INHIBITION OF BLOOD PLATELET ADHESION BY PHENOLICS’ RICH FRACTION OF HIPPOPHAE RHAMNOIDES L. FRUITS
INTRODUCTION
Various parts of sea buckthorn (Hippophae rhamnoides L.) - both fruits and leaves are used in traditional medicine. Moreover, preparations made from H. rhamnoides are also used in conventional medicine. It is known that extracts from different parts of this plant demonstrate various biological properties, including anticancerous, antioxidant, anti-inflammatory and antibacterial activities (1-6). Different in vivo and in vitro studies have also proved that H. rhamnoides has therapeutic and protective action in cardiovascular diseases (7, 8), and yet mechanisms of its action have been still unknown. Sayegh et al. (8) discuss the potential cardiovascular implications of H. rhamnoides fruits consumption in humans. Eccleston et al. (9) demonstrate antihypertensive activity of the fruit juice of H. rhamnoides. Our previous experiments demonstrated that the phenolic fraction from H. rhamnoides fruit might reduce the generation of free radicals and arachidonic acid metabolism in thrombin-stimulated blood platelets (6). Our present study sheds new light on the effect of the phenolic fraction from H. rhamnoides fruit on hemostatic properties of blood platelets i.e. platelet adhesion to collagen type I (which is the most prominent collagen type in the arterial wall in vessels changed by atherosclerosis) as well as fibrinogen, platelet aggregation in vitro. We also determined the effects of the tested fraction on thiol groups metabolism (protein thiols and glutathione) in blood platelets, as changes of thiol redox potential of platelets play an important role in different steps of their activation, signal transduction and the exposure receptors on platelet surface. Moreover, protein disulfide isomerase was found on the platelet surface, where it appears to play an important function in the platelet activation, i.e. the exposure of integrin αIIbβ3 (10). The range of tested concentrations of the H. rhamnoides fraction (0.5 – 50 µg/ml) can be achieved in plasma by way of supplementation with phenolic compounds.
MATERIAL AND METHODS
Chemicals
Adenosine diphosphate (ADP) was obtained from Chrono-Log Corporation (Havertown, USA). Thrombin was purchased from BioMed Lublin, Poland. Collagen type I, bovine serum albumin (BSA), 5,5’-dithio-bis(2-nitro-benzoic acid), and dimethylsulfoxide (DMSO) were purchased from Sigma (St. Louis, MO., USA). For measurements of blood platelet adhesion, the Thermo Scientific Pierce BCA (bicinchoninic acid) Protein Assay kit (Thermo Scientific, Rockford, USA) was used. Fibrinogen was isolated from pooled citrated human plasma with the cold ethanol precipitation technique followed by ammonium sulphate fractionation at 26% saturation at 4°C, according to Doolittle (11). Its concentration was determined spectrophotometrically at 280 nm using an extinction coefficient 1.55 for 1 mg/ml solution. The concentration of purified fibrinogen in the reaction system was 2 mg/ml. All other reagents represented analytical grade and were provided by commercial suppliers.
Plant material
Sea buckthorn (Hippophae rhamnoides L.) berries were obtained from a horticultural farm in Sokolka, Podlaskie Voivodeship, Poland (53°24’N, 23°30’E). Fruits were freeze-dried and stored in a refrigerator.
Preparation and quantification of the fraction of phenolic compounds
The phenolic fraction of sea buckthorn fruit was prepared as described in (6). Briefly, lyophilized sea buckthorn fruit (800 g) was extracted with 4 L of 80% methanol, at room temperature (24 h). The fruit was further subjected to hot extraction with 4L of 80% methanol, under reflux (1 h). The extracts were combined, filtered, and rotary-evaporated to remove methanol. The residue was loaded onto a short C18 column (LiChroprep 40 – 63 µm RP-18, Millipore Corp., Bedford, MA, USA). The column was washed with water, and phenolic compounds were subsequently eluted with 50% methanol. The eluate was concentrated under reduced pressure and freeze-dried, to yield 10.19 g of dry phenolic fraction. LC-MS analyses (negative ion mode) of the fraction were performed according to the methods described in (6). Components of the fraction were identified by HPLC-ESI-MS/MS, using a Thermo Finnigan Surveyor HPLC system, equipped with a photodiode array (PDA) detector and coupled with a Thermo LCQ Advantage Max ion-trap mass spectrometer. Separation was performed on a Waters XBridge BEH C18 column (3.0 × 150 mm, 2.5 µm), 50°C. The injection volume was 5 µL. The elution (300 µL min–1) was carried out with a gradient of solvent B (acetonitrile with 0.1% FA) in solvent A (MilliQ water with 0.1% FA): 0 – 5 min, 5% B; 5 – 85 min, 5 – 60% B; 85 – 95 min, 60% B. Quantitative analysis was performed with the use of ACQUITY UPLC system (Waters, USA), equipped with a PDA detector and a triple quadrupole mass detector (ACQUITY TQD, Waters). Samples were separated on an ACQUITY HSS C18 (100 × 2.1 mm, 1.8 µm; Waters) column, maintained at 40°C. The injection volume was 2.5 µL. The elution (400 µL min–1) was carried out with a gradient of solvent B (acetonitrile with 0.1% FA) in solvent A (MilliQ water with 0.1% FA): 0 – 0.50 min, 1% B; 0.50 – 17.95 min, 1 – 35.5% B; 17.95 – 18.00 min, 35.5 – 99% B; 18.00 – 20.00 min, 99% B. Details of MS setting for both LC-MS systems are described in (6). The PDA detection was used for the quantitation of phenolic compounds (λ = 350 nm for flavonoids, 254 nm for other phenolics). Since isorhamnetin derivatives are the main flavonoids found in sea buckthorn fruits, the standard curve of isorhamnetin 3-O-β-glucosyl(1 → 2)-β-galactoside was applied to calculate relative concentrations of individual flavonoids and the total content of other phenolic compounds. Analyses were performed in triplicate.
