HIGH DIETARY FRUCTOSE DOES NOT EXACERBATE THE DETRIMENTAL CONSEQUENCES OF HIGH FAT DIET ON BASILAR ARTERY FUNCTION
INTRODUCTION
Overweight and obesity are major risk factors for a number of chronic diseases, including diabetes, cardiovascular diseases and cancer. Once considered a problem only in high income countries, overweight and obesity are now dramatically on the rise in low- and middle-income countries. According to WHO, more than 1.9 billion adults, 18 years and older, were overweight in 2014. Of these over 600 milion were obese (1). Recent data from the National Center for Health Statistics estimate that in the United States more than one-third of adults and 17% of youth are obese (2).
Among multiple contributing factors, epidemiological studies have indicated a strong correlation between the consumption of sugar- or fructose-enriched products and the increased rates of obesity. High fat and/or fructose diets can reduce the number of insulin receptor substrate proteins and decrease the activity of glucose transporters and intercellular metabolism of glucose (3). Because glucose intolerance is related to plasma leptin levels, both high fat diet and high fructose diet can lead to leptin resistance (4). Moreover, high dietary fructose without added fat and in the absence of obesity induces leptin resistance and exacerbates subsequent HF-induced obesity (5).
Leptin has a role in arterial stiffness. Elevated leptin or adiponectin is associated with arterial stiffness in some patients (6, 7). Of the brain vessels, the basilar artery is the most important artery in the posterior cerebral circulation. It supplies blood to the medulla, cerebellum, pons, midbrain, thalamus, occipital cortex as well as several areas of the brain that are critical for mediating episodic memory, including portions of the hippocampus and the parahippocampal gyrus. Reduced posterior brain blood flow contributes to vertebrobasilar insufficiency (8), resulting in a set of symptoms including vertigo, headaches, sleep disturbances, pupillary and oculomotor abnormalities, dysarthria, and dysphagia to quadriparesis. Furthermore, it leads to memory impairment and neurological disorders, including stroke (9). Approximately 20 – 25% of strokes occur in the vertebrobasilar region. Strokes due to acute basilar artery occlusion have higher mortality rates compared to all the stroke cases (10).
Diet, exercise, stress, and sleep are receiving attention as environmental modifiers of chronic inflammatory diseases, including atherosclerosis, a critical contributor to of myocardial infarction and stroke.
Accumulating data indicate that a high-fat, high-cholesterol diet aggravates cardiovascular disease (11). Many studies have examined the effect of high fat diets on aorta and peripheral vessels. However, little is known about the effect of high fat or fructose containing diets on basilar artery. Since atherosclerosis is the most common cause of ischemic stroke and vertebrobasilar insufficiency, we evaluated the effects of a high fat diet versus high fat with added fructose on basilar artery function and other cardiovascular parameters.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats 7 week old (N = 8 – 10/group) were obtained from Harlan Labs (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for one week. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals and protocols were approved by the University of Florida Institutional Animal Care and Use Committee (#201203230). Rats were maintained on a 12:12 hour light-dark cycle and provided either a standard rodent chow, high fat (HF) or high fat and high fructose (HF/F), with water available ad libitum throughout the experimental protocol.
All diets were purchased from Harlan (Harlan Teklad; Madison, WI). The detailed compositions of the diets are shown in Table 1. Neither diet contains sucrose. Both diets other than chow contain moderately high fat (30%) with and without fructose. The vitamin and mineral content are identical for HF and HF/F diets.
Experimental design
After baseline food intake was assessed for a week rats were divided into 3 groups (week 0); Control (n = 10) with provided the standard chow, HF (n = 8) with provided the HF diet, and HF/F (n = 8) with provided the HF/F diet for 12 weeks. This time period was chosen based on our previous experience that at least 8 weeks is required to observe the metabolic changes with HF diets. Rats were housed individually and food consumption and body weight were recorded daily throughout the experiment.
Body composition of fat and lean mass
Body composition was assessed using time-domain nuclear magnetic resonance (TD-NMR) in restrained but fully conscious rats (TD-NMR Minispec, Bruker Optics, The Woodlands, TX, USA) at week 0, 6 and 11.
Blood glucose and lipids
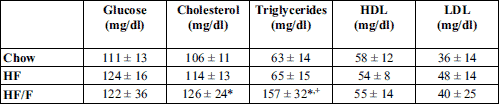
Blood glucose and lipids were measured after an 8-hour fasting at week 10. Glucose, total cholesterol, triglycerides, and high and low density lipoproteins (HDL, LDL) were analysed by CardioCheck Blood Testing Device (Health Check Systems Inc, Brooklyn, NY). Low density lipoprotein (LDL) was calculated by the Friedewald formula from total cholesterol (TC), high density lipoprotein (HDL) and triglyceride (TG): LDL = TC – HDL – TG/5.0 (mg/dL)
Serum leptin
Enzyme immunoassays were used to determine the levels of leptin (rat leptin ELISA kit, EZRL-83K; Milipore, Massachusetts, USA). Leptin was assayed in blood which is collected during sacrifice in a fed state.
Serum ADMA assay
Asymmetrical dimethylarginine (ADMA), which is an endogenous inhibitor of nitric oxide, was measured using ADMA Elisa kit (Enzo Life Sciences, PA, USA, catalogue number: ALX-850-327-KI01) according to the manufacturer’s instructions. Results were expressed as µmol ADMA/L.
Heart rate, blood pressure, and stress-induced blood pressure
Heart rate, blood pressure, and stress-induced blood pressure were measured at week 0, 6 and 10 by a non-invasive tail cuff system (CODA, Kent Scientific, Torrington, Connecticut). After an acclimation period of 10 minutes, systolic, diastolic, mean arterial pressure (MAP), and heart rate were recorded by the computer system via the CODA software (Kent Scientific).
Stress-induced blood pressure and heart rate
Restraining stress-induced blood pressure and heart rates were recorded the next day of the measurements at week 10. The animals were restrained in Plexiglas chambers (chambers used for tail cuff) for an hour and their blood pressure was measured afterwards.
