MANTIDIS OOTHECA INDUCES VASCULAR RELAXATION THROUGH
PI3K/AKT-MEDIATED NITRIC OXIDE-CYCLIC GMP-PROTEIN KINASE G
SIGNALING IN ENDOTHELIAL CELLS
INTRODUCTION
The vascular endothelium performs a side array of homeostatic functions within normal blood vessels. Located between the vascular lumen and the smooth muscle cells of the vessel wall, the monolayer of endothelial cells is able to transduce blood-borne signals, sense mechanical forces within the lumen, and regulate vascular tone through the production of a variety of factors (1, 2). Endothelium produces potent vasodilators such as EDRF, prostacyclin, and endothelium-derived hyperpolarizing factor. The most important vasodilator substance produced by endothelial cells is an EDRF, which has been identified as a nitric oxide (NO) (3). Endothelium derived NO has been recognized as a pleiotropic biological mediator, regulating diverse activities ranging from neuronal function to vasoactivity regulation (4). NO has been identified as a neurotransmitter in both the peripheral and central nervous systems (5). It accounts for many autonomic responses in the cardiovascular system, as well as in the gastrointestinal and urogenital tracts, such as regulation of blood flow and blood pressure (6), inhibition of gastrointestinal motility and relaxation of the urethra during the micturition reflex (7). Especially, in vascular smooth muscle cell, NO-mediated activation of the enzyme soluble guanylyl cyclase (sic) catalyzes the formation of the second messenger guanosine 3',5-cyclic monophosphate (cGMP), leading to vasorelaxation on aorta (8, 9). In addition to its important vasodilatory function, NO also inhibits smooth muscle cell contraction, migration, and proliferation as well as endothelin production, platelet aggregation, and adhesion of leukocytes to the endothelium and then prevents atherogenic procedures (10, 11). The direct role for L-arginine in normalizing the high blood pressure has also been known. In the healthy as well as in patients with essential hypertension, treatment with L-arginine causes a rapid reduction of systolic and diastolic blood pressures (12).
Mantidis ootheca (Sang Piao Xiao) is a Pinyin transliteration referring to the oothecae, or egg case. According to the Shennong BencaoJing (The Classic of Herbal Medicine and Shen-nung Pen-tsao Ching), Mantidis ootheca regulates urination and the water passageways (13). It has been used in traditional medicine to treat incontinence, frequent urination, cloudy urine as it to the kidneys (14). Also, Mantidis ootheca increased the index of testis and thymus gland, and has an antidiuretic, decrease the content of lipid peroxidation in liver of the hypercholesterolemia rats, and has an antidiuretic effect (15).
However, to the best of our knowledge, the effect of Mantidis ootheca on vascular endothelial cell has not yet been defined. Therefore, the purpose of the present study was to investigate action mechanism of an aqueous extract of Mantidis ootheca (AMO). We used cultured human umbilical vein endothelial cells (HUVECs) for determine whether AMO elicits the production of NO. Furthermore, we examined the vascular relaxant activity of AMO in thoracic aortic ring.
MATERIALS AND METHODS
Reagents
Acetylcholine chloride (ACh), phenylephrine HCl (PE), NG-nitroarginine methyl ester (L-NAME), 4-aminopyridine,, glibenclamide, 1H-[1,2,4]-oxadiazole-[4,3α]-quinoxalin-1-one (ODQ), LY-294002, tetraethylammonium (TEA), (±)-propranolol HCl and 3-isobutyl-1- methylxanthine (IBMX) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 4-aminopyridine and KT5823 were purchased from Tocris Bioscience Chemical Co. (Missouri, USA). Acetylcholine, phenylephrine, L-NAME, IBMX, and TEA were dissolved in distilled water. Stock solutions of indomethacin, ODQ, wortmannin, LY-294002, KT5823, glibenclamide, propranolol and 4-aminopyridine were dissolved in dimethylsulfoxide (DMSO); working solutions were made in Krebs solution. Control experiments demonstrated that the highest DMSO level (0.2%) had no effect on vascular smooth muscle contraction. buy a thing from Sigma Chemical Co. (St.Louis, MO, USA).
