MELANOCORTIN-4 RECEPTOR AGONIST (RO27-3225) AMELIORATES SOLEUS BUT NOT GASTROCNEMIUS ATROPHY IN ARTHRITIC RATS
2Department of Basic Sciences in Health, Faculty of Health Sciences, Rey Juan Carlos University, Madrid, Spain
INTRODUCTION
Inflammatory diseases, such as sepsis and rheumatoid arthritis, are associated with cachexia, body weight loss and skeletal muscle wasting, which increase mortality and morbidity. The ubiquitin-proteasome pathway is a proteolytic system that plays an important role in muscle wasting induced by chronic diseases (1). The E3 ubiquitin ligating enzyme genes, atrogin-1 and MuRF1, called atrogenes, are sensitive markers of muscular atrophy, since they are increased in many conditions associated with cachexia (1, 2). Adjuvant-induced arthritis in rats is an experimental model of rheumatoid arthritis that induces anorexia and muscle proteolysis throughout the ubiquitin-proteasome system (3, 4). Experimental arthritis decreases circulating IGF-1 levels, and this decrease contributes to body weight loss and muscle wasting (5). Increased secretion of glucocorticoids also contributes to muscle proteolysis in various catabolic diseases such as sepsis and cancer (6). In addition to endocrine modifications, pro-inflammatory cytokines and inflammatory mediators, such as COX-2, play an important role in muscle wasting in inflammatory diseases (7-9). NF-κB is a transcription factor that is critical for inflammatory response and skeletal muscle atrophy (10).
Melanocortins belong to a family of endogenous peptides (melanocyte stimulating hormones (MSH) and adrenocorticotropin hormone (ACTH) derived from the proopiomelanocortin (POMC), which are involved in a host of physiological activities, such as energy homeostasis pigmentation and immune function. Between the five subtypes of melanocortin receptors (MC-R), MC3-R and MC4-R are expressed in the central nervous system and have anti-inflammatory effects (11). Alpha melanocyte stimulating hormones (αMSH), a pan-agonist that can activate all these receptors, has been shown to ameliorate the course of several chronic inflammatory diseases in experimental animals (12, 13), and to also inhibit inflammation in experimental arthritis (14, 15). We have observed that αMSH treatment, in addition to its anti-inflammatory effects, decreases arthritis-induced muscle wasting (17). The MC3-R agonist
D-Trp(8)-gMSH also exerts anti-inflammatory activities and decreases gastrocnemius wasting in arthritic rats, downregulating atrogene expression (17). Those effects can be due to the fact that D-Trp(8)-γMSH decreases the effect of arthritis on IGF-1 and glucocorticoid secretion as well as on muscle NF-κB(p65)/TNF-α signalling transduction pathway (17).
MC4-R is expressed in the brain, both in neurons and in astrocytes, and also in peripheral tissue such as skeletal muscle (18, 19). MC4 R activation protects against haemorrhagic shock (20) and neuronal damage in animal models of ischaemic stroke (21). To our knowledge, there is no data on the effect of MC4R agonist on muscle mass regulation. Foetal rat limb muscles express MC4-R, and melanocortins accelerate maturation of the neuromuscular system and myoblast proliferation during development (22, 23). Therefore, it is logical to think that MC4-R activation may affect skeletal muscle. Thus, the aim of this study was to elucidate whether administration of RO27-3225, a MC4-R agonist, (24), is able to prevent the effect of experimental arthritis on muscle wasting. Skeletal muscle has two main types of fibers, type I, oxidative or slow, and type II, glycolytic or fast. Arthritis induces atrophy of both types of muscles, where fast muscle type is more affected than slow type (5, 25). Therefore, we studied the expression of atrogin-1 and MuRF1, as well as the myogenic regulatory factor MyoD in two muscles, gastrocnemius and soleus. We selected gastrocnemius as representative of fast-twitch muscle and soleus muscle as representative of slow-twitch muscle.
MATERIAL AND METHODS
Animals
Arthritic and control male Wistar rats weighing 160 g (6 week old) were purchased from Charles River Laboratories (Barcelona, Spain). Arthritis was induced in rats by an intradermal injection of 0.1 ml of heat-inactivated Mycobacterium butyricum (40 mg/ml in paraffin oil) in the right hind paw under isoflurane anaesthesia (3). Control animals were injected with vehicle. After arrival (day 3 after adjuvant injection), rats were housed three per cage and maintained under standardized conditions of temperature (20 – 22°C) and light (lights on from 7:30 am to 7:30 pm). All efforts were taken to minimize animal suffering.