Tissue harvesting and preparation
Rats were euthanized with isoflurane. Twenty ml of ice-cold saline was perfused through heart, and retroperitoneal, perirenal, epididymal white adipose tissues (RTWAT, PWAT and EWAT, respectively) and brown adipose tissue (BAT) were excised. Fats and hearts were weighed.
Malondialdehyde (MDA) assay
Heart samples were homogenized with potassium phosphate buffer (pH 6.0) and ice-cold trichloracetic acid (1 g tissue plus 10 ml 10% TCA) in an ultrasonic tissue homogenizer. The MDA levels of heart samples were assayed for products of lipid peroxidation by monitoring thiobarbituric acid reactive substance (TBARS) formation at 532 nm as described previously (11). Lipid peroxidation was expressed in terms of MDA equivalents i.e. nmol MDA/g tissue.
Reduced glutathione (GSH) assay
Glutathione measurements of heart samples were performed using a modification of the Ellman procedure (13). Briefly, after centrifugation of heart homogenates at 2000 g for 10 min, 0.5 ml of supernatant was added to 2 ml of 0.3 mol/l Na2HPO4.2H2O solution. A 0.2 ml solution of dithiobisnitrobenzoate (DTNB) (0.4 mg/ml 1% sodium citrate) was added and after mixing, the absorbance at 412 nm was measured immediately. The results were expressed in µmol GSH/g tissue.
Microvessel preparation
Rats were anesthetized (isoflurane 3%/O2 balance) and euthanized by the removal of the heart. After rinsing, the brain was placed in cold (4°C) physiological saline solution (PSS) containing 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer, and 1 g/100 ml bovine serum albumin (BSA), pH 7.4. The basilar arteries were isolated with the aid of a dissection microscope (Olympus SVH10) as previously described (14). The arteries were transferred to a Lucite chamber containing PSS equilibrated with room air. The ends of the artery were cannulated with micropipettes and secured with nylon sutures. The chamber containing the cannulated artery was then placed on an inverted microscope (Olympus IX70) equipped with a video camera and micrometer (Panasonic BP310; Texas A&M Cardiovascular Research Institute) to measure intraluminal diameter. The basilar arteries were then pressurized at 90 cm H2O (≌ 66 mmHg) with two hydrostatic columns (15). Arteries unable to hold pressure due to leaks or branches were discarded. Arteries without leaks were warmed to 37°C and allowed to equilibrate for 40 minutes before beginning the assessment of vasoconstrictor or vasodilator responses (14).
KCl induced vasoconstriction
Non-receptor dependent contractile responses were evaluated with six different doses of KCl (30, 50, 80, 100, 120, 150 mM, isotonic substitution for NaCl) administered at two minute intervals. The change in the internal diameter of the vessel was recorded and a dose response curve was generated.
Endothelin-1 induced vasoconstriction
Endothelin-1 (ET-1) (Phoenix Pharmaceuticals, catalogue no: 023-01) was used as a receptor-dependent contractile agent. It was added to the bath cumulatively in increasing concentrations (10–11 – 10–7 M) at 2 minute intervals. The change in the internal diameter of the vessel was recorded and a dose response concentration-response curve was generated.
Phenylephrine vasoconstriction
A concentration response curve to phenylephrine (adrenergic alpha-1 agonist) was generated by the cumulative addition of phenylephrine (1 × 10–9 M–1 × 10–4 M) at 2 minute intervals to the vessel bath. The change in the internal diameter of the vessel was recorded.
Acetylcholine vasodilation
Endothelium-dependent dilation to ACh (Sigma A6625) was evaluated by the addition of ACh to the bath in incremental doses (10–10 – 10–4 M) at 2 minute intervals.
DEA-NONO-ate vasodilation
Endothelium-independent dilation was evaluated by the addition of a nitric oxide donor, diethylamine NONO-ate (DEA-NONO-ate) (Sigma D184) to the bath in incremental doses (10–10 – 10–4 M) at 2 minute intervals.
Papaverine vasodilation
Responsiveness to papaverine, a direct smooth muscle relaxant that signals through cyclic AMP, was evaluated by the addition of papaverine to the bath in incremental doses every 2 min. (1 × 10–10 M to 1 × 10–4 M).
Calculations
Vasoconstrictor responses to KCl and ET-1 are expressed as percent constriction as calculated by the formula:
Constriction % = ((Db – Ds)/Db) × 100
Where Db is the baseline diameter immediately prior to addition of the first dose of vasoconstrictor agonist, and Ds is the steady state diameter measured after addition of each dose of the vasoconstrictor agent.
For vasodilation experiments, a minimum of 15% spontaneous tone was necessary prior to assessment of dose-response curves.
Spontaneous tone (%) (Dmax – Db) / Dmax × 100
Db is the steady-state baseline diameter recorded at 90 cm H2O. Maximum vasodilation (Dmax) was obtained by the addition of sodium nitroprusside (SNP) (10–4M), a direct NO donor, to the calcium-free PSS bath at the end of the experiments.
Relaxation responses to ACh and DEA-NONOate are expressed as percent relaxation as calculated by the following formula:
Relaxation (%) = [(Ds – Db)/(Dmax – Db)] × 100
where Ds is the arteriolar diameter measured after addition of the vasodilatory agent, Db is the diameter recorded immediately prior to initiation of the concentration-diameter curves, and Dmax is the maximal diameter for the arteriole.