Preparation of extract of Mantidis ootheca
Mantidis ootheca was perchased from the Hanyakjaemart (Handan Shi of Hebei Province, China, 2015). Mantidis ootheca (200 g) was extracted with 2L of boiling distilled water at 100°C for 2 hours. The aqueous extract was centrifuged at 2500 rpm for 20 min at 4°C and filtered with Whatman No.2 filter paper, and then concentrated using rotary evaporator. The aqueous extract (10.3 g, HBG161-01) was lyophilized using freeze-drier and used in this experiment.
Cell cultures and assessment of cell viability
Primary cultured HUVEC were purchased from Gibco Cascade, which contains 2.5% fetal bovine serum and growth supplements such as recombinant epidermal growth factor (rEGF), VEGF, human fibroblast growth factor-basic, ascorbic acid, human recombinant insulin-link growth focator, hydrocortisone, heparin, gentamicin and amphotericin. To determine cell viability, MTT (20 µl) and AMO (0 – 500 µg/ml) was added to HUVECs (5 × 105 cells/well) in 6-well plates suspension for 4 hours. Optical density (OD) of each culture well was measured usiong a microplate reader at 590 nM (Multiskan, Thermo Labsystems Inc, Franklin, MA). The OD in control cells was taken as 100% of viability.
Intracellular nitric oxide and nitrite production
The fluorescent probe, DAF-FM Diacetate, was used to determine the intracellular generation of NO. The confluent HUVEC in the 6 well culture plates were pretreated with DAF-FM for 1 hour. After removing excess probe from the wells, the HUVEC were treated with AMO for 30 min. The fluorescence intensity was measured by spectrofluorometer (Infinite F200 pro, TECAN) and examined under a fluorescence microscope (Eclipse Ti, Nikon).
Preparation of vascular tissues
The animal procedures were in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Utilization committee for Medical Science of Wonkwang University. Male Sprague-Dawley (S.D.) rats were purchased from Samtako, Inc. (OSan, Korea). The thoracic artery was isolated from male Sprague-Dawley rats weighting 220 – 280 g, carefully dissected from surrounding fat and connective tissue, and cut into 3-mm-long circular segments. All vessel segments were immediately placed in Krebs-Ringer bicarbonate solution, aerated with 95% O2 and 5% CO2. The thoracic aorta were rapidly and carefully dissected and placed into ice-cold Krebs-Ringer bicarbonate solution (pH 7.4) containing 118 mM NaCl, 4.7 mM KCl, 1.1 mM MgCl, 1.2 mM KH2PO4, 1.5 mM CaCl2, 25 mM NaHCO3, and 10 mM glucose. All dissecting procedures were done with extreme care to protect the endothelium from inadvertent damage.
Recording of isometric vascular tone
The aortic rings were suspended by means of two L-shape stainless-steel wires inserted into lumen in a tissue bath containing Krebs solution (pH 7.4) at 37°C, while being continuously bubbled with 95% O2, 5% CO2. The baseline load placed on the aortic rings was 1.0 g, and the changes in isometric tension were recorded using a force-displacement transducer (Grass FT 03, Quincy, MA, U.S.A.) connected to a Grass polygraph recording system (Model 7E). In the first set of experiments, the aortic rings were contacted with phenylephrine (PE, 1 µM) to obtain maximal response. Once the maximal response to PE has been obtained acetylcholine (Ach, 1 uM) was treated as a single concentration to determine the endothelial cell condition. After confirming the vascular condition, the aortic rings were washed every 10 min with Krebs solution until the tension returned to the basal level. The concentration-dependent response curve to AMO (1 to 100 µg/ml) was performed in aortic rings contracted by PE. The rings were exposed to various modulating agents for 20 min prior to exposure to PE, and then vascular relaxation was carried out by cumulative addition of AMO (1 to 100 µg/ml). The effect of vehicle, 0.1% dimethylsulfoxide (DMSO), was also tested. After each test, the arterial rings were washed three times with fresh Krebs-Ringer solution and allowed for 30 min to equilibrate.
