MODULATORY EFFECTS OF STEROID HORMONES, OXYTOCIN, ARACHIDONIC ACID, FORSKOLIN AND CYCLIC AMP ON THE EXPRESSION OF AQUAPORIN 1 AND AQUAPORIN 5 IN THE PORCINE UTERUS DURING PLACENTATION
INTRODUCTION
Aquaporins are channel-forming trans-membrane proteins facilitating the water movement across the cellular plasma membrane (1). To date, there are 13 known mammalian aquaporins (AQP0-AQP12) that enable increased permeability of water as well as other small molecules such as glycerol and urea (2). Numerous studies have characterized the tissue-specific distribution of AQPs in rats, mice and humans (3). Furthermore, studies with animal knockout for genes encoding these proteins have proved the physiological significance of AQPs in the regulation of water transport in different organs, including those belonging to the reproductive system (4-7). Currently, AQPs are also considered as important players in processes associated with reproduction. Several AQP isoforms were found in the female reproductive tissues: ovary, uterus, placenta, amnion and chorion cytotrophoblast (8, 9). In the pig, the presence of AQP1 and AQP5 in uterus has been documented (10, 11). These AQPs are water selective and belong to the classical AQP family (2). AQP1 is a 28-kDa protein expressed in kidneys (12), pleural membranes, alveolar microvessels, gall bladder, fetal membranes, placenta, endothelia and different epithelia (13-15). AQP5 has mainly been localized in the apical plasma membranes of various secretory glands (16, 17) as well as in the apical membrane of the airway epithelium, corneal and pancreatic epithelium (18). In the pig uterus, AQP1 mostly appears in the capillary endothelium, but AQP5 in smooth muscles as well as in luminal and glandular epithelial cells (10, 11). Data accumulated so far, pertaining to the uterus, suggest that AQPs are involved in water movement between the intraluminal, interstitial and capillary compartments and their uterine expression is essential throughout the pregnancy, including its early stages (19, 15, 20) and parturition (21).
In the pig, the type of placenta is epitheliochorial, in which the chorion does not invade into the uterine endometrium, and the placental barrier includes both trophectoderm and uterine epithelium (22). The placentation in this species starts around Day 30 of pregnancy, when the free-floating embryos are already attached to the endometrium. This process relies on conceptus elongation to increase the available surface area for gas and nutrient exchange (23). Moreover, during early pregnancy, the intra-uterine microenvironment is enriched in a complex array of substances, mainly produced by the uterine glands, which play an important role in onset of pregnancy, i.e. conceptus development, implantation and placentation (24-28). Thus, we supposed that the uterine AQPs are implicated in water handling in connection with the high endometrial secretory activity. A participation of steroid hormones, OT, AA, cAMP and FSK in the regulation of AQP1 and AQP5 expression in the porcine uterus has been tested during the estrous cycle and implantation period (29, 30). In recently published papers, different aspects of placentation process in the pig were considered (31, 32), however data concerning the uterine expression of AQPs during this period are not available. Therefore, in this study, we evaluated the effects of chosen factors on AQP1 and 5 expressions in the pig uterus during placentation using the in vitro procedures. The study aimed to assess the influence of short (3 h) and long (24 h) in vitro incubation of the porcine uterine tissue, representing the placentation period, to P4, E2, OT, AA, cAMP and FSK on the expression of AQP1 and AQP5 mRNA and protein. Preliminary results of this research have been reported during national meeting of Polish Physiological Society (33).
MATERIALS AND METHODS
Animals and collection of uterine tissue
All experiments were performed in accordance with Animal Ethics Committee (AEC approval No. 66/2010 DTN), University of Warmia and Mazury in Olsztyn, Poland.
Tissue samples were recovered from mature cross-bred gilts (Large White × Polish Landrace) (n = 5) on Days 30 – 32 of gestation (placentation). Gilts were observed daily for estrus behavior (Day 0) and exhibited at least two estrous cycles of normal duration (Days 18 – 21). During third estrus they were naturally bred. The animals were slaughtered at a local abattoir on Days 30 – 32 of pregnancy. Pregnancy was additionally confirmed by the presence of morphologically normal conceptuses. Isolated uteri were placed immediately in ice-cold phosphate-buffered saline (PBS) supplemented with 100 IU/ml penicillin (Polfa, Poland) and 100 µg/ml streptomycin (Polfa, Poland) and transported to the laboratory on ice within 1 to 1.5 h for in vitro tissue culture.