Hematoxylin & eosin staining of basilar artery
Isolated arteries were fixed in formalin solution, immersed in fast green, embedded in optimal cutting temperature (OCT) compound, and then immediately stored at –80°C, until the cutting of 5 µm sections occurred. Sections were then stained by Hematoxylin & eosin (H&E) method. Wall and lumen thicknesses were determined under the microscope (Axiovert 40 CFL, Zeiss, Germany) connected to a computerized system using the Image J/Lab software (NIH, Bethesda, MD, USA).
eNOS immunohistochemistry
Five µm sections of basilar arteries on a slide were rinsed 3 times for 5 min with PBS at room temperature then incubated in 1% sodium dodecyl sulphate (SDS) in PBS for 5 min. After rinsing, sections were incubated in blocking solution (PBS containing 5% goat serum, and 0.3% Triton) for 1 h in room temperature followed by overnight incubation in anti-eNOS (1:400, Sigma E9780) primary antibody at 4°C After rinsing, sections were incubated in fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:400, Abcam ab6717) for fluorescence development. The cell nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole). Images were taken using a Nikon TiE-PFS-A1R (Tokyo, Japan) confocal microscope equipped with a 488 nm laser diode with a 510-560 nm band pass filter, and a 561 laser with a 575-625 nm band pass filter. The fluorescence intensity was analysed using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5.0 (GraphPad Software, San Diego; CA; USA). All data were expressed as means ± S.E.M. Groups were compared with one-way or two ways ANOVAs (analysis of variance) followed by post-hoc tests. Kruskal Wallis test followed by Dunn’s test was performed for nonparametric analysis. Values of P < 0.05 were regarded as statistically significant.
For the vessel experiments, sigmoidal dose-response curves and groups/doses were compared with two-way repeated-measures ANOVA followed by Bonferroni post hoc-tests.
RESULTS
Food intake, energy intake, and delta body weight
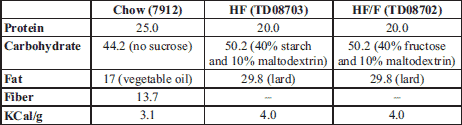
Food consumption expressed as Kcal was unchanged across diets whether as daily consumption (Fig. 1a) or cumulative intake over 71 days (inset). When food intake was expressed as grams, control rats consumed more grams due the reduced calories per gram of the chow diet. All rats gained weight, as expected but no significant difference was observed in body weight or delta body weight across diets during the experimental period (Fig. 1b).
Body composition of fat and lean mass
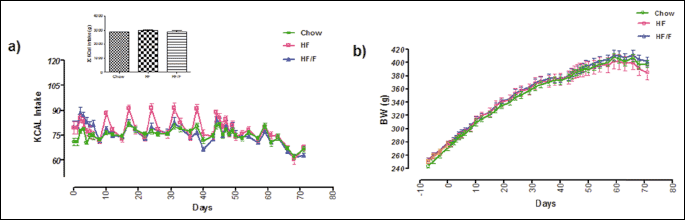
Rats had similar baseline level of % fat and % lean mass at week 0. Fat mass was increased and lean mass was decreased in HF and HF/F fed rats by week 6 compared with their baseline. Although both HF and HF/F were significantly different than the chow control by week 11, there was no significant difference between HF and HF/F groups. There were significant main effects for both high fat diet groups (P = 0.07) and week (P < 0.001), and an interaction (P = 0.032) by two-way ANOVA (Fig. 2).
Adipose tissue parameters
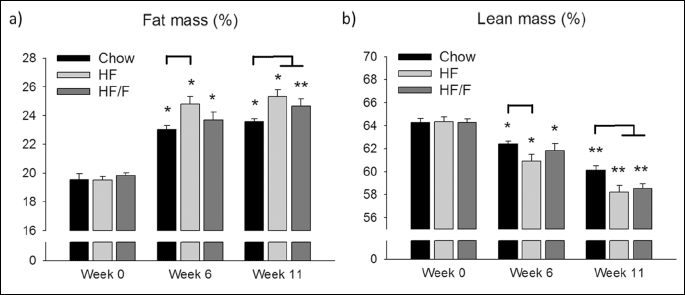
Composition of body fats was compared in terms of retroperitoneal, perirenal, epididymal white adipose tissues (RTWAT, PWAT and EWAT, respectively) and brown adipose tissue (BAT). There were significantly (P < 0.05 – 0.001) more RTWAT, PWATs and EWAT in HF and HF/F diet-fed rats compared with chow-fed rats. Consistently, the sum of WAT weights in HF and HF/F groups was greater than the chow group (P < 0.001). However, the amount of BAT was not significantly different between groups (Table 2).
Blood leptin, glucose and lipids
Fed state leptin, fasting glucose, and lipid parameters including cholesterol, triglycerides, high density cholesterol (HDL), and low density cholesterol (LDL) are provided in Table 3. Leptin levels were significantly higher in HF and HF/F groups compared with the chow group (P < 0.01, P < 0.001, respectively). Glucose, HDL, and LDL levels were not different between groups. There was a significant (P < 0.05) increase in both cholesterol and triglyceride levels in HF/F group compared with chow group; however no change was observed in HF group compared with chow at week 10.
Serum ADMA
Serum ADMA levels, endogenous competitive inhibitor of NOS, was significantly increased in both the HF and HF/F groups (P < 0.05) when compared with the chow group (Fig. 3).
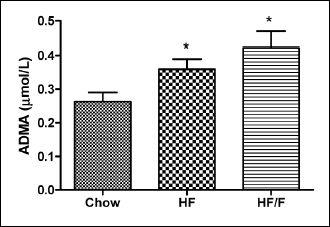 |
Fig. 3. Serum asymmetrical dimethyl arginine (ADMA) levels of the chow, 30% high fat (HF) and HF + fructose (HF/F) fed rats at week 12. Each group consists of 8 – 10 rats. A significant (P < 0.05 and P < 0.01, respectively) increase was observed in serum ADMA levels both in HF and HF/F groups when compared with chow group in Kruskal Wallis test followed by Dunn’s multiple comparison. *P <0.05 versus chow. |
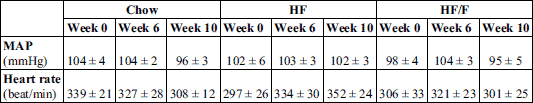
Heart rate, blood pressure, and stress-induced blood pressure
Heart rate (HR) and mean arterial pressure (MAP) were monitored at weeks 0, 6 and 10. Additionally a stress-induced HR and MAP measurements were performed during week 10, one day following above assessments) (Table 4 and Fig. 4).