Measurements of cyclic GMP
The levels of cyclic GMP (cGMP) in aortic tissues were measured by the direct cGMP ELISA kit (Enz, catalog no. ADI-900-014, Farmingdale, NY, USA) according to the manufacturer's instruction. Collecting samples of the thoracic aorta isolated from healthy rats for 30 min in Krebs-Ringer solution gassed with 95% O2 – % CO2, rings were incubated in 5 ml of fresh Krebs-Ringer solution containing at constant temperature water bath (37°C). The vessels were then allowed to equilibrate for an additional 5 min before addition of 3-isobutyl-1-methylxanthine (IBMX, 10 mM) and PE (1 µM). After the rings were subjected to AMO in the presence or absence of modulators of cGMP production for 4 min, reactions were stopped by freezing the tissues at liquefied N2. Results are expressed as picomoles of cGMP per milligram of total protein.
Western blot analysis
The arterial rings were homogenized with a buffer containing protein extraction solution (RIPA, Elpis Biotech, Daejeon, Korea) supplemented with sodium fluoride (NaF, 1 M) and sodium orthovanadate (Na3VO4, 0.2 M). The homogenates were then centrifuged at 1300 rpm for 10 min at 4°C. The protein (30 µg) of aortic rings from rats was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocellulose membranes using a Mini-Protean II (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked with 5% non-fat skim milk powder (Becton Dickinson, Le Pont-De-Claix, France) in 0.5% Tween 20-TBS for 1 hour, and then incubated primary antibodies to Akt and phosphorylated Akt or eNOS and phosphorylated Akt or β-actin (Santa Cruz Biotechnology, CA, USA) at final dilution of 1:1000 overnight, 4°C. The blot was washed several times with 0.05% Tween 20-TBS and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 hours. The membrane was washed several times with 0.05% Tween 20-TBS and then detected by enhanced chemiluminescence (Amersham Lab, Buckinghamshire, England) procedure. The protein expression levels were determined by analyzing the signals captured on nitrocellulose membrane (Millipore, MA, USA) using a Chemi Doc image analyzer (Bio-Rad Laboratories, Hertfordshire, UK).
Statistical analysis
Vasorelaxant responses are manifestated as percentage relaxation from PE (1 µM) precontraction production. Significant difference was compared using repeated measures ANOVA followed by Bonferroni's multiple-comparison test. Student's t-test for unpaired data was also applied. Statistical significance was defined as P < 0.05. The results are given as means ± S.E.M.
RESULTS
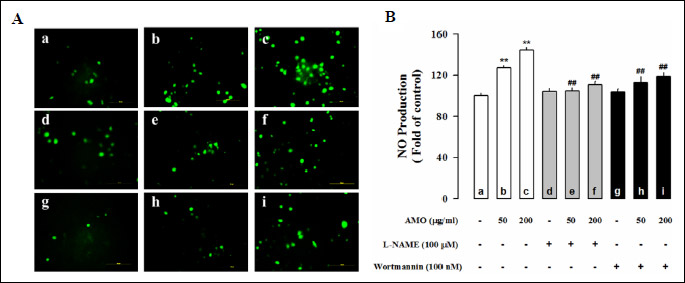
Aqueous extract of Mantidis ootheca stimulates the production of nitric oxide in human umbilical vein endothelial cells
The fluorescence intensity of adhesive HUVEC cells was monitored by fluorescence microscopy, there was a significant increase in the level of NO production treated with AMO (50 and 200 µg/ml) compared to the control (Fig. 1). Pretreatment with L-NAME (100 µM), a nonselective inhibitor of NOS, and wortmannin (0.1 µM), an inhibitor of phosphoinositide 3-kinase (PI3K), significantly decreased the NO produced to AMO-induced HUVEC (P < 0.01). AMO-induced NO production was inhibited by L-NAME and wortmannin, suggesting that this effect is mediated by Akt/NO pathway.