Preparation and incubation of uterine slices
Sections of the middle part of uterine horn collected from pigs were opened longitudinally on the mesometrial surface. Uteri were washed three times with sterile PBS, cut into small pieces (400 mg weight) and washed three times in medium M199 (Sigma, USA). The uterine slices were placed in culture vials containing 2 ml Medium 199 supplemented with 0.1% BSA (Sigma), 20 µg nystatin (Sigma) and 20 µg gentamicin (Krka, Novo Mesto, Slovenia) and then preincubated in vitro under atmosphere of 95% O2 and 5% CO2 at 37°C for 18 h. After preincubation, the culture medium was replaced with fresh medium, and the explants were treated with vehicle (control) or P4 (10–5 M), E2 (10 nM), OT (10–7 M; Sigma), AA (10–5 M; Sigma), cpt-cAMP analogue (200 µM; Sigma) and FSK (10 ng/mL) and incubated for additional 3 or 24 h. Concentrations for the treatments were previously determined (29, 34, 35). All treatments were performed in duplicates. Furthermore, uterine tissue explants were snap-frozen in liquid nitrogen (for RNA and protein extraction) and stored at –80°C until further use.
Total RNA isolation, cDNA synthesis and quantitative real-time polymerase chain reaction analysis
Total RNA was extracted from the uterine explants collected after in vitro culture (n = 5 cultures), using fenozol (A&A Biotechnology, Gdansk Poland) in accordance with manufacturer`s instructions. RNA quality and quantity were determined with spectrophotometry (NanoDrop ND-1000, Thermo Scientific, Wilmington, DE, USA). Total RNA samples were transcribed to cDNA using an Enhanced Avian HS RT-PCR Kit (Sigma) and a mix of dNTPs and random hexamers as primers. Real-time PCR was performed in duplicate for each sample by 7300 Real-time PCR system and SYBR®Green PCR Master Mix (Life Technologies, Grand Island, NY, USA) using specific primers for AQP1 and AQP5 as described previously (29). Samples for the specificity control, non-template controls and dissociation curve analysis of the amplified products were used for each amplification. The specificity of amplifications was further validated with electrophoresis of obtained amplicons in a 2% agarose gel and, after extraction from gel, submitted to automated sequencing using 3730xl DNA Analyzer (Life Technologies). Levels of gene expression were calculated with the ΔΔCt method and normalized using the geometrical means for the expression of two reference genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18S rRNA.
SDS-PAGE and Western blot
Following isolation, the tissues were immediately placed in ice-cold dissection buffer (0.3 M sucrose, 25 mM imidazol, 1 mM EDTA in ddH2O, pH 7.2) containing 8.4 µM leupeptin and 0.4 mM pefabloc. As described previously (29), the nitrocellulose membranes were incubated overnight at 5°C with anti-AQPs or β-actin antibodies, then with horseradish peroxidase-conjugated secondary antibody (P448, diluted 1:3,000, Dako A/S, Glostrup, Denmark) in PBS-T for 1 h. After washing with PBS-T, the sites of antibody-antigen reaction were visualized with an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Little Chalfont, UK) and exposed to photographic film (Hyperfilm ECL, RPN3103K, Amersham Pharmacia Biotech, Little Chalfont, UK). The products of Western blotting were quantified by densitometric scanning of immunoblots with GelScan for Windows version 1.45 software (Kucharczyk, Poland). Data were expressed as a ratio of AQP proteins relative to actin protein in OD units. In our previous study, we demonstrated that anti-AQP1 and anti-AQP5 antibodies preincubated with the respective immunizing peptides prevented labeling in the pig uterus (11).
Immunohistochemistry
Paraffin embedded blocks of porcine uterus after culture were prepared as described in details elsewhere (11, 29). The tissue sections (4 µm) were exposed to the primary antibody overnight, then to a horseradish peroxidase conjugated goat anti-rabbit secondary antibody (P448; Dako). Nonspecific binding of IgG was eliminated by incubating the sections in 50 mM NH4Cl for 30 min, followed by blocking in PBS supplemented with 1% BSA, 0.05% saponin and 0.2% gelatin. Labeling was visualized by 0.05% 3.3 diaminobenzidine tetrahydrochloride (DAB), and finally counterstained with Mayer`s haematoxylin. The microscopy was carried out using an Olympus light microscope (BX51, Japan). In addition, immunoglobulins from non-immunized rabbit were used as a negative control.
Statistical analysis
All numerical data were analyzed by one-way ANOVA and least significant difference (LSD) post hoc test and reported as the means (± S.E.M.) for five independent observations. Statistical analyses were performed using the Statistica program (StatSoft Inc., Tulsa, USA). Value of P < 0.05 was considered to be statistically significant.
RESULTS
AQP1 mRNA expression in the porcine uterine explants
The control abundance of AQP1 transcript in the uterine tissue harvested from gilts on Days 30 – 32 of pregnancy (the placentation period) did not significantly change during 3-h and 24-h incubations (Fig. 1A and 1B). The AQP1 mRNA expression in the uterine explants significantly (P < 0.05) decreased after 3-h treatments with P4, AA and FSK. Unlike, treatment with E2 for 24 h significantly (P < 0.05) increased AQP1 gene expression (Fig. 1A and 1B). Treatment with OT for 24 h significantly decreased AQP1 expression (P < 0.05). In turn, treatment with cAMP did not change AQP1 expression in the uterine explants (Fig. 1B).
