SIMPLE OBESITY AND RENAL FUNCTION
INTRODUCTION
It has been estimated that the number of people with obesity reaches 500 million worldwide and is continuously increasing (1). It is recognized that obesity predisposes to type 2 diabetes, hyperlipidemia and hypertension. The mechanisms underlying these disorders are complex and include insulin resistance, endothelial dysfunction, and contributions from the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) (2). Interestingly, it has recently been demonstrated that the presence of endothelial nitric oxide synthase type 3 (NOS3) gene variant T894 is associated with increased risk of hypertension in the obese (3).
While the importance of obesity in the context of metabolic syndrome is well appreciated, the effects of simple obesity are less clear. Increasing evidence suggests that obesity per se, i.e. without significant comorbidities, also can exert serious health effects. They include obesity-associated inflammation, endothelial cell dysfunction and altered endocrine functions of the adipose tissue (4, 5). A substantial and chronic increase in the volume of adipocytes that is unaccompanied by adequate angiogenesis leads to adverse remodelling of the adipose tissue. It is characterized by tissue hypoxia and death of the most poorly supplied adipocytes, which initiates the inflammatory response. Although of low intensity, this smouldering and chronic inflammation leads to the adipose tissue infiltration by macrophages (mainly of the pro-inflammatory phenotype M1), their activation and uncontrolled production of cytokines (monocyte chemoattractant protein-1, interleukin-1β, interleukin-6, tumor necrosis factor-α) (6). This interferes with the secretion of adipocytokines (leptin, resistin, apelin, visfatin, adiponectin and omentin) by adipose tissue, which contributes to systemic inflammation and endothelial cell dysfunction that may jeopardize kidney function (4, 7). Results of a meta-analysis clearly show that the risk of kidney disease for people with obesity is at least 40% greater than for subjects with normal body weight (8). This effect is largely independent of classic obesity-associated risk factors, such as dyslipidemia, diabetes, and hypertension. Moreover, it is characterized be a dose-response relationship, which means that the risk of kidney disease increases in parallel with increasing BMI (strictly speaking, this relationship appears to be U-shaped as underweight with low BMI may also promote kidney damage). Interestingly, the risk of kidney disease in the obese differs between the sexes and is greater for obese women. The reason for this phenomenon is not clear, but a proportionally greater amount of body fat in females seems to play a role (8).
OBESITY-RELATED GLOMERULOPATHY
The impact of obesity on renal function has been known for a long time. First reports on a possible relationship between obesity and proteinuria date back to 1970s (9). Later, many studies confirmed that obesity confers an increased risk of chronic kidney disease (CKD). They showed that patients with BMI > 30 kg/m2 suffered from renal failure more often and proportionally to the magnitude of BMI (10). Recent data indicate that structural and functional alterations in the kidney may occur even in mild obesity (BMI > 25 kg/m2) and in the absence of diabetes (11). With increased prevalence of obesity, these changes became the focus of attention and were designated as obesity-related glomerulopathy (ORG) (12). Although the clinical course of ORG appears to be less severe than that of diabetic kidney disease, yet the long-term prognosis is also negative (13).
First observations on the association between obesity and structural changes in the kidney came from autopsy studies (14). These identified glomerular hypertrophy and segmental sclerosis as the most typical lesions in the obese. The diameter of glomeruli in obese individuals was found to be approximately 1.3-fold greater compared with that of non-obese subjects. In addition, the glomerular density appeared to be reduced (15). Segmental sclerosis in ORG resembles that seen in focal segmental glomerulosclerosis (FSGS), although its intensity and frequency is usually less compared with primary FSGS. Lesions of segmental sclerosis are most often located in the perihilar region and may be associated with increased diameter of the afferent arteriole and reduced podocyte density. A decrease in podocyte density is thought to result mainly from the enlargement of glomerular volume that cannot be matched by a corresponding increase in the number of podocytes (13). Other morphological findings in ORG include tubular atrophy and interstitial fibrosis, although their severity may be minimal. Obesity-associated changes in renal histology are accompanied by changes in renal function that include an initial increase in glomerular filtration rate (GFR), followed by albuminuria and proteinuria, and a gradual decrease in GFR leading ultimately to CKD (12, 16). The prevalence of ORG is difficult to estimate. A retrospective analysis of kidney biopsies detected features of ORG in more than 2.5% of the specimens, with a clear upward trend over the past 30 years (12). These values may, however, be underestimated, because the biopsies were performed only in patients with overt symptoms suggestive of kidney disease.