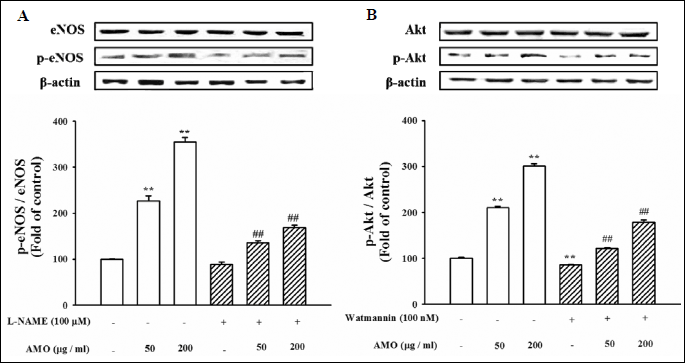
Aqueous extract of Mantidis ootheca -induces phosphorylation of Akt and endothelial nitric oxide synthase in human umbilical vein endothelial cells
To further characterize the eNOS activation by AMO in vasorelaxation, the change in the PI3-kinase/Akt pathway was traced in HUVECs. The phosphorylation levels of Akt (56 kDa) and eNOS (140 kDa) were assessed in HUVECs using Western blot analysis. First, we examined the activation status of Akt and eNOS after AMO stimulation. The levels of phosphorylation were significantly increased with AMO. However, the effects of AMO on the phosphorylation levels of Akt and eNOS were abolished by pretreatment of cells with PI3-kinase/Akt inhibitors, wortmannin (0.1 µM) (Fig. 2), suggesting that PI3-kinase/Akt pathway mediates AMO-induced eNOS phosphorylation.
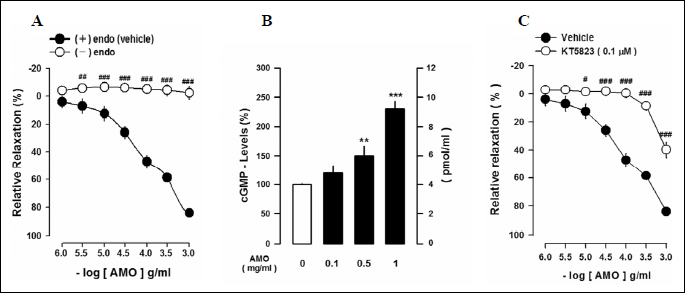
Effects of aqueous extract of Mantidis ootheca on the vascular reactivity and cGMP levels in thoracic aortic rings
AMO relaxed the phenylephrine (PE, 1 µM) precontracted thoracic aorta in an endothelium-dependent manner (Fig. 3A). Effect of AMO-induced vascular relaxation after removal of endothelium from the thoracic aorta tissue was measured. Endothelium-denudation completely abolished the AMO-induced vasorelaxation (Fig. 3A). Incubation of vascular tissues with AMO induced the production of cGMP in a dose-dependent manner (Fig. 3B). Furthermore the effect of KT5823 (0.1 µM), selective inhibitor of cGMP-dependent protein kinase (PKG), was measured. Pretreatment with KT5823 completely abolished the AMO-induced vasorelaxation (Fig. 3C).
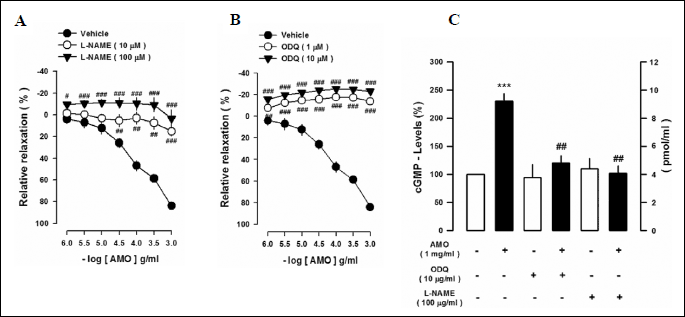
Effects of nitric oxide synthase and soluble guanylyl cyclase inhibition on aqueous extract of Mantidis ootheca-induced vascular reactivity and cGMP levels in thoracic aortic rings
To test the involvement of NO in the AMO-induced vasorelaxation, the effect of L-NAME (10 and 100 µM), a nonselective inhibitor of NOS, was measured. Pretreatment with L-NAME completely abolished the AMO-induced vasorelaxation (Fig. 4A). Because the AMO-induced vasorelaxation was associated with NOS, its downstream signaling was defined. Pretreatment with ODQ (1 and 10 µM), a selective sGC inhibitor, completely abolished the AMO-induced vascular relaxant response of thoracic aortic rings (Fig. 4B). To further define the AMO-induced vasorelaxation, changes in cGMP levels were measured in PE-pretreated thoracic aortic rings. L-NAME and ODQ completely blocked the AMO-induced increase in cGMP levels (Fig. 4C). These findings indicate that AMO induces vascular relaxation and also that activation of endothelium-dependent eNOS-sGC-cGMP-PKG signaling is involved in the AMO-induced vasorelaxation.