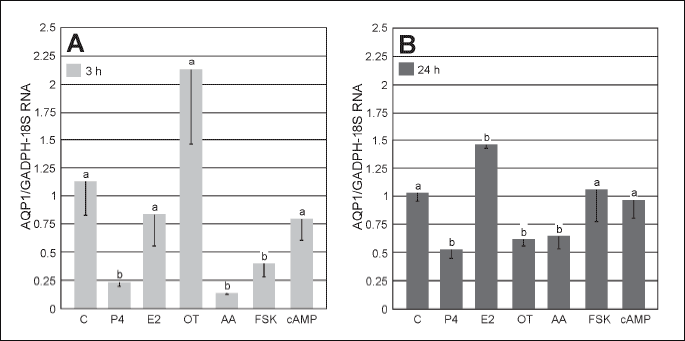
AQP5 mRNA expression in the uterine explants
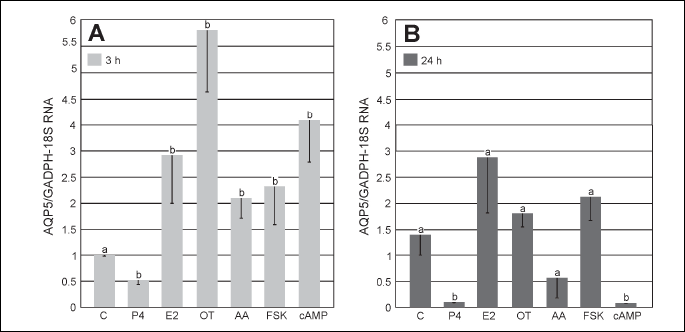
The expression of AQP5 mRNA in the uterine explants on Days 30 – 32 of pregnancy is presented on Fig. 2A and 2B. The content of AQP5 transcript in the uterine explants following control incubations for 3 and 24 h did not significantly differ. The uterine explants incubated for 3 h with E2, OT, AA, FSK and cAMP exhibited significantly (P < 0.05) increased AQP5 mRNA expression. In contrast, P4 treatment for 3 and 24 h significantly (P < 0.05) reduced AQP5 mRNA expression (Fig. 2A and 2B). Similarly, AA (P < 0.05) and cAMP (P < 0.05) treatments for 24 h significantly decreased AQP5 mRNA expression (Fig. 2B).
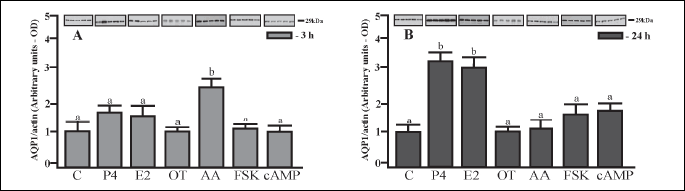
AQP1 protein content in the porcine uterine explants
The effects of P4, E2, OT, AA, FSK and cAMP on AQP1 protein expression in uterine explants harvested from pregnant gilts during the placentation period (Days 30 – 32) are shown in Fig. 3A and 3B. AQP1 protein expression in the uterine explants was significantly increased (P < 0.05) only by AA, after 3-h incubation (Fig. 3A). In turn, P4 and E2 significantly (P < 0.05) stimulated the expression of AQP1 protein (by ~3-fold) following 24-h incubation (Fig. 3B).
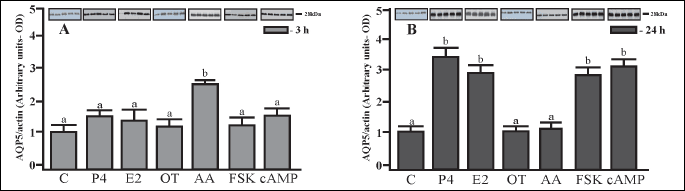
AQP5 protein content in the porcine uterine explants
The AQP5 expression in the uterine explants from gilts on Days 30 – 32 of pregnancy is depicted in Fig. 4A and 4B. The incubation of the explants with AA for 3 hours significantly (P < 0.05) increased in AQP5 protein expression (by ~2.7-fold), (Fig. 4A). Longer incubation (24 hours) with P4, E2, FSK and cAMP also significantly (P < 0.05) elevated AQP5 protein expression (by ~2.5 – 3.0 fold), Fig. 4B.