The procedures followed the guidelines recommended by the EU for the care and use of laboratory animals, and were approved by the Complutense University Animal Care Committee (approval ID: CEA-UCM 16/12).
On Day 7 after adjuvant injection, rats were randomly divided into five groups of 10 rats.
- Control rats injected with 250 µl of saline i.p. twice a day (at 9:00 a.m. and at 5:00 p.m.).
- Control rats injected i.p. with 180 µg/kg RO27-3225, the MC4 receptor agonist, dissolved in saline twice a day.
- Pair-fed rats injected with saline.
- Arthritic rats injected with saline.
- Arthritic rats injected with 180 µg/kg RO27-3225. At this dosage, RO27-3225 is able to counteract the intestinal inflammatory response after cerebral ischemia (26).
Pair-fed group received the same amount of food (g/100 g body weight) eaten by the arthritic rats treated with saline on the previous day. Food intake per cage was calculated by measuring the difference between the initial and the remaining amount of pellets in the feeder. Body weight, food intake, and arthritis index scores were examined daily. On day 16 after adjuvant injection all animals were euthanized by decapitation at 12:00 h, trunk blood was collected, allowed to clot, and the serum was stored at –20°C for IGF-1, adrenocorticotropin hormone (ACTH) and corticosterone assays. The left hind paw volume was measured by water displacement. Epidydimal white adipose tissue weight was measured. Gastrocnemius and soleus muscles of the left hind paw were removed, weighed, frozen immediately in liquid nitrogen, and stored at –80°C for isolation of proteins.
Measurement of arthritis index
Assessment of arthritis was performed by measuring the arthritis index of each animal, which was clinically scored by grading each paw from 0 to 4. Grading was determined as: 0 - no erythema or swelling, 1 - slight erythema or swelling of one or more digits, 2 - swelling of paw, 3 - swelling of entire paw and ankle, 4 - ankylosis, incapacity to bend the ankle. The severity score was the sum of the clinical scores of each limb, the maximum value being 16.
Western blot
Gastrocnemius and soleus were homogenized in RIPA buffer (10 µl/mg) with protease inhibitor cocktail, sodium deoxycolate 12.5 mM, phenylmethane sulfonyl fluoride 100 mM, sodium orthovanadate 12.5 mM and with phosphatase inhibitors (Sigma-Aldrich, Madrid, Spain). The homogenate was later centrifuged at 13,000 rpm at 4°C for 30 min to remove tissue debris. Protein concentration was determined using the Bradford protein assay with bovine serum albumin as standard. The protein extract was boiled for 5 min with a 1:1 volume of Laemmli loading buffer. Proteins (100 µg) were resolved by electrophoresis on 14% polyacrylamide gels under reducing conditions, and then transferred onto nitrocellulose membranes that were blocked by incubation in 5% non-fat dry milk, 0.1% Tween (Sigma-Aldrich, Madrid, Spain), in Tris-buffered saline. Ponceau-S staining was performed to ensure equal transfer prior to blocking. Membranes were probed overnight at 4°C sequentially with antibodies against pNF-κBp65ser(536), NF-κBp65 (C20), atrogin1, MuRF and MyoD (Santa Cruz Biotechnology, Santa Cruz, CA, USA), COX-2 (Cell Signaling Technology Inc, Boston, MA), and a-tubulin (Sigma-Aldrich, Madrid, Spain). Membranes were then incubated for 90 min in the appropriate secondary antibody conjugated to horseradish peroxidase (anti-mouse IgG (Amersham Biosciences, Little Chalfont, UK); anti-rabbit IgG (GE Healthcare, Madrid, Spain); anti-goat IgG (Santa Cruz), and peroxidase activity was detected using enhanced chemiluminescent reagent (Amersham Biosciences, Little Chalfont, UK). Following ECL detection of phospho-NF-κB(p65), membranes were stripped with stripping buffer (Restore Western Blot Stripping Buffer, Thermo-scientific Rockford, Il, USA) and reprobed for total protein. Band intensities were quantified by densitometry using a PC-Image VGA24 program for Windows. The density of the protein band in each lane was expressed as the percentage of the mean density of control rats, after load normalization using α-tubulin.
Serum insulin-like growth factor-1, adrenocorticotropin hormone and corticosterone measurements
Serum IGF-1, ACTH and corticosterone levels were analysed by a commercial kit from MP Biomedicals, LLC (Orangeburg, NY, USA), following the manufacturer’s protocols.