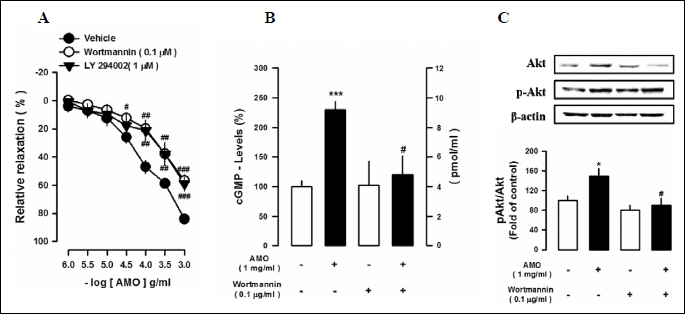
Role of PI3K/Akt signaling signaling on aqueous extract of Mantidis ootheca-induced vascular reactivity and increase in cGMP levels
To define the role of PI3K/Akt signaling on the AMO-induced vasorelaxation, effect of wortmannin (0.1 µM), an inhibitor of phosphoinositide 3-kinase (PI3K), or LY294002 (1 µM), a highly selective inhibitor of PI3 kinase, was tested. Pretreatment with wortmannin and LY294002 completely abolished the AMO-induced vasorelaxation (Fig. 5A). Similarly, wortmannin blocked the AMO-induced increase in pAkt levels and cGMP levels (Fig. 5B and 5C). These data suggest that activation of the PI3K/Akt signaling is involved in the AMO-induced vasorelaxation.
Effects of K+ channel blockers on aqueous extract of Mantidis ootheca-induced vasorelaxation
Effects of K+ channel inhibitors on the AMO-induced vasorelaxation were tested. Pretreatment of thoracic aortic rings with TEA (100 µM) and 4-AP (100 µM), non-selective K+ channel inhibitors, significantly attenuated the AMO-induced vasorelaxation (Fig. 6A). Furthermore, glibenclamide (10 µM), a selective adenosine triphosphate (ATP)-sensitive K+ (K+ATP) channel blocker, attenuated the AMO-induced vasorelaxation (Fig. 6B). These data indicate that activation of K+ channels, especially, K+ ATP channels, are involved in the AMO-induced vasorelaxation.