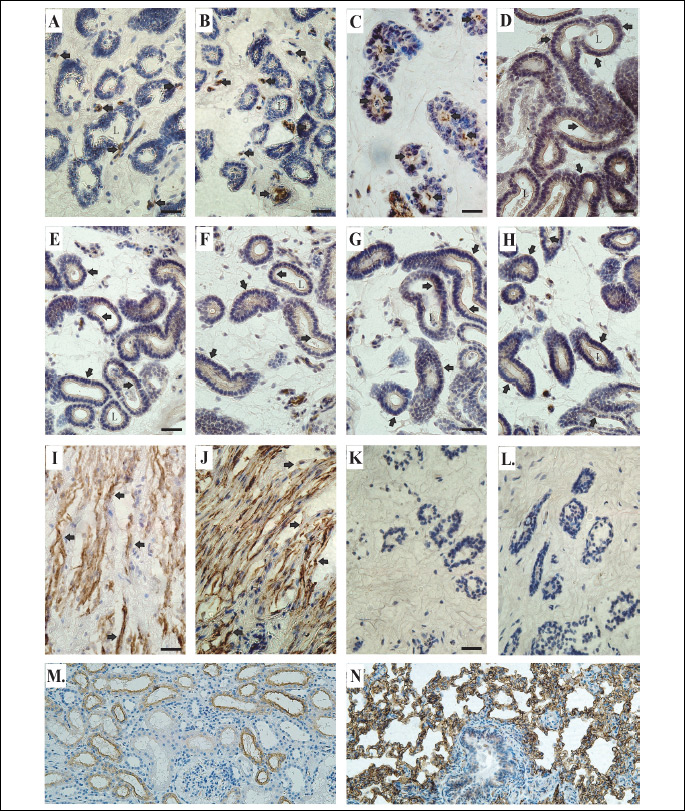
Immunohistochemical localization of AQP1 and AQP5 in the porcine uterine explants
In the uterine explants from gilts on Days 30 – 32 of pregnancy (the placentation period), immunoperoxidase labeling for AQP1 was associated with uterine endothelial cells under control conditions as well as following applied treatments. As exemplarily shown in Fig. 5, both the apical and basal cell membranes exhibited stable AQP1 labeling in control (Fig. 5A) and E2-treated uterine explants (Fig. 5B). In turn, AQP5 was associated with uterine epithelial cells (Fig. 5C-5H) and smooth muscle cells (Fig. 5I-5J). The prominent AQP5 labeling was seen in the apical (in control; Fig. 5C) and additionally in basolateral plasma membranes of the epithelial cells following treatments of the tissue explants with P4 (Fig. 5D), E2 (Fig. 5E), FSK (Fig. 5F) and cAMP (Fig. 5G) for 24 h, and with AA for 3 h (Fig. 5H). In the smooth muscle cells, in contrast to the epithelial cells, applied treatments did not cause any changes in AQP5 localization within the cell membranes.
DISCUSSION
In this in vitro study, performed with the uterine explants derived from pregnant gilts during placentation period (Days 30 – 32), we observed the following effects of tested factors; 1) stimulatory of E2 on AQP1 and 5 mRNA expression, 2) predominantly inhibitory of progesterone on AQP1 and 5 mRNA levels, 3) stimulatory of P4, E2 and AA on both AQPs, but cAMP and FSK only on AQP5 protein content, and 4) prominent AQP5 labeling in both the apical and basolateral plasma membranes of the glandular epithelial cells in response to 24-h exposure to steroid hormones, AA, cAMP and FSK.
In previous study, we have described the effects of steroid hormones on the expression of AQP1 and 5 mRNAs on Days 14 – 16 of pregnancy when the implantation occurs (30). In that study, E2 decreased the expression of both AQPs, but P4 affected neither AQP1 nor AQP5 mRNA expression. In the present study, both steroids influenced the expression of studied AQPs during placentation. Namely, E2 up-regulated, but P4 down-regulated AQP1 and 5 expressions. Notably, the response of AQP mRNAs to estradiol required longer (24 h) exposition to the treatment. This comparison implies the existence of distinct expression patterns of genes encoding these AQPs in response to P4 and E2 during implantation and placentation. The change of estradiol action on the expression of AQPs from inhibitory to stimulatory with progressing gestation seems to be the most intriguing observation. It was already found that uterine tissues in pigs produce considerable amounts of steroids (36, 25), including estradiol, of which daily uterine production in this species during early pregnancy reaches about 1 ug (25). It is also likely, that estrogens secreted by developing placenta may additionally modify the uterine AQP expression (37). In previous studies, stimulatory effect of E2 on the uterine expression of AQP1 in rat and AQP5 in mouse were documented by Li et al. (38) and Kobayashi et al. (39), respectively. In addition, Kobayashi et al. (39) have revealed the presence of functional estrogen response element in AQP5 promoter what suggests a possibility of direct action of estrogens on this AQP gene expression. Moreover, Richard et al. (15) found increased AQP1 mRNA expression in mice myometrium in response to estrogens. In contrast to the opposite AQP mRNAs response to E2 and P4 found in the present experiment, the expression of studied AQPs at the protein level was stimulated by both steroids. In many other studies, the lack of correlation between mRNA and protein expression has been already reported (40-44). Our data suggest, that progesterone is engaged in stimulation of AQP protein synthesis in the uterine tissue during placentation period, despite the lack of evident increase in AQP transcript content. Possibly, this effect was induced at the posttranscriptional level.