Statistical analysis
Statistics were computed using the statistics program STATGRAPHICS plus for Windows. Data are presented as mean ± S.E.M. and differences among experimental groups were analysed by one-way analysis of variance. Post-hoc comparisons were made by using subsequent LSD multiple range tests. Statistical significance was set at P < 0.05.
RESULTS
Treatment with the MC4 receptor agonist, RO27-3225, has an anti-inflammatory effect, since arthritic rats injected with RO27-3225 had lower arthritis scores than arthritic rats treated with saline (P < 0.05) from day 13 after adjuvant injection (Fig. 1A). In addition, RO27-3225 administration decreased arthritis-induced increase in left hind paw volume (P < 0.01), whereas left hind paw volume was not modified in control rats by RO27-3225 treatment (Fig. 1B).
Daily body weight gain over the 8 days of treatment is shown in Fig. 1C. Arthritic rats treated with saline had lower body weight gain than control or pair-fed rats from day 9 after adjuvant injection (P < 0.01). In arthritic rats, RO27-3225 treatment increased body weight gain to values similar to those of pair-fed rats, where the increase was statistically significant (P < 0.05) on days 9, 10 and 15 after adjuvant injection. However, administration of RO27-3225 did not modify body weight gain in control rats. Arthritis decreased daily food intake (P < 0.01, Fig. 1D). RO27-3225 administration increased food intake in arthritic rats (P < 0.05), whereas it was unable to modify food intake in control rats.
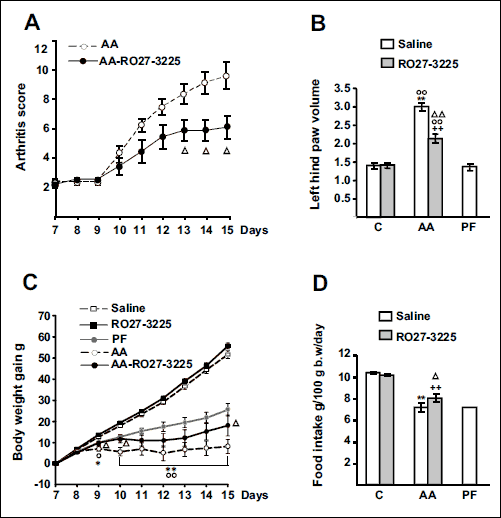 |
Fig. 1. Effect of arthritis and RO27-3225 administration (180 µg/kg, i.p. twice a day s.c.) for 8 days on arthritis score (A), left hind paw volume (B), cumulative body weight gain (C) and food intake (D). C - control, PF - pair-fed, AA - arthritic rats. AA rats had lower body weight gain than control and PF rats (P < 0.01). RO27-3225 administration to AA rats decreased arthritis scores and left hind paw volume (P < 0.01). AA rats treated with RO27-3225 had higher body weight gain than AA rats injected with saline (P < 0.05) and similar to that of PF rats. Arthritis decreased daily food intake (P < 0.01, Fig. 1D) and RO27-3225 administration increased food intake in arthritic rats (P < 0.01), whereas in control rats RO27-3225 was unable to modify food intake. Data are expressed as means ± S.E.M. for n = 7 – 10 rats/group. **P < 0.01 versus control rats injected with saline. °P < 0.05, °°P < 0.01 versus PF rats, ++P < 0.01 versus C rats treated with RO27-3225, ΔΔP < 0.01, ΔP < 0.05 versus AA rats injected with saline. LSD multiple comparison test. |
Fig. 2 shows the effect of arthritis and RO27-3225 administration on serum concentrations of IGF-1, ACTH and corticosterone. Both arthritis and pair feeding the rats decreased serum concentrations of IGF-1 (Fig. 2A). RO27-3225 administration increased serum concentrations of IGF-1 in control rats (P < 0.05), but not in arthritic rats. Arthritis increased serum concentrations of ACTH and corticosterone (P < 0.01, Fig. 2B and 2C) in rats treated with either saline or RO27-3225. Administration of RO27-3225 did not modify serum concentrations of ACTH or corticosterone in control or in arthritic rats. Pair feeding the rats increased serum concentrations of corticosterone (P < 0.01), but not serum ACTH levels.