The pathogenesis of ORG remains incompletely understood (12). It has been hypothesized that glomerular hyperfiltration results either from primary vasodilation of the afferent arteriole or from increased reabsorption of sodium in the proximal tubule leading to decreased exposure of the macula densa to sodium, blunted tubuloglomerular feedback and the afferent arteriole dilation (Fig. 1). The mechanisms that initiate these processes are unknown, however, they are likely to include activation of the renin-angiotensin-aldosterone system, increased sympathetic activity, increased leptin-to-adiponectin ratio, hypertriglyceridemia, hyperinsulinemia, and possibly a high-protein diet (17-19). An increase in the intraglomerular pressure causes glomerular stretch and enlargement, which imposes mechanical stress on podocytes leading eventually to their dysfunction and damage. In addition, persistent inflammation in the adipose tissue results in lipolysis and hyperlipidemia, which promotes lipid accumulation in mesangial cells, podocytes and proximal tubule epithelial cells. Dysfunction of these cells together with increased permeability of glomerular capillaries and albuminuria induce mediators of fibrogenesis and glomerulosclerosis (12, 20, 21).
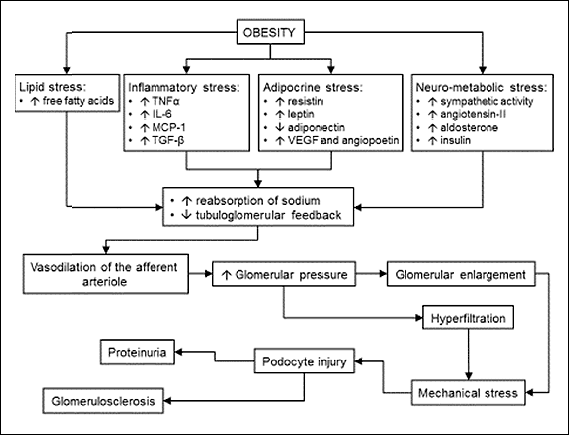 |
Fig. 1. Pathogenesis of obesity-related glomerulopathy. |
Recent data support the importance of adipose tissue-derived endocrine signalling, pointing to a key role of adiponectin (22). Under physiological conditions adiponectin maintains podocyte integrity acting primarily through adiponectin type 1 receptor (AdipoR1) and the signalling pathway controlled by 5’AMP-activated protein kinase (AMPK) (23). Adiponectin concentration decreases when BMI increases, which makes the glomerular filtration barrier vulnerable to injury (24). Obesity can also lead to a rise in the concentration of leptin, which stimulates the expression of transforming growth factor-β (TGF-β) that acts as the main driver of extracellular matrix accumulation, mesangial cell proliferation and progressive glomerulosclerosis (12, 25). Renal changes resembling those seen in human obesity can also be detected in mice after few months on high fat diet. It has recently been demonstrated that the development of such changes in mice is related to mitochondrial dysfunction in glomerular endothelial cells, proximal tubule epithelial cells, and podocytes. Accordingly, the administration of SS-31, a mitochondrial membrane-stabilizing peptide, preserved mitochondrial structure and function, and prevented renal cell injury and subsequent glomerulosclerosis (26). It remains to be determined whether such a therapeutic approach is effective in human disease.
Prompt diagnosis of ORG is difficult, as early stages of the disease may not give overt manifestations that would justify renal biopsy. However, by carefully analysing the parameters of renal function such as GFR and albuminuria one may get a hint of what might be a developing disease.
PARAMETERS OF RENAL FUNCTION IN OBESITY-RELATED GLOMERULOPATHY
Earlier formulas for estimating GFR had some limitations when applied to obese patients (27). The Cockcroft and Gault equation may overestimate GFR in the obese (28), while a MDRD (Modification of Diet in Renal Diseases) Study equation (27) may underestimate the real GFR (29) and, thus, may be not optimal for the assessment of obesity-associated hyperfiltration (27). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (30) is more accurate, especially for GFR > 60 ml/min/1.73 m2 and appears to be currently the best option for estimating GFR in the obese (31).
