permeability as a novel regulator fact accumulation. J Biol Chem 2005; 280: 15493-15496.
- Hibuse T, Maeda N, Funahashi T, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA 2005; 102: 10993-10998.
- Saito K, Kageyama Y, Okada Y, et al. Localization of aquaporin-7 in human testis and ejaculated sperm: possible involvement in maintenance of sperm quality. J Urol 2004; 172: 2073-2076.
- Sohara E, Ueda O, Tachibe T, et al. Morphologic and functional analysis of sperm and testis in aquaporin 7 knockout mice. Fertil Steril 2007; 87: 671-676.
- Ishibashi K, Imai M, Sasaki S. Cellular localization of aquaporin 7 in rat kidney. Exp Nephrol 2000; 8: 252-257.
- Sohara E, Rai T, Miyazaki J, Verkman AS, Sasaki S, Uchida S. Defective water and glycerol transport in the proximal tubules of AQP7 knockout mice. Am J Physiol Renal Physiol 2005; 289: F1195-F1200.
- Burch HB, Narins RG, Chu C, et al. Distribution along the rat nephron of the free enzymes of gluconeogenesis in acidosis and starvation. Am J Physiol 1978; 235: 246-253.
- Vandewalle A, Wirthensohn G, Heidrich HG, Guder WG. Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol 1981; 240: F492-F500.
- Geyer RR, Musa-Aziz R, Qin X, Boron WF. Relative CO2/NH3 selectivities of mammalian aquaporins 0-9. Am J Physiol Cell Physiol 2013; 304: C985-C994.
- Esbjornsson M, Bulow J, Norman B, et al. Adipose tissue extracts plasma ammonia after sprint exercise in women and man. J Appl Physiol 2006; 101: 1576-1580.
- Elkjaer ML, Nejsum LN, Gresz V, et al. Immunolocalization of aquaporin-8 in rat kidney, gastrointenstinal tract testis and airways. Am J Physiol Renal Physiol 2001; 281: 1047-1057.
- Agemark M, Kowal J, Kukulski W, et al. Reconstitution of water channel function and 2D-crystalization of human aquaporin 8. Biochim Biophys Acta 2012; 1818: 839-850.
- Calamita G, Ferri D, Gena P, et al. The inner mitochondrial membrane has aquaporin-8 water channel and is highly permeable to water. J Biol Chem 2005; 280: 17149-17153.
- Saparov SM, Liu K, Agre P, Pohl P. Fast and selective ammonia transport by aquaporin-8. J Biol Chem 2007; 282: 5296-5301.
- Bienert GP, Moller ALB, Kristiansen KA, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 2007; 282: 1183-1192.
- Soria LR, Fanelli E, Altamura N, Svelto M, Marinelli RA, Calamita G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem Biophys Res Commun 2010; 393: 217-221.
- Molinas SM, Trumper L, Marinelli RA. Mitochondrial aquaporin-8 in renal proximal tubule cells: evidence for a role in the response to metabolic acidosis. Am J Physiol Renal Physiol 2012; 303: F458-F466.
- Curthoys NP, Gstraunthaler G. Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol 2001; 281: F381-F390.
- Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev 1989; 69: 179-249.
- Good WD, Burg MB. Ammonia production by individual segments of the rat nephron. J Clin Invest 1984; 73: 602-610.
A c c e p t e d : January 29, 2016