Oxytocin is an important factor that stimulates contraction of uterus (45), as well as affects phosphoinositide hydrolysis (46), expression of COX-2 in pig uterine tissue (47) and PGF2α secretion (46). It was also evidenced that endometrially secreted OT may play a role in the CL maintenance during early pregnancy. In the porcine uterine lumen, significantly higher concentration of OT is observed on Days 11 – 14 of pregnancy than during corresponding days of the estrous cycle (48). In the present studies, we observed the up-regulation of uterine AQP5 gene expression after 3-h incubation with OT. In turn, OT treatment decreased AQP1 mRNA expression after longer incubation. However, similarly to implantation period (30), OT affected neither AQP1 nor AQP5 protein expression during placentation. Very recently, Ducza and coworkers (49) proposed that OT selectively influences the expression of AQP5 at the end of pregnancy and may serve as a marker that indicates the initiation of delivery in rats.
Prostaglandins (PGs), especially PGE2 and PGF2α, are implicated in the regulation of reproductive processes at the utero-ovarian level, including the crucial role of PGE2 in maintenance of pregnancy and conceptus development (50). Both prostaglandins are produced in the porcine uterus, mainly by endometrial tissue (47), but also by myometrium (34). It is well known that prostaglandin synthesis depends on accessibility of arachidonic acid (51) and the activity of enzymes involved in its metabolism, such as COX-1 and COX-2 (52). Previous studies have suggested that prostaglandins may affect the expression of AQPs. Namely, it was found that the distribution of AQP2 in rat inner medulla is regulated by a balance between AVP and PGE2 (53), and that mice lacking the initial enzyme in prostaglandin synthesis, cPLA2, have abnormal AQP1 expression in the kidney (54). In our recent in vitro study performed with uterine explants derived from gilts on Days 10 – 12 and 14 – 16 of the estrous cycle, the expression of AQP1 and 5 was stimulated at the protein level by AA only during luteolysis (29). During the placentation, the AQP1 expression at the mRNA level was significantly reduced, but at the protein level was stimulated by AA. In turn, the expression of AQP5 was stimulated at the mRNA and protein levels by AA during 3-h exposition. Interestingly, during implantation the inverse relation was found in case of AQP5; AA decreased its mRNA, but increased its protein (30). Collectively, these data indirectly suggest that during placentation the AQP1 and 5 expression may be specifically affected by prostaglandins. Nevertheless, an examination of colocalization of these AQPs with major enzymes of prostaglandin pathway, COX-1 or COX-2, would provide deeper insight into the role of prostaglandins in the uterine AQPs expression during early pregnancy.
In the present study, mainly AQP5 was submitted to regulatory effect of cAMP and forskolin, since these compounds markedly increased AQP5 mRNA during 3-h exposure and protein abundance after 24-h incubation. This indicates that elevated AQP protein abundance most likely resulted from increased number of its transcripts, which was accompanied by subsequent decrease in AQP5 mRNA level (24 h). Similar relationship was found by Yang et al. (35) in studies concerning mouse lung tissue. Moreover, Sidhaye et al. (55) observed that the exposure of lung epithelial cells for several hours to cAMP or forskolin increased AQP5 protein abundance. In the present study, only forskolin decreased AQP1 mRNA after 3-h incubation, whereas other treatments of the uterine tissue with cAMP or FSK were ineffective. This notation is in agreement with the results published by Wang et al. (56), who failed to see any effects of cAMP analogue on the expression of AQP1 gene in vitro in human amnion epithelia, although AQP1 mRNA was increased by FSK. In contrast, during implantation, cAMP and FSK increased both AQP1 mRNA and protein expression (30). Altogether, the previous (30) and present our studies indicate that participation of cAMP in the regulation of AQP1 expression in the uterine tissues is modified depending on the stage of gestation.