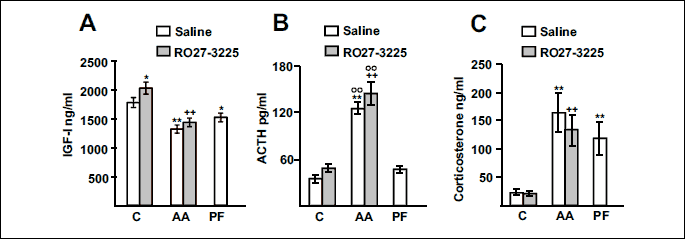
As we have previously reported (5), arthritis decreased both gastrocnemius and soleus weights (Fig. 3A and 3B, P < 0.01), with the decrease being higher in gastrocnemius than in soleus weight. Administration of RO27-3225 prevented the inhibitory effect of arthritis on soleus weight (P < 0.01), but RO27-3225 was not able to significantly increase gastrocnemius weight in arthritic rats. Pair feeding the rats or administering the MC4R agonist to control rats did not modify gastrocnemius or soleus weights. Both arthritis and pair feeding the rats decreased epididymal white adipose tissue weight (P < 0.01, Fig. 3C). RO27-3225 administration increased epididymal white adipose tissue weight (P < 0.05) in arthritic rats, but not in control rats.
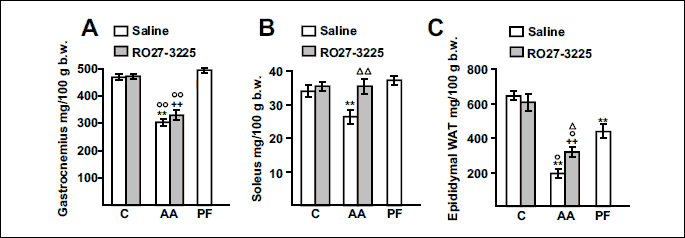
Fig. 4A and 4B shows the effect of arthritis and RO27-3225 treatment on NF-κB(p65) phosphorylation in the gastrocnemius. Arthritis increased phospho-NF-κB(p65) levels in the gastrocnemius (P < 0.01), whereas RO27-3225 administration prevented arthritis-induced increase in phospho-NF-κB(p65) (P < 0.01). Neither pair feeding the rats nor RO27-3225 treatment to control rats modified phospho-NF-κB(p65). Total NF-κB(p65) levels were similar in all groups. Fig. 4C shows COX-2 protein levels in the gastrocnemius. Arthritis increased the levels of this inflammatory mediator (P < 0.01) and this increase was not prevented by RO27-3225 treatment. Arthritis also increased both atrogin-1 (P < 0.01) and MuRF1 (P < 0.05) levels in the gastrocnemius (Fig. 4D and 4E). RO27-3225 administration to arthritic rats was not able to significantly decrease atrogin-1 or MuRF1 levels in this muscle.
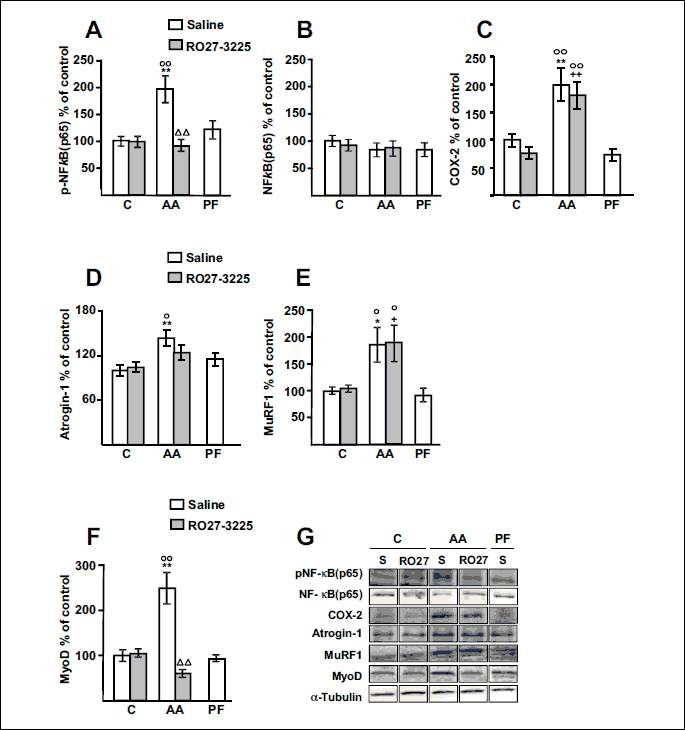
MyoD levels were increased in the gastrocnemius of arthritic rats treated with saline (P < 0.01, Fig. 4F). RO27-3225 treatment did not modify MyoD levels in control rats, but prevented the arthritis-induced increase in MyoD in the gastrocnemius (P < 0.01). Pair feeding the rats did not modify MyoD levels in the gastrocnemius.