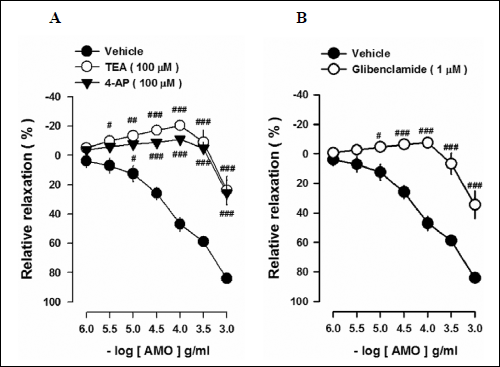 |
Fig. 6. Effect of K+ channel inhibitors on AMO-induced vascular relaxation. (A) Effects of TEA (100 µM) or 4-AP (100 µM) on AMO-induced vascular relaxation. (B) Effects of glibenclamide (1 µM) on AMO-induced vascular relaxation. #P < 0.05, ##P < 0.01, ###P < 0.001 versus vehicle (AMO). |
DISCUSSION
We demonstrated here that AMO induces vasorelaxation via endothelium-dependent eNOS-sGC-cGMP-PKG signaling in the vascular smooth muscle cells. Importantly, the present findings further indicate that AMO-induced vasodilatation was associated with an accentuation of NO production via the PI3K/Akt-dependent activation of eNOS by phosphorylation. Our results provide a molecular mechanism of AMO on the cardiovascular system. PI3/Akt pathway is an important upstream mediator of NO production (16). It was reported that eNOS is regulated by endothelium PI3K/Akt signaling (17, 18). In addition, several natural products have been reported to increase eNOS expression (19, 20). We also found that the increase of expression of p-AKTser473 and p-eNOSser1117 and NO production by AMO. Our results indicated that AMO activates PI3K/Akt signaling pathway to elevate eNOS expression for several reasons: Akt phosphorylation at Ser-473 was stimulated by AMO, whereas enhanced eNOS phosphorylation was almost completely eliminated in the presence of PI3K/Akt inhibitor. Therefore, the activation of PI3K/Akt signaling pathway is crucial in the endothelial NO release system induced by AMO. More importantly, a removal of functional endothelium abolished this relaxant response to AMO, suggesting that the vasorelaxation caused by AMO was endothelium-dependent. Originally identified as EDRF, NO is expressed in vascular endothelial cells and plays an important role in the regulation of vascular tone (3). Recent study has shown that NO increases endothelial cell function and reduces inflammation in hypertensive rats with diabetes (21). To verify the involvement of endothelium-derived vasodilators, the effect of various inhibitors on AMO-induced vascular relaxation were examined. Our results showed that pretreatment of arterial tissues with L-NAME and ODQ, abolished the AMO-induced vascular relaxation. Relaxation of vascular smooth muscle by the NO-cGMP signaling involves a sequence of steps (22). Moreover, AMO-induced increases in cGMP production were abolished by pretreatment with L-NAME or ODQ in arterial tissues, suggesting a significant role of the endothelium/NO-cGMP signaling in AMO-induced vascular relaxation. It is well known that many vasodilators increase both cGMP production for vascular relaxation (23). In parallel, the present study showed that wortmannin significantly attenuated the AMO-induced vasorelaxation and increases in cGMP levels and pAkt expressions. Many compounds are known to activate receptors on the smooth muscle cells that couple via Gs-proteins to guanylyl cyclase. This lead to an increase in intracellular cGMP production and a subsequent activation of cGMP-dependent PKG (24). Pretreatment of vascular tissues with KT5823 attenuated the AMO-induced vascular relaxant response in thoracic aorta. Therefore, suggesting that AMO relaxes vascular smooth muscle via activation of Akt-eNOS-sGC-cGMP-PKG signaling. K+ channels play an essential role in NO synthesis and release in endothelial cells (25, 26). NO is known to cause membrane hyperpolarization by activating adenosine triphosphate (ATP)-sensitive K+(KATP) channel in the vascular smooth muscle (27). Our results indicated that K+ channels play an important role at least in part, in the AMO-induced vascular relaxation since it was significantly inhibited by TEA, 4-aminopyridine, and glibenclamide. Although we do not have pure compound to explain the vasorelaxation, AMO showed clear acute hypotensive effect in anesthetized SD rats. Hence, the current study has provided further cellular insight to the vasoprotective effect of AMO and reinforces the use of this traditional medicinal herb in the treatment of hypertension.
Taken together, the present study demonstrates that Mantidis ootheca relaxes vascular smooth muscle via endothelium-dependent activation of through the PI3K/Akt-mediated NO-sGC-cGMP-PKG signaling, possible involvement of K+ channel.
Authors' contributions: H.Y. Kim and Y.J. Lee conceived and designed the experiment; H.Y. Kim, B.H. Han, J.J. Yoon, Y.M. Ahn, M.H. Hong, R. Tan performed the experimental work and data analyses; D.G. Kang, and H.S. Lee supervised the experimental work. All authors read and approved the final manuscript.
Acknowledgements: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2008-0062484) (2014R1A2A2A01005101) (2016R1A2B1016174).
Conflict of interests: None declared.
REFERENCES
- Shepherd JT, Katusic ZS. Endothelium-deried vasoactive factors. I. Endothelium-dependent relaxation. Hypertension 1991; 18: 76-85.
- Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf) 2015; 219: 22-96.
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 1989; 3: 2007-2018.
- Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 2007; 42: 271-279.
- Knott AB, Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal 2009; 11: 541-54.
- Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun 1988; 153: 1251-1256.
- Chakraborti A, Gulati K, Ray A. Possible role of nitric oxide (NO) in the regulation of gender related differences in stress induced anxiogenesis in rats. Nitric Oxide 2014; 43: 74-80.
- Michel T, and Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch 2010; 459: 807-16.
- Morgado M, Cairrao E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci 2012; 69: 247-266.
- Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 1997; 100: 2153-2157.
- John S, Schmieder RE. Impaired endothelial function in arterial hypertension and hypercholesterolemia. J Hypertens 2000; 18: 363-374.
- Halim MA, Gillberg L, Boghus S, Sundbom M, Karlbom U, Webb DL, Hellstm PM. Nitric oxide regulation of migrating motor complex: randomized trial of N(G)-monomethyl-L-arginine effects in relation to muscarinic and serotonergic receptor blockade. Acta Physiol (Oxf) 2015; 215: 105-118.
- Jiao SD, Craig M. Ten Lectures on the Use of Medicinals from the Personal Experience of Jiao Shu-De. Jiao Clinical Chinese Medicine Series. Paradigm Publications, Bilingual edition, 2003.
- Kim CM, Shin MK, Ahn DK, Lee KS. Chinese Medicine Dictionary Chapter 5. Seoul Jungdam 2006; pp. 2184-2186.
- Tan Z, Lei Y, Zhang B, Huang L. Comparison of pharmacological studies on ootheca Mantidis. Zhongguo Zhong Yao Za Zhi 1997; 22: 496-499.
- Blanes MG, Oubaha M, Rautureau Y, Gratton JP. Phosphorylation of tyrosine 801 of vascular endothelial growth factor receptor-2 is necessary for Akt-dependent endothelial nitric-oxide synthase activation and nitric oxide release from endothelial cells. J Biol Chem 2007; 282: 10660-10669.
- Ohkita M, Tawa M, Kitada K, Matsumura Y. Pathophysiological roles of endothelin receptors in cardiovascular diseases. J Pharmacol Sci 2012; 119: 302-313.
- Wang Y, Wang S, Wier WG, et al. Exercise improves the dilatation function of mesenteric arteries in postmyocardial infarction rats via a PI3K/Akt/eNOS pathway-mediated mechanism. Am J Physiol Heart Circ Physiol 2010; 299: H2097-H2106.
- Sun YY, Su XH, Jin JY, et al. Rumex acetosa L. induces vasorelaxation in rat aorta via activation of PI3-kinase/Akt- AND Ca(2+)-eNOS-NO signaling in endothelial cells. J Physiol Pharmacol. 2015; 66: 907-915.
- Kim HY, Oh H, Li X, Cho KW, Kang DG, Lee HS. Ethanol extract of seeds of Oenothera odorata induces vasorelaxation via endothelium-dependent NO-cGMP signaling through activation of Akt-eNOS-sGC pathway. J Ethnopharmacol 2011; 133: 315-323.
- Mason RP, Corbalan JJ, Jacob RF, Dawoud H, Malinski T. Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and rantes levels in hypertensive rats with diabetes. J Physiol Pharmacol 2015; 66: 65-72.
- Gillespie JS, Liu XR, Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol 1989; 98: 1080-1082.
- Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 2010; 62: 525-563.
- Inserte J, Garcia-Dorado D. The cGMP/PKG pathway as a common mediator of cardioprotection: translatability and mechanism. Br J Pharmacol 2015; 172: 1996-2009.
- Loeb AL, Izzo NJ, Johnson RM, Garrison JC, Peach MJ. Endothelium-derived relaxing factor release associated with increased endothelial cell inositol trisphosphate and intracellular calcium. Am J Cardiol 1988; 62: 36G-40G.
- Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 1988; 95: 1165-1174.
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science 1989; 245: 177-180.
A c c e p t e d : April 21, 2017