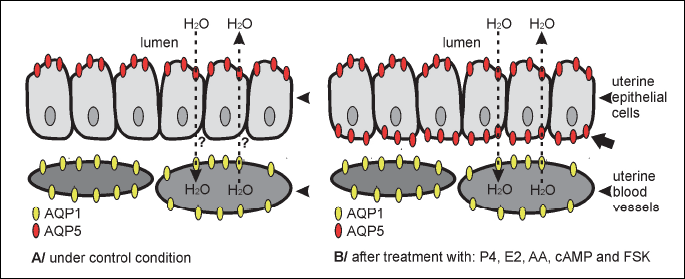
In the present study, we also established the uterine localization of AQP1 and 5 by immunohistochemistry in pigs during placentation. Previous studies have documented predominant localization of AQP1 in the apical and basal membranes of uterine endothelial cells and AQP5 in the apical membranes of epithelial cells (10, 11). Such distribution of AQP5 protein in the uterine glands is in line with its epithelial localization in type I and type II pulmonary alveolar cells as well as in the yolk sac and salivary glands (35, 57-59). In the present study, in vitro treatment of the uterine explants with steroid hormones (P4 and E2) has caused an intense accumulation of AQP5 signal additionally in the basolateral membrane of the epithelial cells. The effects of steroid hormones on AQPs expression were also studied in rodents mainly during implantation period (15, 19, 20, 60). Lindsay and Murphy (20, 60) reported increased expression of AQP5 in uterine epithelial cells by progesterone alone and in combination with estrogens. They also noted progesterone-dependent expression of AQPs in the rat uterus; AQP5 in glandular epithelium and AQP1 in the inner circular layer of myometrium. Moreover, in our study, AA, cAMP and FSK have caused - in contrast to control - a prominent accumulation of AQP5 in the basolateral membrane of the uterine epithelial cells. Similar effects of studied factors on localization of AQP5 were noted in uterine tissue representing the implantation period (30). The above observations, concerning AQP5 distribution in the uterine tissue, are summarize in Fig. 6. The appearance of AQP5 on surface of the basolateral membrane following treatment with examined substances indicates that this aquaporin is markedly engaged in water efflux and influx within uterus. These changes seem to be connected with high secretory activity of endometrial glands during early pregnancy, which release multiple substances that promote uterine receptivity and embryonic development (61, 25).
In summary, this research has demonstrated the uterine expression of mRNAs and proteins for AQP1 and AQP5 in pregnant gilts during placentation as well as the effects of studied factors (P4, E2, OT, AA, cAMP and FSK) on this expression. Estradiol stimulated AQP1 and 5 mRNA expression, but progesterone predominantly inhibited AQP1 and 5 mRNA levels. In turn, at the protein level, steroid hormones and AA stimulated the expression of both AQPs, but cAMP and FSK stimulated only AQP5. Interestingly, the basal uterine expression of AQP1 during placentation appeared to be markedly lower than during implantation in previous study. Moreover, studied factors (P4, E2, AA, cAMP or FSK) have caused appearance of AQP5 in the basolateral membranes of epithelial cells, in addition to its presence in the apical membranes of these cells.
Based on our previous and present studies, it might be concluded that AQP1 and AQP5 play an important physiological role in maintaining water homeostasis and transcellular water movement within endometrial and endothelial cells in the pig uterus during early pregnancy. The present study also indicates that uterine expression of AQP1 and AQP5 remains under control of steroid hormones (P4 and E2), AA derivatives and cAMP, which contribute to embryo placentation in pigs.
Author contributions: conception and design of research: MTS and AS, the protein analysis: MTS and AS, the gene expression analysis: PM and AS, contribution to the analysis and interpretation of data: AS, the manuscript was written by AS, revised by: SO, SN and MTS, prepared figures: MTS and AS.
Acknowledgements: This work was support by Grant Number N N308 5848 40 awarded to MTS from the Polish Ministry of Science and Higher Education.
Conflict of interests: None declared.
REFERENCES
- Carbrey JM, Agre P. Discovery of the aquaporins and development of the field. Handb Exp Pharmacol 2009; 190: 3-28.
- Agre P, King LS, Yasui M, et al. Aquaporin water channels-From atomic structure to clinical medicine. J Physiol 2002; 542: 3-16.
- Ishibashi K, Hara K, Kondo S. Aquaporin water channels in mammals. Clin Exp Nephrol 2009; 13: 107-117.
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of salivia in transgenic mice lacking aquaporin-5 water channels. J Biol Chem 1999; 274: 20071-20074.
- Verkman AS. Physiological importance of aquaporin water channels. Ann Med 2002; 34: 192-200.
- Sha X, Xiong Z, Liu H, Di X, Ma T. Maternal-fetal fluid balance and aquaporins: from molecule to physiology. Acta Pharmacol Sin 2011; 32: 716-720.
- Zheng Z, Liu H, Beall M, Hao R, Ross MG. Role of aquaporin 1 in fetal fluid homeostasis. J Matern Fetal Neonatal Med 2014; 27: 505-510.
- Zhang D, Tan YJ, Qu F, Sheng JZ, Huang HF. Functions of water channels in male and female reproductive systems. Mol Aspects Med 2012; 33: 676-690.
- Hua Y, Jiang W, Zhang W, Shen Q, Chen M, Zhu X. Expression and significance of aquaporins during pregnancy. Front Biosci (Landmark Ed.) 2013; 18: 1373-1383.
- Skowronski MT, Kwon TH, Nielsen S. Immunolocalization of aquaporin 1, 5 and 9 in the female reproductive system. J Histochem Cytochem 2009; 57: 61-67.
- Skowronski MT. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod Biol Endocrinol 2010; 8: 109.
- Nielsen S, DiGiovani SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunilocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 1993; 90: 11663-11667.
- Oshio K, Song Y, Verkman AS, Manley GT. Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir Suppl 2003; 86: 525-528.
- Jablonski EM, McConnell NA, Hughes FM Jr, Huet-Hudson YM. Estrogen regulation of aquaporins in the mouse uterus: potential roles in uterine water movement. Biol Reprod 2003; 69: 1481-1487.