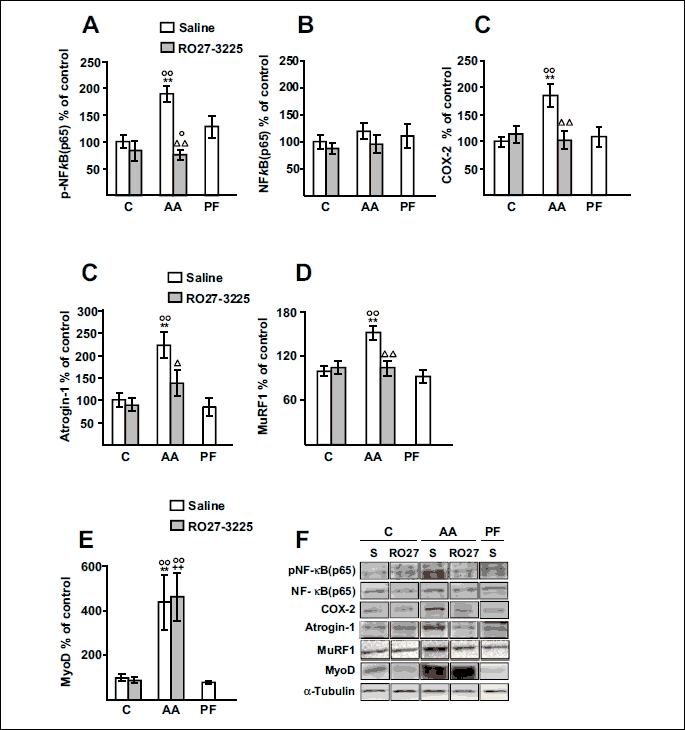
Arthritis also increased phospho-NF-κB(p65) levels in the soleus of rats treated with saline (P < 0.01 Fig. 5A), whereas arthritic rats treated with RO27-3225 had lower phospho-NF-κB(p65) levels than arthritic rats injected with saline (P < 0.01), and similar to those of control rats. RO27-3225 administration did not modify phospho-NF-κB(p65) in the soleus of control rats. Total NF-κB(p65) was not modified in any group of rats (Fig. 5B). COX-2 levels in soleus were increased in arthritic rats compared to control or pair-fed rats, whereas RO27-3225 administration prevented this increase (P < 0.01, Fig. 5C).
Atrogin-1 and MuRF1 levels in soleus were increased by arthritis (P < 0.01, Fig. 5D and 5E). RO27-3225 treatment prevented arthritis-induced increase in atrogin-1 (P < 0.05) and MuRF1 (P <0.01), whereas it did not modify atrogene in control rats. MyoD expression was increased in the soleus of arthritic rats injected either with saline or with RO27-3225 (P < 0.01, Figs. 5F). Pair feeding the rats did not modify MyoD levels in soleus.
DISCUSSION
Our results suggest that activation of MC4R by RO27-3225 administration to arthritic rats has an anti-inflammatory effect and decreases the inhibitory effect of arthritis on body weight gain and soleus wasting.
The anti-inflammatory effect of the MC4-R agonist in arthritic rats is evidenced by its actions decreasing the external signs of inflammation such as arthritis index and hind paw volume. The broad anti-inflammatory effect of melanocortins is due to the fact that they are able to reduce NF-κB activation in different cell types, predominantly via MC3-R and MC4-R (11). Although MC4-R mediates most of the central anti-inflammatory effects of melanocortins (27), this receptor has also been observed in peripheral rat organs. In this sense, it has recently been reported that MC4R activation is able to reduce sepsis-induced acute liver injury in rats by inhibiting NF-κB signalling pathway and decreasing inflammation (28).
Arthritis-induced decrease in body weight gain and in epididymal white adipose tissue (WAT) mass are secondary to both the decrease in food intake and inflammation, since pair-fed rats have lower body and WAT weights than control rats, but higher than arthritic rats treated with saline. In contrast, skeletal muscle (gastrocnemius and soleus) wasting or ‘rheumatoid cachexia’ observed in arthritic rats is driven by inflammation, rather than by anorexia, since pair feeding the rats did not decrease relative skeletal muscle mass.