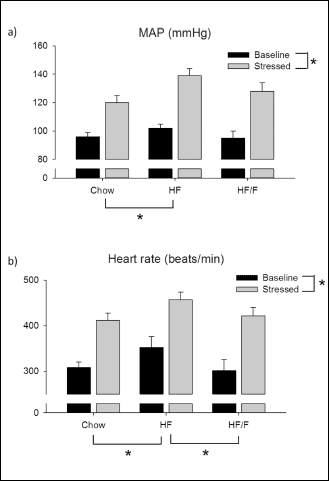 |
Fig. 4. The comparison of baseline and stress-induced (a) mean arterial blood pressure (MAP) and (b) heart rate of the rats fed with chow, high fat (30%) and HF + fructose (HF/F) at week 10. Each group consists of 8 – 10 rats. Regarding MAP, there was a statistically significant main effect of high fat relative to chow (P = 0.042). Stress conditions resulted in an increase in MAP for all groups (main effect; P < 0.001). Regarding heart rate, the high fat group was significantly higher than both other groups (P = 0.035). Stress conditions resulted in an increase in heart rate for all groups (main effect; P < 0.001). Two way ANOVA was performed. |
Although baseline HR at week 10 was slightly higher in HF and HF/F groups by week 10, these differences were not significant between groups for any of the weeks (Table 4).
Restraint stress induced a significant increase in HR (P < 0.05 – 0.01) and MAP (P < 0.01 – 0.001) compared to baseline at week 10. However, delta increase in MAP and HR were unchanged across groups (Fig 4a).
Regarding heart rate, the baseline heart rate was significantly greater in the high fat group compared with either of the other two groups (P = 0.035). Stress conditions resulted in an increase in heart rate for all groups (main effect; P < 0.001) but no differences across groups (Fig. 4b).
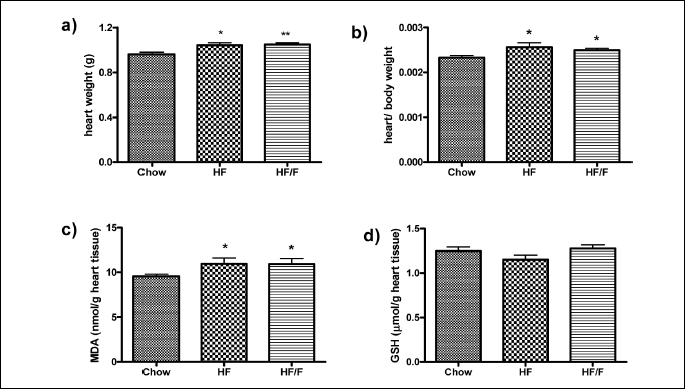
Heart weight and oxidative stress in the heart
Heart weight, heart/body weight, and the oxidative stress parameters, MDA and GSH in the heart tissue are shown in Fig 4. The heart weight and heart/body weight ratio were increased significantly (P < 0.05 – 0.01) in both the HF and HF/F groups compared with the chow group (Fig 5a and 5b).
Increased levels of MDA in heart tissue were observed both in HF and HF/F groups when compared with the chow group (P < 0.05); however, GSH levels in heart tissue remained unchanged across groups (Fig 5c and 5d).
Vascular reactivity of the basilar artery
Contractility responses
The vasoconstriction response to 30 – 150mM KCl was similar in all groups. The logEC50 values were 50.34; 49.90 and 49.91 in chow, HF and HF/F groups respectively. Although endothelin-1 contractile responses were similar with doses of 10–11 – 10–8M in all groups, the HF and HF/F groups were less sensitive to the higher doses of 3 ×10–8 and 10–7M. In contrast, the EC50 values were not significantly different across groups. The contractile response with phenylephrine in the basilar arteries was considerably weaker than that observed with KCl or endothelin-1 and was unchanged across groups (Fig. 6a, 6b, 6c, respectively).
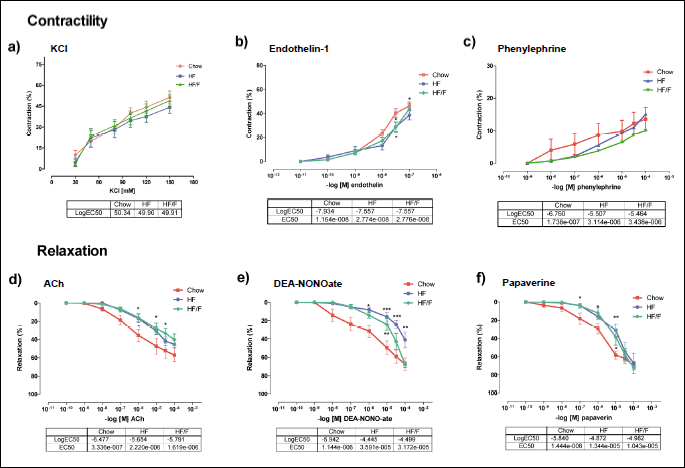
Relaxation responses
The relaxation response to all the vasodilators was impaired with both HF and HF/F diets compared with the chow group. Basilar arteries from both HF and HF/F groups demonstrated significantly (P < 0.05) impaired endothelium-dependent vasodilation to increasing doses of ACh (10–10 to 10–4 M). The EC50 values in chow, HF and HF/F were 3.3 × 10–7, 2.2 × 10–6 and 1.6 ×10–6 respectively (Fig. 6d). The NO donor, DEA-NONO-ate (10–10 to 10–4 M) induced relaxation in basilar arteries and was significantly (P < 0.05 – 0.001) decreased in HF and HF/F group as compared to the chow control group, with an EC50 of 3.6 × 10–5 in the HF group and 3.2 × 10–5 in the HF/F group versus 1.1 × 10–6 in the control group (Fig. 6e). Relaxation response to papaverine, a smooth muscle relaxant, was also significantly decreased in HF and HF/F groups with the EC50 values 1.3 × 10–5 and 1.0 × 10–5 versus 1.4 × 10–6M in the control group (Fig. 6f).