- Richard C, Gao JU, Brown N, Reese J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the peri-implantation period in the mouse. Endocrinology 2003; 144: 1533-1541.
- Gresz V, Kwon TH, Gong H, et al. Immunolocalization of AQP-5 in rat parotid and submandibular salivary glands after stimulation or inhibition of secretion in vivo. Am J Physiol Gastrointest Liver Physiol 2004; 287: G151-G161.
- Ishikawa I, Ishida H. Aquaporin water channel in salivary glands. Jpn J Pharmacol 2000; 83: 95-101.
- Burghard B, Elkaer ML, Kwon TH, et al. Distribution of aquaporin water channels AQP1 and AQP5 in the ductual system of the human pancreas. Gut 2003; 52: 1008-1016.
- Lindsay LA, Murphy CR. Aquaporin-1 increases in the rat myometrium during early pregnancy. J Mol Histol 2004; 35: 75-79.
- Lindsay LA, Murphy CR. Redistribution of aquaporins in uterine epithelial cells at the time of implantation in the rat. Acta Histochem 2004; 106: 299-307.
- Helguera G, Eghbali M, Sforza D, Minosyan TY, Toro L, Stefani E. Changes in global gene expression in rat myometrium in transition from late pregnancy to parturition. Physiol Genomics 2009; 36: 89-97.
- Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc Natl Acad Sci USA 2006; 103: 3203-3208.
- Kridli RT, Khalaj K, Bidarimath M, Tayade C. Placentation, maternal-fetal interface, and conceptus loss in swine. Theriogenology 2016; 85: 135-144.
- Peter S, Michel G, Hahn A, et al. Puerperal influence of bovine uterine health status on the mRNA expression of pro-inflammatory factors. J Physiol Pharamacol 2015; 66: 449-462.
- Okrasa S, Franczak A, Zmijewska A, et al. The uterine secretory activity and its physiological changes in the pig. Act Biol Cracoviensia 2014; 55/56: 40-57.
- Iciek R, Wender-Ozegowska E, Mikolajczak P, et al. Placental vascular endothelial growth factor expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol 2014; 65: 577-583.
- Bazer FW, Song G, Kim J, et al. Uterine biology in pigs and sheep. J Anim Sci Biotechnol 2012; 3: 23. doi: 10.1186/2049-1891-3-23.
- Sinowatz F, Friess AE. Uterine glands of the pig during pregnancy. An ultrastructural and cytochemical study. Anat Embryol (Berl) 1998; 166: 121-134.
- Skowronska A, Mlotkowska P, Wojciechowicz B, Okrasa S, Nielsen S, Skowronski MT. Progesterone, estradiol, arachidonic acid, oxytocin, forskolin and cAMP influence on aquaporin 1 and 5 expression in porcine uterine explants during the mid-luteal phase of the estrous cycle and luteolysis: an in vitro study. Reprod Biol Endocrinol 2015; 13: 7. doi: 10.1186/s12958-015-0004-5
- Skowronska A, Mlotkowska P, Majewski M, Nielsen S, Skowronski MT. Expression of aquaporin 1 and 5 and their regulation by ovarian hormones, arachidonic acid, forskolin and cAMP during implantation in pigs. Physiol Res 2016; Mar 15. [Epub ahead of print].
- Wollenhaupt K, Brussow KP, Albrecht D, Tomek W. The Akt/mTor signaling cascade is modified during placentation in the porcine uterine tissue. Reprod Biol 2013; 13: 184-194.
- Samborski A, Graf A, Krebs S, et al. Transriptone changes in the porcine endometrium during the preattachment phase. Biol Reprod 2013; 89: 1-16.
- Skowronska A, Mlotkowska P, Wojciechowicz B, Okrasa S, Nielsen S, Skowronski MT. Steroid hormones, arachidonic acid, forskolin and c AMP influence on aquaporin 1 and 5 expression in porcine uterus during the lutelysis and pregnancy: in vitro study. J Physiol Pharmacol 2014; 65 (Suppl. 1): 70.
- Franczak A, Kotwica G, Kurowicka B, Oponowicz A, Waclawek-Potocka J, Petroff BK. Expression of enzymes of cyclooxygenase pathway and secretion of prostaglandin E2 and F2a by porcine myometrium during luteolysis and early pregnancy. Theriogenology 2006; 66: 1049-1056.
- Yang F, Kawedia JD, Menon AG. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem 2003; 278: 32173-32180.
- Franczak A, Kotwica G. Secretion of estradiol-17β by porcine endometrium and myometriumduring early pregnancy and luyeolysis. Theriogenology 2008; 69: 283-289.
- Fisher HE, Bazer FW, Fields MJ. Steroid metabolism by endometrial and conceptus tissues during early pregnancy and pseudopregnancy in gilts. J Reprod Fertil 1985; 75: 69-78.