Albuminuria can be the first manifestation of kidney injury in ORG (32). Since a 24-hour urine collection is inconvenient to obtain, the albumin-to-creatinine ratio (ACR) is increasingly used in clinical practice as a test of similar validity (33). The degree of correlation between ACR and albuminuria in daily urine collection can be increased by computing the logarithm for ACR (34).
THE IMPORTANCE OF WEIGHT LOSS
Some aspects of ORG can be alleviated by dietary restriction and a decrease in body fat mass (12, 35). Low-calorie diet was found to ameliorate histological alterations in the kidneys of animals with experimental obesity and diabetes (36). It is not clear, however, whether a similar effect occurs in humans. It has been observed that already few weeks of dietary regimen reduces albuminuria (37). It has been estimated that weight reduction by 1 kg decreases albuminuria by approximately 4% (38). By contrast, such a modest weight loss does not appear to produce a significant change in GFR (37, 38). However, a more substantial weight loss as a result of bariatric surgery can normalize GFR and markedly reduce albuminuria (39).
Weight loss-induced renoprotection is probably related to several factors, including dampening of systemic chronic inflammation (40) and normalization of adipocytokine profiles (12). In this respect, a significant improvement in adipocytokine levels can be achieved already with moderate dietary restriction (41), while a substantial decrease in BMI after bariatric surgery lead to a more profound decrease in serum CRP and a significant improvement in endothelial cell function (42).
OTHER TREATMENT OPTIONS
Considering the postulated role of microbiota in obesity-induced inflammation, probiotics may act to decrease inflammation and slow down the progression of ORG (43, 44). Similarly, by promoting the growth of healthy microbiota in the intestine, high-fiber diets may ameliorate partly kidney dysfunction (45). Some studies suggest that green tea polyphenols can have a positive effect on the intestinal microbiota and improve kidney function in diabetic nephropathy (46-48). Finally, the transplantation of fecal microbiota might be considered as a therapeutic option for ORG (43, 49).
While metformin can improve metabolic control in diabetes, its role in ORG not associated with diabetes is not certain (43). Data from experimental models of kidney disease suggest that metformin may display antifibrotic activity and as such may of potential benefit for patients with ORG (50).
Inhibitors of the RAAS are commonly used for treating patients with proteinuria and diabetic nephropathy. They can also decrease albuminuria and reduce the prevalence of CKD in patients with ORG (12, 43). Moreover, some data suggest that obese patients might be even more sensitive to the renoprotective activity of the RAAS blockade than patients with normal BMI (51).
Based on the observation that selective endothelin-A receptor antagonists (e.g. avosentan and antrasentan) reduce albuminuria and preserve renal function in diabetes (52), they might also be considered as a therapeutic option for ORG (53).
PERSONAL EXPERIENCE
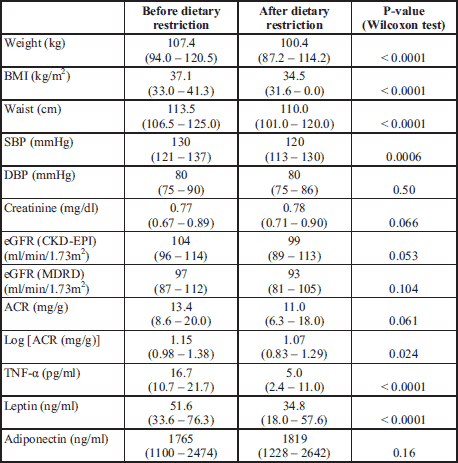
To illustrate the problems associated with renal function in obesity, we have assessed eGFR and ACR in 57 adults (16 male and 41 female, mean age 37 ± 11 years) with simple obesity who participated in our previous study(41). These patients had no significant comorbidities and underwent a moderate 8-week dietary intervention with calorie restriction by 300 – 500 kcal/day. On average, the procedure resulted in a reduction in body weight by 5.3 ± 4.0 kg, which corresponded to a decrease in BMI by 1.84 ± 1.36 kg/m2 (Table 1). The GFR estimated both at the beginning and at the end of the study was formally well within normal limits. However, it tended to decrease with time. Similarly, the intervention resulted in a decrease in ACR, although absolute ACR values remained in the normal range. Thus, taking the whole clinical context into account, it is tempting to hypothesize that those decreases represented a beneficial reversal of a potentially detrimental trend towards hyperfiltration and proteinuria. Obviously, more adequately powered studies would be required to verify this hypothesis. Interestingly, this relatively modest dietary restriction resulted in a small, but consistent decrease in blood pressure (independent of any additional medication), as well as a reduction in systemic levels of TNF-α and leptin.