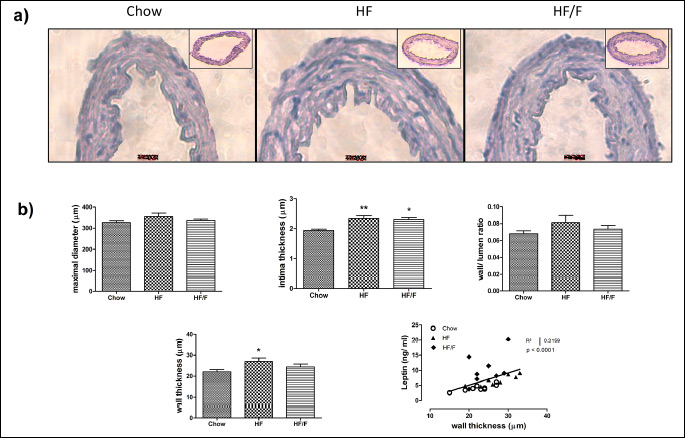
General characteristics of basilar artery and vessel histomorphology
The spontaneous diameters of basilar arteries were not significantly different between groups (control: 299 ± 12; HF: 302 ± 24; HF/F: 312 ± 7) at the beginning of organ bath experiments. The maximal diameters obtained at the end of the experiments were not significantly different either (control: 327 ± 8; HF: 348 ± 22; HF/F: 336 ± 8) (Fig. 7). The H&E staining analysis of the basilar arteries and the wall/lumen ratio, wall and intima thickness of basilar arteries are shown in Fig. 7. There was a significant (P < 0.05) increase in wall thickness in HF group compared with the chow group. However, no change was observed in either wall thickness or wall/lumen ratio in HF/F group compared with chow. The intima thickness was increased significantly (P < 0.05 – 0.01) both in HF and HF/F groups compared to chow group. Wall thickness correlated with serum leptin levels (P < 0.001) (Fig. 7). When examined individually, chow and HF groups exerted a significant positive correlation between wall thickness and serum leptin levels, whereas HF/F group failed to be significant.
eNOS immunofluorescence
The cell nuclei counterstained with DAPI (blue fluorescence, first column) were merged with eNOS (green fluorescence, middle column) in Fig. 8. The eNOS immunofluorescence of the basilar artery, an indicator of endogenous relaxant-NO production by the endothelium, was significantly (P < 0.05) decreased both in HF and HF/F groups compared with the chow group. This was consistent with the decreased relaxation response to ACh, indicating impairment of endothelium-dependent NO-mediated dilation, and increased ADMA (endogenous competitive inhibitor of NOS) levels.
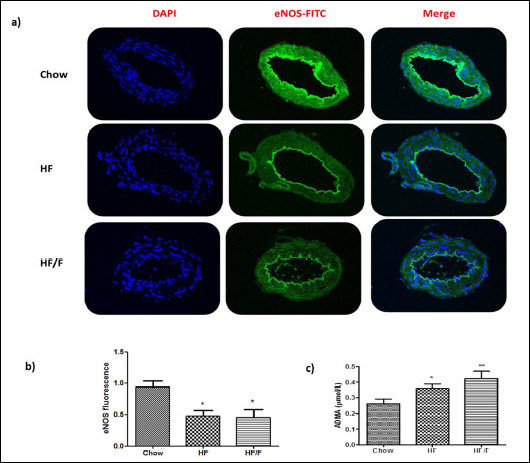 |
Fig. 8. (a) Endothelial nitric oxide synthase (eNOS) immunoreactivity and (b) eNOS fluorescence intensity in the basilar arteries. of the chow, 30% high fat (HF) and HF + fructose (HF/F) fed rats at week 12. The cell nuclei counterstained with DAPI (blue fluorescence, first column) was merged with eNOS (green fluorescence, middle column). The eNOS immunofluorescence in basilar artery cross-sections was significantly increased in both HF and HF/F groups as compared to the chow group. Kruskal Wallis test followed by Dunn’s multiple; *P < 0.05. Each group consists of 8 – 10 rats. |
DISCUSSION
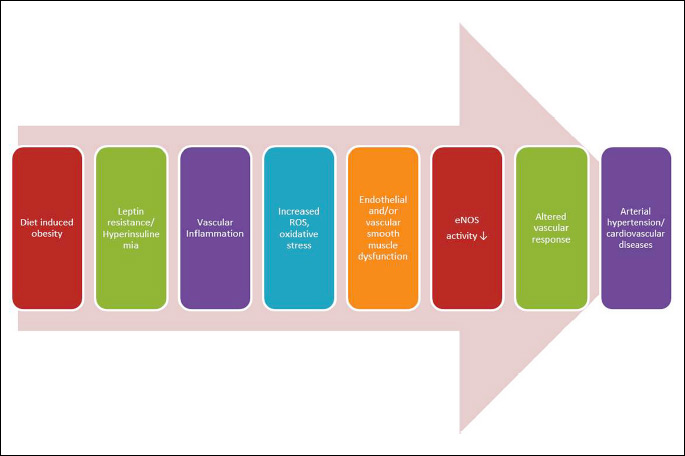
The present study evaluated the effect of high fat versus high fat plus fructose diet on cardiovascular health. We used a moderately HF diet (30% fat) which is similar to the fat content average that Americans consume. Few individuals consume a low fat, high fructose diet without glucose. Therefore, we evaluated moderate HF diet with or without fructose. In the current study, we observed no differences in caloric consumption or body mass among groups unlike the other studies where 60% fat diet was used (16). We suspect that this particular sugar/fat combination is not sufficiently palatable to sustain an increased caloric intake (total caloric consumption was unchanged across groups). Thus, the consequences of the high-fat diet were metabolic rather than weight gain. Although there was no difference in weight gain and caloric intake among the groups by week 12, the HF and HF/F diets induced changes in body composition, including an increase in absolute body fat and body % fat. Absolute lean mass was not different across diets, however, because of the increase in fat mass with unchanged body mass, percent lean mass decreased. There was more lipid deposition in HF fed animals as evidenced by the adipose tissue changes presented in Table 2. The addition of fructose to the high-fat diet significantly increased total amount of WAT levels. Also we observed that addition of fructose to the high-fat diet accelerated the development of dyslipidemia, especially the elevation in fraction of triglycerides and total cholesterol. This is consistent with previous findings by us (16) and others (17). High-fat diets are known to increase leptin levels (4). Moreover, increased leptin is associated with hyperinsulinemia, glucose intolerance, and increased body fat composition, as well as leptin resistance. Leptin, adiponectin, and resistin have been shown to have an association with abdominal adiposity and arterial stiffness (18). Furthermore, leptin is suggested to be involved in response to systemic inflammation (19). Obesity triggers an inflammatory pathway in the vessels that leads to atherosclerosis. Our data indicates that two types of high-fat diets, either with or without fructose, leading to both increased reactive oxygen species and impaired vascular responses. We suggest that the increased generation of reactive oxygen species causes oxidative stress and endothelial dysfunction along with vascular smooth muscle atrophy and dysfunction. Thus, the altered cardiovascular responsiveness leads to arterial dysfunction (Fig. 9).