The LC-MS analyses showed that flavonoids were the dominant compounds in the phenolic fraction of sea buckthorn fruits and their total amount, expressed as isorhamnetin 3-O-b-glucosyl(1 → 2)-β-galactoside equivalent, equaled 214.04 mg/g. Other phenolic compounds, including putative proanthocyanidins, were difficult to identify, and most of them was present in small amounts. Their total content was 28.65 mg/g of the fraction (expressed as isorhamnetin 3-O-β-glucosyl(1 → 2)-β-galactoside equivalent) (6).
A stock solution of the H. rhamnoides phenolic fraction was made in 50% DMSO. The final concentration of DMSO in samples was lower than 0.05% and its effects were determined in all experiments.
Blood platelet isolation
Fresh human plasma was obtained from regular, medication-free donors at the Regional Centre of Blood Donation and Blood Treatment (Lodz, Poland). The donors had not taken any medications or addictive substances (including tobacco, alcohol, antioxidant supplementation, aspirin or any other anti-platelet drugs). The protocol was approved by the Committee for Research on Human Subjects of the University of Lodz number 2/KBBN-UL/III/2014. Platelet-rich plasma (PRP) was prepared by centrifugation of fresh human blood at 250 × g for 10 min at room temperature. Platelets were then sedimented by centrifugation at 500 × g for 10 min at room temperature. The platelet pellet was washed with Tyrode’s buffer (10 mM HEPES, 140 mM NaCl, 3 mM KCl, 0.5 mM MgCl2, 5 mM NaHCO3, 10 mM glucose; pH 7.4) twice; afterwards, the platelets were suspended in the same buffer. The concentration of platelets in suspensions, estimated spectrophotometrically (12), amounted to 2.5 – 3 × 108/ml.
Suspensions of blood platelets or PRP were pre-incubated (30 min, at 37°C) with: H. rhamnoides fraction at the final concentrations of 0.5 – 50 µg/ml.
Platelet adhesion
Adhesion of blood platelets to collagen type I and fibrinogen was determined with Tuszynski’s and Murphy’s method (13) as described earlier (14). Wells of a 96-well microtiter dish (CLINIPLATE EB FB 50 PCS/CRS, Labsystems) were incubated for 2 – 3 h with 50 µl of fibrinogen (final concentration of 2 mg/ml), dissolved in phosphate-buffered saline, pH 7.5 (PBS) or 40 µg/ml collagen dissolved in 0.05% CH3COOH. The wells were aspirated, treated with 200 µl PBS containing 1% BSA for 1 h and then washed three more times with 200 µl of PBS. Immediately after washing, the wells were supplemented with 50 µl of thrombin or ADP (final concentration 0.2 U/ml or 10 µM, respectively). Then platelet suspension (after 30 min preincubation at 37°C), with the tested fraction at the final concentrations of 0.5 – 50 µg/ml, was added to each well (100 µl) and the plate was incubated at 37°C for
1 hour. The control suspension did not contain H. rhamnoides fraction. Nonadherent cells were removed by aspiration and the wells were washed three times with 200 µl of PBS. The total cell-associated protein was determined by dissolving the attached blood platelets directly in the microtiter wells with 200 µl of the BCA working solution, and incubated at 37°C for 60 min. Plates were allowed to cool to room temperature, cover sheets were removed, and the absorbance of each well was determined at 560 nm with a microtiter plate reader (SPECTROstar Nano microplate reader; BMG Labtech Inc., Ortenberg, Germany). The absorbance of control platelets (without the tested plant fraction) was expressed as 100%. Before adding the BCA working solution, microscopic examination of adherent blood platelet to microplate wells coated with collagen type I was also performed.
Platelet aggregation
Blood platelet aggregation was measured by platelet turbidity, with 0% aggregation calibrated as the absorbance of platelet poor plasma and 100% aggregation as the absorbance of PRP. The aggregation of PRP (preincubated with tested plant fraction) in response to 10 µM ADP was recorded using a microtiter plate reader (BioRad, Model 550) (15).