SUMMARY
Obesity-related glomerulopathy is characterized by the enlargement of glomeruli and secondary focal segmental glomerular sclerosis in patients with a BMI > 30 kg/m2. Since there is usually no indication for renal biopsy, other diagnostic criteria for early diagnosis of the disease are needed.
The classic criteria for ORG include (1) obesity (BMI > 30 kg/m2), (2) normal serum albumin, (3) proteinuria with or without changes in GFR. However, structural changes in the kidneys of patients with obesity develop probably much earlier than could be demonstrated by the above criteria.
An early diagnosis of ORG may be facilitated by monitoring GFR (according to the CKD-EPI formula) and albuminuria (as measured by ACR or log(ACR)), especially after the dietary intervention or bariatric surgery. Although at early stages of ORG these parameters may be normal, their reduction in response to weight loss could suggest that they were in fact relatively increased and reflected developing ORG.
Weight loss exerts renoprotective effects in ORG with even short-term moderate dietary restriction providing clinical benefits related mainly to a decrease in blood pressure, dampening of systemic inflammation and normalization of adipocytokine profiles.
REFERENCES
- Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990 – 2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 2287-2323.
- Lopes HF, Correa-Giannella ML, Consolim-Colombo FM, Egan BM. Visceral adiposity syndrome. Diabetol Metab Syndr 2016; 8: 40.
- Wrzosek M, Sokal M, Sawicka A, et al. Impact of obesity and nitric oxide synthase gene G894T polymorphism on essential hypertension. J Physiol Pharmacol 2015; 66: 681-689.
- Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther 2016; 5: 47-56.
- Martinez-Santibanez G, Lumeng CN. Macrophages and the regulation of adipose tissue remodeling. Annu Rev Nutr 2014; 34: 57-76.
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014; 156: 20-44.
- Iwan-Zietek I, Ruszkowska-Ciastek B, Michalska M, et al. Association of adiponectin and leptin-to-adiponectin ratio with the function of platelets in morbidly obese patients. J Physiol Pharmacol 2016; 67: 555-561.
- Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 2008; 73: 19-33.
- Weisinger JR, Kempson RL, Eldridge FL, Swenson RS. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 1974; 81: 440-447.
- Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21-28.
- Okabayashi Y, Tsuboi N, Sasaki T, et al. Glomerulopathy associated with moderate obesity. Kidney Int Rep 2016; 1: 250-255.
- D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016; 12: 453-471.
- Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498-1509.
- Cohen AH. Massive obesity and the kidney. A morphologic and statistical study. Am J Pathol 1975; 81: 117-130.
- Tsuboi N, Utsunomiya Y, Kanzaki G, et al. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol 2012; 7: 735-741.
- Chen HM, Liu ZH, Zeng CH, Li SJ, Wang QW, Li LS. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis 2006; 48: 772-779.
- Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens 2006; 28: 29-40.
- Strazzullo P, Barba G, Cappuccio FP, et al. Altered renal sodium handling in men with abdominal adiposity: a link to hypertension. J Hypertens 2001; 19: 2157-2164.
- Barbato A, Cappuccio FP, Folkerd EJ, et al. Metabolic syndrome and renal sodium handling in three ethnic groups living in England. Diabetologia 2004; 47: 40-46.
- Guebre-Egziabher F, Alix PM, Koppe L, et al. Ectopic lipid accumulation: a potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 2013; 95: 1971-1979.