Although rats do not develop rapid atherosclerosis with HF diet, they are often used as a hyperlipidemia model. The fat deposition in cerebral arteries was observed with high blood pressure, hemodynamic derangements induced by hypertension, or carotid artery obstruction in high-fat fed rats (8, 9, 20). As suggested above, the increased oxidative stress may lead to fat deposition in the vessels and result in a vascular dysfunction in terms of contractile and relaxation responses. Such a dysfunction has been widely studied with the peripheral vessels in fructose-fed (21) or high-fat fed rats (22), but fewer studies have been conducted with cerebral vessels (23, 24). An epidemiological study by Coksuer et al. (25) showed that the intima-media thickness and wall thickness of the carotid and vertebra-basilar system arteries were significantly increased in young Caucasian woman with polycystic ovary syndrome who had increased leptin levels, and glucose intolerance (25). In the present study, interestingly, we also found that wall thickness correlated with serum leptin levels. Although we cannot yet reveal the underlying mechanism how leptin induced wall thickness, whether the increased number of smooth muscle cells was the cause or result; this is the first report to show this correlation. Leptin has a role in the pathophysiology of atherosclerosis (26, 27). Under pathological conditions, such as obesity and metabolic syndrome, resistance to the acute NO-mimetic effect of leptin is accounted for by chronic hyperleptinemia and may result from different mechanisms, such as downregulation of leptin receptors in endothelium and/ or vascular smooth muscle cells (28), increased levels of cytokines, oxidative stress and overexpression of epidermal growth factor receptor signalling (29-31). In the present study along with the increased levels of leptin, the basilar artery wall thickness and intima thickness significantly increased in HF group, which likely occurred through a leptin induced proliferation of smooth muscle cells causing vascular remodelling and stiffness. Vascular stiffness is a well-known contributor to impairments in relaxation of the vessels, which is consistent with the observed decrease to relaxation (endothelium dependent and independent) response in our study.
Since the endothelium is critical for the release of vasoactive factors that modulate vascular smooth muscle tone, endothelial dysfunction is characterized by decreased production and/or bioavailability of NO. Obesity and NOS polymorphism are risk factors for hypertension in humans (32). Endothelial dysfunction in the diabetic basilar artery is related to increased oxidative stress, and insulin can preserve endothelial function by alleviating oxidative stress (33, 34). Moreover, we also previously reported that increased oxidative stress due to aging impairs basilar artery function in rats (14). Omega 3 fatty acid supplementation has been shown to have beneficial effects on endothelial dysfunction (35).
High fat diets are also known to contribute to increased oxidative stress in several organs and particularly the brain (36, 37). Most of these studies have been limited to the brain tissue. In the present study we aimed to investigate the effects on brain vessels; and to our knowledge, this is the first study to demonstrate the effect of HF and HF/F diets on the vascular response of the basilar arteries in rats. Since the basilar artery is a very tiny tissue (~300 µm); we could only use for functional and immunostaining. Hence, we assayed the oxidative stress in another cardiovascular tissue, i.e. heart.
To test the vascular reactivity we used KCl as a non-receptor dependent contractile agent, ET-1 and phenylephrine as receptor mediated contractile agents. Our findings showed that consumption of either diet does not have a significant effect on contractility, but impairs relaxation. The relaxation responses to acetylcholine (NO mediated endothelium-dependent relaxant), DEA-NONO-ate (NO donor, endothelium-independent cGMP mediated relaxant), and papaverine (direct smooth muscle relaxant that increases the cAMP mediated response via the inhibition of phosphodiestherase (PDE) were decreased in HF and HF/F groups. Such changes in the vasodilatory properties of the basilar artery could contribute to the diminished perfusion and higher vascular resistance in the posterior cerebral circulation of at rest (38) and during periods of stress, such as under environmental conditions of hypoxia and hypercapnea (39). When we further examined the vessels under the microscope, we observed that the intima thickness was significantly increased with both HF and HF/F diets indicating a vascular hypertrophic remodelling. In large conduit arteries, remodelling leads to vascular smooth muscle cells with decreased ability to contract or relax. They lose myofilaments and exhibit a high rate of proliferation and production of extracellular matrix proteins (40, 41). It is known that increases in wall thickness and decreases in smooth muscle cells along with increased collagen network area and decreased elastin function may be responsible for the stiffening of the basilar artery (42, 43), and contribute to the development of atherosclerosis and vertebrobasilar insufficiency. The decreased relaxation of the smooth muscle cells could be either be cAMP or cGMP-mediated. Our findings indicate that both pathways were involved in diet-induced vascular dysfunction; i.e. papaverine and DEA response were decreased as well as the endothelium dependent ACh response which is NO-mediated.