The effects of RO27-3225 on increasing body weight gain and food intake in inflammatory cachexia have not been previously reported. In contrast, it has been reported that MC4-R agonists in normal rodents decrease food intake and increase metabolic rate (29). However, in our data chronic RO27-3225 administration did not modify food intake or body weight in control rats. Discrepancies cannot be due to the fact that RO27-3225 was administered by systemic injection, since the pentapeptide RO27-3225 reaches the CNS after systemic injection (24). A possible explanation can be that in our present study RO27-3225 was administered chronically and, although acute i.c.v. or i.p. RO27-3225 administration decreases food intake, RO27-3225 is unable to decrease cumulative food intake after 24 hours (24). In arthritic rats RO27-3225 treatment increased food intake, and this response can be the result of its anti-inflammatory effect, rather than to a direct effect on the central pathways that regulate food intake.
In arthritic rats treated with saline, the decrease in gastrocnemius weight is higher than in soleus weight. These data are in contrast to those reported in muscle atrophy induced by disuse, in which soleus is more affected than gastrocnemius and other fast muscle (30). However, in cachexia induced by inflammatory illness, fast glycolytic muscles are more prone to atrophy than slow oxidative muscles (5, 31). RO27-3225 treatment prevented arthritis-induced decrease in soleus weight, but it was unable to significantly increase gastrocnemius mass. This difference can be due to the fact that RO27-3225 was able to prevent arthritis-induced expression of COX-2, atrogin-1 and MuRF1 in soleus, but not in gastrocnemius. In addition, RO27-3225 administration to arthritic rats decreased MyoD expression in gastrocnemius but not in soleus.
NF-κB is one of the most relevant signalling pathways that induces muscle wasting by the ubiquitin-proteasome system, leading to muscle atrophy of both fast and slow types of skeletal muscle (10, 32). As mentioned above, COX-2 is also an important mediator of inflammation-induced muscle atrophy (7-9). Skeletal muscle proteolysis through the ubiquitin-proteasome system is upregulated by FoxO3 activation (33) and glucocorticoids (34). Glucocorticoid-induced skeletal muscle atrophy is selective for fast muscles, whereas slow muscles are relatively resistant (35). In fact, the ability of glucocorticoid administration to induce atrogin-1 and MuRF1 expression is higher in extensor digitorum longus (a fast muscle) than in soleus (a slow muscle) (36, 37). Furthermore, soleus atrophy induced by disuse is dependent on NF-κB activation (32), but not on glucocorticoids (38). Therefore, different responses between soleus and gastrocnemius to RO27-3225 treatment can be secondary to the lack of modifications in glucocorticoid secretion, since RO27-3225 was unable to prevent the increase in serum concentrations of ACTH and corticosterone in arthritic rats.
In addition to NF-κB and glucocorticoids, atrogin-1 and MuRF1 expression are downregulated by IGF-1 (39). IGF-1 administration is able to decrease arthritis-induced atrogin-1 and MuRF1 expression in gastrocnemius, but not in soleus (5). All these data suggest that atrogin-1 and MuRF1 expression in fast muscles are more susceptible to the effects of glucocorticoid and IGF-1 than slow muscles. Soleus muscle atrophy seems to be more dependent on the local inflammation process, since the blockade of p-NF-κB and COX-2 increase prevents the arthritis-induced increase of atrogenes. Therefore, the inability of RO27-3225 to modify the effects of arthritis on corticosterone, IGF-1 and gastrocnemius COX-2 can be one of the causes that explains the different responses of gastrocnemius and soleus to RO27-3225 administration.
As we have reported, arthritis increased MyoD expression in gastrocnemius and soleus (5). Increased expression of MyoD has also been reported in muscle wasting induced by other conditions, such as burn and chronic kidney disease (40, 41). The different effects of RO27-3225 in arthritic rats decreasing MyoD expression in gastrocnemius, but not in soleus can contribute to the effect of RO27-3225 on soleus mass. It has been recently reported that MyoD, in addition to stimulating muscle differentiation, functions in skeletal muscle as a regulator of multiple genes involved in muscle oxidative metabolism (42). In arthritic rats the increase of fatty acid oxidation by fenofibrate administration has a protective effect against arthritis-induced decrease in muscle weight, where the increase is higher in soleus weight than in gastrocnemius weight (25, 43). Whereas fenofibrate decreased MyoD expression in the gastrocnemius of arthritic rats (25), it increased MyoD in soleus (43).