- Nolan E, O’Meara YM, Godson C. Lipid mediators of inflammation in obesity-related glomerulopathy. Nephrol Dial Transplant 2013; 28 (Suppl. 4): iv22-iv29.
- Guzik TJ, Mangalat D, Korbut R. Adipocytokines - novel link between inflammation and vascular function? J Physiol Pharmacol 2006; 57: 505-528.
- Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008; 118: 1645-1656.
- Ahima RS. Linking adiponectin to proteinuria. J Clin Invest 2008; 118: 1619-1622.
- Wang TN, Chen X, Li R, et al. SREBP-1 mediates angiotensin II-induced TGF-β1 upregulation and glomerular fibrosis. J Am Soc Nephrol 2015; 26: 1839-1854.
- Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 2016; 90: 997-1011.
- Delanaye P, Pottel H, Botev R, Inker LA, Levey AS. Con: Should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial Transplant 2013; 28: 1396-1403.
- Cirillo M, Anastasio P, De Santo NG. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant 2005; 20: 1791-1798.
- Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 2003; 14: 2573-2580.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604-612.
- Andrassy KM. Comments on “KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease”. Kidney Int 2013; 84: 622-623.
- KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 (2 Suppl 2): S12-S154.
- Herrington W, Illingworth N, Staplin N, et al. Effect of processing delay and storage conditions on urine albumin-to-creatinine ratio. Clin J Am Soc Nephrol 2016; 11: 1794-1801.
- Hogan MC, Reich HN, Nelson PJ, et al. The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int 2016; 90: 1080-1089.
- Kanda E, Muneyuki T, Suwa K, Nakajima K. Effects of weight loss speed on kidney function differ depending on body mass index in nondiabetic healthy people: a prospective cohort. PLoS One 2015; 10: e0143434. doi: 10.1371/journal.pone.0143434
- Neff KJ, Elliott JA, Corteville C, et al. Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in the Zucker diabetic fatty rat. Surg Obes Relat Dis 2016; 13: 21-27.
- Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2009; 4: 1565-1574.
- Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 2010; 25: 1173-1183.
- Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 2006; 17 (12 Suppl. 3): S213-S217.
- Holdstock C, Lind L, Engstrom BE, et al. CRP reduction following gastric bypass surgery is most pronounced in insulin-sensitive subjects. Int J Obes (Lond). 2005; 29: 1275-1280.
- Korybalska K, Swora-Cwynar E, Luczak J, et al. Association of endothelial proliferation with the magnitude of weight loss during calorie restriction. Angiogenesis 2016; 19: 407-419.
- Habib P, Scrocco JD, Terek M, Vanek V, Mikolich JR. Effects of bariatric surgery on inflammatory, functional and structural markers of coronary atherosclerosis. Am J Cardiol 2009; 104: 1251-1255.
- Camara NO, Iseki K, Kramer H, Liu ZH, Sharma K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol 2017; 13: 181-190.
- Barengolts E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endocr Pract 2016; 22: 1224-1234.
- Xu H, Huang X, Riserus U, et al. Dietary fiber, kidney function, inflammation, and mortality risk. Clin J Am Soc Nephrol 2014; 9: 2104-2110.
- Yokozawa T, Nakagawa T, Oya T, Okubo T, Juneja LR. Green tea polyphenols and dietary fibre protect against kidney damage in rats with diabetic nephropathy. J Pharm Pharmacol 2005; 57: 773-780.
- Axling U, Olsson C, Xu J, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 2012; 9: 105. doi: 10.1186/1743-7075-9-105
- Park JH, Jin JY, Baek WK, et al. Ambivalent role of gallated catechins in glucose tolerance in humans: a novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J Physiol Pharmacol 2009; 60: 101-109.
- Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913-916.
- Christensen M, Jensen JB, Jakobsen S, et al. Renoprotective effects of metformin are independent of organic cation transporters 1 &2 and AMP-activated protein kinase in the kidney. Sci Rep 2016; 6: 35952. doi: 10.1038/srep35952
- Praga M, Hernandez E, Morales E, et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant 2001; 16: 1790-1798.
- Kohan DE, Pritchett Y, Molitch M, et al. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 2011; 22: 763-772.
- Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017; 28: 1023-1039.
A c c e p t e d : March 17, 2017