NO is produced by the endothelial NO synthase enzyme (eNOS) from L-arginine. Circulating levels of asymmetrical dimethyl arginine (ADMA), an endogenous NOS inhibitor, is positively correlated with the increased carotid intima-media thickness (44). Thus the progression of atherosclerosis increases the risk of myocardial infarction, stroke and other cardiovascular diseases. In our study, we report that the serum ADMA levels were significantly elevated in HF and HF/F diets, and accordingly, eNOS immunofluorescence and NO response were decreased in the basilar arteries. Such a decrease in NO synthesis was also shown in the basilar arteries of obese Zucker rats (45, 46) and diabetic rats (33).
Furthermore, previous experiments indicated that a 60% HF diet increases heart rate and blood pressure in young rats (36, 47). In the present experiment, our 30% high-fat diets resulted in no significant increase in blood pressure or heart rate by week 10. Therefore, we investigated stress-induced response to blood pressure and heart rate. Although both HF and HF/F rats demonstrated a slightly elevated increase in MAP and heart rate, these differences were not statistically significant. On the other hand, weight of the heart tissue and its ratio to body weight was significantly higher in HF and HF/F groups; possibly due to the accumulation of triglycerides in myocytes. Moreover the MDA content, an index for lipid peroxidation, was significantly increased in the hearts of HF and HF/F fed rats, indicating increased oxidative stress. However, the endogenous antioxidant glutathione levels were not different among groups. GSH rapidly decreases during an acute insult that triggers oxidative stress and lipid peroxidation. The oxidative tissue injury is generally due to an imbalance of oxidative/antioxidant status. However, antioxidant defence system replenishes in the long term by the compensatory mechanisms.
Generally, we observed that for almost all of the parameters, either diet resulted in similar outcomes. In a companion study that we have recently published HF diets worsened cancellous bone parameters in skeletally immature male rats and fructose incorporation into HF diets did not exacerbate bone loss (48). Consistently, our findings on the cardiovascular parameters also support that addition of fructose to the high-fat diet did not have an additional worsening effect in this experiment. On the other hand, there are reports on the adverse effects of fructose (49, 50). The reason that we did not observe any additional worsening effect may be due to the relatively short feeding period (12 weeks) or lower fructose and high fat content. Long term studies will be useful to reveal the consequence of the metabolic changes on cardiovascular system.
These findings demonstrate that both HF and HF/F diets result in an altered body composition towards increased fat/lean ratio, and cardiovascular changes. Impairment in vascular responses is the result of structural changes in the vasculature, i.e. endothelial dysfunction (as evidenced by impaired ACh response) and vascular remodelling (evidenced by increased intima thickness). Vascular remodelling is associated with decreased synthesis of NO; and response to NO and other vasodilators like papaverine. These processes lay the groundwork for the progression towards cerebrovascular and cardiovascular complications. Interestingly, the addition of fructose to the HF diet did not exacerbate the effect.
Acknowledgements: Supported by the National Institutes of Health Grant DK 091710 and North Florida/South Georgia Veterans Health System, Research/GRECC, Gainesville. The authors are grateful to Dr. Warren Green and Dr. Jeffrey Martens for their assistance with the fluorescent microscope, to Dr. Michael D. Delp and Dr. Michael Katovich for using their cryostat and tail-cuff equipment.
Conflict of interests: None declared.
REFERENCES
- WHO Fact Sheet N°311 [Updated August 2014]. Obesity and overweight. http://www.who.int/mediacentre/factsheets /fs311/en/
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalance of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311: 806-814.
- Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: an old-age or new-age problem? Biochem Pharmacol 2012; 84: 737-745.
- Vasselli JR, Scarpace PJ, Harris RB, Banks WA. Dietary components in the development of leptin resistance. Adv Nutr 2013; 4: 164-175.
- Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1370-R1375.
- Mahmud A, Feely J. Adiponectin and arterial stiffness. Am J Hypertens 2005; 18: 1543-1548.
- Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation 2002; 106: 1919-1924.
- Lekic T, Ani C. Posterior circulation stroke: animal models and mechanism of disease. J Biomed Biotechnol 2012; 2012: 587590. doi: 10.1155/2012/587590
- Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G. Basilar artery occlusion. Lancet Neurol 2011; 10: 1002-1014.
- Israeli-Korn SD, Schwammenthal Y, Yonash-Kimchi T, et al. Ischemic stroke due to acute basilar artery occlusion: proportion and outcomes. Isr Med Assoc J 2010; 12: 671-675.
- Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res 2015; 116: 884-894.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52: 302-310.
- Beutler E. Glutathione in Red Cell Metabolism: a Manual of Biochemical Methods, New York, Grune and Stratton, 1975, p. 112-114.
- Tumer N, Toklu HZ, Muller-Delp JM, et al. The effects of aging on the functional and structural properties of the rat basilar artery. Physiol Rep 2014; 2(6): pii: e12031. doi: 10.14814/phy2.12031.
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 1990; 66: 8-17.
- Shapiro A, Tumer N, Gao Y, Cheng KY, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr 2011; 106: 390-397.
- Huang BW, Chiang MT, Yao HT, Chiang W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes Metab 2004; 6: 120-126.
- Windham BG, Griswold ME, Farasat SM, et al. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens 2010; 23: 501-507.
- Koenig S, Luheshi GN, Wenz T, Gerstberger R, Roth J, Rummel C. Leptin is involved in age-dependent changes in response to systemic inflammation in the rat. Brain Behav Immun 2014; 36: 128-138.