Inability of RO27-3225 to prevent arthritis-induced gastrocnemius wasting contrasts to the effects of the treatment with D-Trp(8)-γMSH, a MC3-R agonist, since this peptide is able to decrease atrogin-1 and MuRF1 expression in the gastrocnemius, as well as to decrease gastrocnemius and soleus weights (17, and authors’ unpublished observation). On the contrary to RO27-3225, MC3-R stimulation by D-Trp(8)-γMSH administration decreases the effects of arthritis on NF-κB(p65) activation as well as on serum levels of ACTH, corticosterone and IGF-1 (17).
In conclusion, our data suggest that administration of the MC4-R agonist RO27-3225 to arthritic rats decreases inflammation, anorexia and fat mass loss. Although RO27-3225 prevents the arthritis-induced increase in atrogene expression and soleus atrophy, it is unable to ameliorate the effects of arthritis on the gastrocnemius, it is unable to ameliorate the effects of arthritis on the gastrocnemius. The different effect of RO27-3225 on soleus and gastrocnemius can be related to the lack of effect of RO27-3225 on IGF-1 and corticosterone circulating levels.
Acknowledgements: The authors are indebted to Christina Bickart for the English correction of the manuscript.
This work was supported by grant BFU2012-38468, and fellowships from UCM to ABGSM.
Conflicts of interest: None declared.
REFERENCES
- Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004; 18: 39-51.
- Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001; 294: 1704-1708.
- Castillero E, Martin AI, Lopez-Menduina M, Granado M, Villanua MA, Lopez-Calderon A. IGF-1 system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol Cell Endocrinol 2009; 309: 8-16.
- Castillero E, Martin AI, Lopez-Menduina M, Villanua MA, Lopez-Calderon A. Eicosapentaenoic acid attenuates arthritis-induced muscle wasting acting on atrogin-1 and on myogenic regulatory factors. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1322-R1331.
- Lopez-Menduina M, Martin AI, Castillero E, Villanua MA, Lopez-Calderon A. Systemic IGF-1 administration attenuates the inhibitory effect of chronic arthritis on gastrocnemius mass and decreases atrogin-1 and IGFBP-3. Am J Physiol Regul Integr Comp Physiol 2010; 299: R541-R551.
- Braun TP, Grossberg AJ, Krasnow SM, et al. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J 2013; 27: 3572-3582.
- Granado M, Martin AI, Villanua MA, Lopez-Calderon A. Experimental arthritis inhibits the insulin-like growth factor-1 axis and induces muscle wasting through cyclooxygenase-2 activation. Am J Physiol Endocrinol Metab 2007; 292: E1656-E1665.
- Pajak B, Orzechowska S, Pijet B, et al. Crossroads of cytokine signaling-the chase to stop muscle cachexia. J Physiol Pharmacol 2008; 59 (Suppl 9): 251-264.
- Martin AI, Nieto-Bona MP, Castillero E, et al. Effect of cyclooxygenase-2 inhibition by meloxicam, on atrogin-1 and myogenic regulatory factors in skeletal muscle of rats injected with endotoxin. J Physiol Pharmacol 2012; 63: 649-659.
- Cai D, Frantz JD, Tawa NE, et al. IKKb/NF-κB activation causes severe muscle wasting in mice. Cell 2004; 119: 285-298.
- Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. ScientificWorldJournal 2010; 10: 1840-1853.
- Rajora N, Boccoli G, Catania A, Lipton JM. Alpha-MSH modulates experimental inflammatory bowel disease. Peptides 1997; 18: 381-385.
- Taylor AW, Kitaichi N. The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) therapy. Brain Behav Immun 2008; 22: 639-646.
- Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation 1994; 1: 28-32.
- Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol 2011; 179: 259-269.
- Gomez-SanMiguel AB, Martin AI, Nieto-Bona MP, et al. Systemic a-melanocyte-stimulating hormone administration decreases arthritis-induced anorexia and muscle wasting. Am J Physiol Regul Integr Comp Physiol 2013; 304: R877-R886.
- Gomez-SanMiguel AB, Martin AI, Nieto-Bona MP, Fernandez-Galaz C, Villanua MA, Lopez-Calderon A. The melanocortin receptor type 3 agonist d-Trp(8)-gMSH decreases inflammation and muscle wasting in arthritic rats. J Cachexia Sarcopenia Muscle 2016; 7: 79-89.
- Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology 2003; 144: 5488-5496.
- Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 2010; 31: 506-543.