- Li XJ, Yu HM, Koide SS. Regulation of water channel gene (AQP-CHIP) expression by estradiol and anordiol in rat uterus. Yao Xue Xue Bao 1997; 32: 586-592.
- Kobayashi M, Takahashi E, Miyagawa S, Watanabe H, Iguchi T. Chromatin immunoprecipitaation-mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes Cells 2006; 11: 1133-1143.
- Aralla M, Borromeo V, Groppetti D, Secchi C, Cremonesi F, Arrighi S. A Collaboration of aquaporins handles water transport in relation to the estrous cycle in the bitch uterus. Theriogenology 2009; 72: 310-321.
- Klein C, Troedsson MH, Rutllant J. Expression of aquaporin water channels in equine endometrium is differentially regulated during the oestrous cycle and early pregnancy. Reprod Domest Anim 2013; 48: 529-537.
- Gry M, Rimini R, Stromberg S, et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 2009; 10: 365.
- Siawrys G, Kaminski T, Smolinska N Przala J. Expression of leptin and long form of leptin receptor genes and proteins i pituitary of cyclic and pregnant pigs. J Physiol Pharmacol 2007; 58: 845-857.
- Smolinska N, Siawrys G, Kaminski T, Przala J. Leptin gene and protein expression in the trophoblast and uterine tissues during early pregnancy and the oestrous cycle of pigs. J Physiol Pharmacol 2007; 58: 563-581.
- Dittrich R, Muller A, Oppelt P, Hoffmann I, Beckman MW, Maltaris T. Differences in muscarimic receptor agonist-, oxytocin-, and prostaglandin-induced uterine contractions. Fertil Steril 2009; 95: 1694-1700.
- Uzumcu M, Braileanu GT, Carnahan KG, Ludwig TE, Mirando MA. Oxytocin-stimulated phosphoinositide hydrolysis and prostaglandin F secretion by luminal epithelial, glandular epithelial and stromal cells from pig endometrium. I. Response of cyclic pig on day 16 postestrus. Biol Reprod 1998; 59: 1259-1265.
- Blitek A, Waclawik A, Kaczmarek MM, Stadejek T, Pejsak Z, Ziecik AJ. Expression of cyclooxygenase-1 and -2 in the porcine endometrium during the oestrous cycle and early pregnancy. Reprod Domest Anim 2006; 41: 251-257.
- Waclawik A, Blitek A, Ziecik AJ. Oxytocin and tumor necrosis factor alpha stimulate expression of prostaglandin E2 synthase and secretion of prostaglandin E2 by luminal epithelial cells of the porcine endometrium during early pregnancy. Reproduction 2010; 140: 613-622.
- Ducza E, Seres AB, Hajagos-Toth J, Falkay G, Gaspar R. Oxytocin regulates the expression of aquaporin 5 in the late-pregnant rat uterus. Mol Reprod Dev 2014; 81: 524-530.
- Waclawik A. Novel insights into the mechanism of pregnancy establishment: regulation of prostaglandin synthesis and signaling in the pig. Reproduction 2011; 142: 389-399.
- Blitek A, Ziecik AJ. Prostaglandins F and E secretion by porcine epithelial and endometrial cells on different days of the oesrous cycle. Reprod Domest Anim 2004; 39: 340-346.
- Gleeson AR, Thorburn GD, Cox RI. Prostaglandin F concentrations in the utero-ovarian venous plasma of the sow during the late luteal phase of the estrous cycle. Prostaglandins 1974; 5: 521-529.
- Zelenina M, Christensen BM, Palmer J, Nairn AC, Nielsen S, Aperia A. Prostaglandin E2 interaction with AVP: effects on AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol 2000; 278: F388-F394.
- Downey P, Sapirstein A, O’Leary E, Sun TX, Brown D, Bonventre JV. Renal concentrating defect in mice lacking group IV cytosolic phospholipase A(2). Am J Physiol Renal Physiol 2001; 280: F607-F618.
- Sidhaye V, Hoffert JD, King LS. cAMP has a distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 2005; 280: 3590-3596.
- Wang S, Amidi F, Yin S, Beall M, Ross MG. Cyclic adenosine monophosphate regulation of aquaporin gene expression in human amnion epithelia. Reprod Sci 2007; 14: 234-240.
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 1999; 68: 425-458.
- Kraine CM, Towne JE, Menon AG. Cloning and characterization of murine AQP 5: evidence of a conserved aquaporin gene cluster. Mamm Genome 1999; 10: 498-505.
- Budik S, Walter I, Tschulenk W, et al. Significance of aquaporins and sodium potassium ATPase subunits for expansion of the early equine conceptus. Reproduction 2008; 135: 497-508.
- Lindsay LA, Murphy CR. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: a study with light and electron microscopy. Reproduction 2006; 131: 369-378.
- Gray CA, Bartol FF, Tarleton BJ, et al. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311-1323.
A c c e p t e d : April 12, 2016