- Yamori Y, Horie R, Sato M, Fukase M. Hemodynamic derangement for the induction of cerebrovascular fat deposition in normotensive rats on a hypercholesterolemic diet. Stroke 1976; 4: 385-389.
- Nyby MD, Abedi K, Smutko V, Eslami P, Tuck ML. Vascular angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens Res 2007; 30: 451-457.
- Kleinschmidt TL, Oltman CL. Progression and reversal of coronary and mesenteric vascular dysfunction associated with obesity. Obesity 2014; 22: 2193-2200.
- Suzuki M, Yamamoto D, Suzuki T, et al. High fat and high fructose diet induced intracranial atherosclerosis and enhanced vasoconstrictor responses in non-human primate. Life Sci 2006; 23: 200-204.
- Li W, Prakash R, Chawla D, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol 2013; 304: R1001-R1008. doi: 10.1152/ajpregu.00523.2012.
- Coksuer H, Koplay M, Oghan F, Haliloglu B, Keskin N. Evaluation of carotid wall thickness and vertebro-basilar system insufficiency in patients with obese polycystic ovary syndrome. J Obstet Gynaecol Res 2011; 37: 997-1003.
- Beltowski J. Leptin and atherosclerosis. Atherosclerosis 2006; 189: 47-60.
- Krawczynska A, Olczak E, Rembiszewska A, Herman AP, Gromadzka-Ostrowska J. Time-dependent supplementation of vitamin E influences leptin expression in the aortic layers of rats fed atherogenic diet. J Physiol Pharmacol 2014; 65: 33-39.
- Trovati M, Doronzo G, Barale C, Vaccheris C, Russo I, Cavalot F. Leptin and vascular smooth muscle cells. Curr Pharm Des 2014; 20: 625-634.
- Beltowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol 2012; 39: 168-178.
- Beltowski J. Leptin and the cardiovascular system - a target for therapeutic interventions. Curr Pharm Des 2014; 20: 601-602.
- Beltowski J, Jazmroz-Wisniewska A. Transactivation of Erb B receptors by leptin in the cardiovascular system: mechanisms, consequences and target for therapy. Curr Pharm Des 2014; 20: 616-624.
- Wrzosek M, Sokal M, Sawicka A, et al. Impact of obesity and nitric oxide synthase gene G894T polymorphism on essential hypertension. J Physiol Pharmacol 2015; 66: 681-689.
- Matsumoto T, Yoshiyama S, Wakabayashi K, Kobayashi T, Kamata K. Effects of chronic insulin on endothelial dysfunction of basilar arteries from established streptozotocin-diabetic rats. Eur J Pharmacol 2004; 504: 119-127.
- Busija DW, Miller AW, Katakam P, Erdos B. Adverse effects of reactive oxygen species on vascular reactivity in insulin resistance. Antioxid Redox 2006; 8: 1131-1140.
- Frimmel K, Vlkovicova J, Sotnikova R, Navarova J, Bernatova I, Okruhlicova L. The effect of omega-3 fatty acids on expression of connexin-40 in Wistar rat aorta after lipopolysaccharide administration. J Physiol Pharmacol 2014; 65: 83-94.
- Erdos B, Kirichenko N, Whidden M, Basgut B, Woods M, Cudykier I, et al. Effect of age on high-fat diet-induced hypertension. Am J Physiol Heart Circ Physiol 2011; 301: 164-172.
- Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci 2014; 17: 241-251.
- Salter JM, Cassone VM, Wilkerson MK, Delp MD. Ocular and regional cerebral blood flow in aging Fischer-344 rats. J Appl Physiol 1998; 85: 1024-1029.
- Haining JL, Turner MD, Pantall RM. Local cerebral blood flow in young and old rats during hypoxia and hypercapnia. Am J Physiol 1970; 218: 1020-1024.
- Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-humanprimate Macacamulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 2012; 67: 811-820.
- Masawa N, Yoshida Y, Yamada T, Joshita T, Sato S, Mihara B. Morphometry of structural preservation of tunica media in aged and hypertensive human intracerebral arteries. Stroke 1994; 25: 122-127.
- Fonck E, Feigl GG, Fasel J, et al. Effect of aging on elastin functionality in human cerebral arteries. Stroke 2009; 40: 2552-2556.
- Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res 1990; 66: 1747-1754.
- Bai Y, Sun L, Du L, et al. Association of circulating levels of asymmetric dimethylarginine (ADMA) with carotid intima-media thickness: evidence from 6168 participants. Ageing Res Rev 2013; 12: 699-707.
- Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes 2004; 53: 1352-1359.
- Karagiannis J, Reid JJ, Darby I, Roche P, Rand MJ, Li CG. Impaired nitric oxide function in the basilar artery of the obese Zucker rat. J Cardiovasc Pharmacol 2003; 42: 497-505.
- de Castro UG, dos Santos RA, Silva ME, de Lima WG, Campagnole-Santos MJ, Alzamora AC. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis 2013; 12: 136. doi: 10.1186/1476-511X-12-136.
- Yarrow JF, Toklu HZ, Balaez A, et al. Fructose consumption does not worsen bone deficits resulting from high-fat feeding in young male rats. Bone 2016; 85: 99-106.
- Axelsen LN, Lademann JB, Petersen JS, et al. Cardiac and metabolic changes in long-term high fructose-fat fed rats with severe obesity and extensive intramyocardial lipid accumulation. Am J Physiol Regul Integr Comp Physiol 2010; 298: R1560-R1570.
- Axelsen LN, Pedersen HD, Petersen JS, Holstein-Rathlou NH, Kjolbye AL. Metabolic and cardiac changes in high cholesterol-fructose-fed rats. J Pharmacol Toxicol Methods 2010; 61: 292-196.
A c c e p t e d : March 29, 2016
Dr. Hale Zerrin Toklu, Department of Pharmacology and Therapeutics, University of Florida, College of Medicine, 1200 Newell Dr., PO Box 100267, Gainesville, FL, 32610, USA; e-mail: haletoklu@yahoo.com