- Sowa P, Adamczyk-Sowa M, Zwirska-Korczala K, et al. Proopiomelanocortin but not vasopressin or renin-angiotensin system induces resuscitative effects of central 5-HT1A activation in haemorrhagic shock in rats. J Physiol Pharmacol 2014; 65: 659-671.
- Giuliani D, Zaffe D, Ottani A, et al. Treatment of cerebral ischemia with melanocortins acting at MC4 receptors induces marked neurogenesis and long-lasting functional recovery. Acta Neuropathol 2011; 122: 443-453.
- Strand FL, Rose KJ, Zuccarelli LA, et al. Neuropeptide hormones as neurotrophic factors. Physiol Rev 1991; 71: 1017-1046.
- De Angelis L, Cusella-De Angelis MG, et al. ACTH-like peptides in postimplantation mouse embryos: a possible role in myoblast proliferation and muscle histogenesis. Dev Biol 1992; 151: 446-458.
- Benoit SC, Schwartz MW, Lachey JL, et al. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. J Neurosci 2000; 20: 3442-3448.
- Castillero E, Nieto-Bona MP, Fernandez-Galaz C, et al. Fenofibrate, a PPAR(alpha) agonist, decreases atrogenes and myostatin expression and improves arthritis-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 2011; 300: E790-E799.
- Spaccapelo L, Bitto A, Galantucci M, et al. Melanocortin MC4 receptor agonists counteract late inflammatory and apoptotic responses and improve neuronal functionality after cerebral ischemia. Eur J Pharmacol 2011; 670: 479-486.
- Caruso C, Durand D, Schioth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology 2007; 148: 4918-4926.
- Li H, Qiu D, Gao Q, Wang H, Sun M. Selectively activating melanocortin 4 receptor acts against rat sepsis-induced acute liver injury via HMGB1/TLR4/NF-κB signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016; 32: 1055-1059.
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997; 385: 165-168.
- Jee H, Sakurai T, Lim JY, Hatta H. Changes in aB-crystallin, tubulin, and MHC isoforms by hindlimb unloading show different expression patterns in various hindlimb muscles. J Exerc Nutrition Biochem 2014; 18: 161-168.
- Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK. Skeletal muscle wasting and contractile performance in septic rats. Muscle Nerve 2005; 31: 339-348.
- Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 2004; 114: 1504-1511.
- Gwag T, Park K, Park J, Lee JH, Nikawa T, Choi I. Celastrol overcomes HSP72 gene silencing-mediated muscle atrophy and induces myofiber preservation. J Physiol Pharmacol 2015; 66: 273-83.
- Castillero E, Alamdari N, Lecker SH, Hasselgren PO. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism 2013; 62: 1495-1502.
- Gardiner PF, Montanaro G, Simpson DR, Edgerton VR. Effects of glucocorticoid treatment and food restriction on rat hindlimb muscles. Am J Physiol 1980; 238: E124-E130.
- Menconi MJ, Arany ZP, Alamdari N, et al. Sepsis and glucocorticoids downregulate the expression of the nuclear cofactor PGC-1beta in skeletal muscle. Am J Physiol Endocrinol Metab 2010; 299: E533-E543.
- Nakao R, Yamamoto S, Yasumoto Y, Oishi K. Dosing schedule-dependent attenuation of dexamethasone-induced muscle atrophy in mice. Chronobiol Int 2014; 31: 506-514.
- Tischler ME. Effect of the antiglucocorticoid RU38486 on protein metabolism in unweighted soleus muscle. Metabolism 1994; 43: 1451-1455.
- Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-1 stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 2004; 287: E591-E601.
- Avin KG, Chen NX, Organ JM, et al. Skeletal muscle regeneration and oxidative stress are altered in chronic kidney disease. PLoS One 2016; 11: e0159411. doi: 10.1371/journal.pone.0159411
- Quintana HT, Bortolin JA, da Silva NT, et al. Temporal study following burn injury in young rats is associated with skeletal muscle atrophy, inflammation and altered myogenic regulatory factors. Inflamm Res 2015; 64: 53-62.
- Shintaku J, Peterson JM, Talbert EE, et al. MyoD regulates skeletal muscle oxidative metabolism cooperatively with alternative NF-κB. Cell Rep 2016; 17: 514-526.
- Castillero E, Martin AI, Nieto-Bona MP, et al. Fenofibrate administration to arthritic rats increases adiponectin and leptin and prevents oxidative muscle wasting. Endocr Connect 2012; 1: 1-12.