Glutathione and thiol groups measurement
The thiol group content in platelet proteins and GSH concentration were measured spectrophotometrically (the SPECTROstar Nano Microplate Reader- BMG LABTECH Germany) by absorbance at 412 nm with Ellman’s reagent: 5,5’-dithio-bis-(2-nitrobenzoic acid). The thiol group concentration and GSH was calculated using a molar extinction coefficient (e = 13,600 M–1 cm–1) (16, 17).
Data analysis
Statistical analysis was done using several tests. In order to eliminate uncertain data, the Q-Dixon test was performed. All the values in this study were expressed as mean ± S.E.M.; n - number of blood donors. Statistical analysis was performed with one-way ANOVA for repeated measurements.
RESULTS
We demonstrate that adhesion to collagen of resting blood platelets preincubated with the phenolic fraction from H. rhamnoides fruits (at a dose ranged from 0.5 to 50 µg/ml) was inhibited, and this process was statistically significant. The inhibitory effect of the H. rhamnoides fraction appears to be concentration-dependent in adhesion test (Fig. 1A). Fig. 1A also reports the percent inhibition of adhesion of thrombin-activated platelets to collagen. Microscopic examination of adherent blood platelets (treated with the tested plant fraction) to microplate wells coated with collagen confirmed our observation (Fig. 1C). We observed the same process, which was inhibition of platelet adhesion, when we measured the adhesion to fibrinogen of resting platelets and thrombin-activated platelets (Fig. 1B). The action of the H. rhamnoides fraction was concentration-dependent (Fig. 1B). In the presence of the highest concentration of the H. rhamnoides fraction (50 µg/ml) inhibition of platelet adhesion to fibrinogen for resting platelets and thrombin-activated platelets was about 65% and 55%, respectively (Fig. 1B). During evaluation of blood platelet response to ADP, the tested plant fraction (at all concentrations: 0.5 – 50 µg/ml) displayed significantly lower anti-adhesive properties than when thrombin was used. Moreover, the reduction of adhesion in the presence of the phenolic fraction (at the highest concentrations: 10 and 50 µg/ml) was statistically insignificant (Fig. 1B).
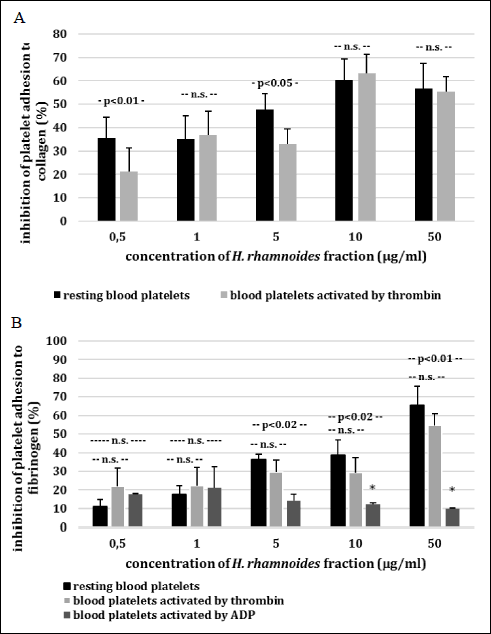 |
Fig. 1AB. Effects of the phenolic fraction from fruits of H. rhamnoides (0.5 – 50 µg/ml; 30 min) on adhesion of resting blood platelets and thrombin-activated platelets to collagen (A) resting blood platelets and thrombin/ADP-activated platelets to fibrinogen (B). |
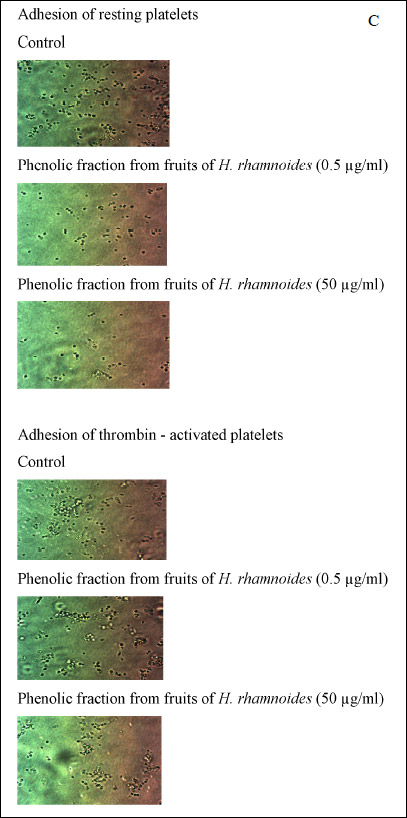 |
Fig. 1C. Inhibition of platelet adhesion by the phenolic fraction from fruits of H. rhamnoides was expressed as percentage of that recorded for control blood platelets (without the phenolic fraction from fruits of H. rhamnoides). Data represent mean ± S.E.M. of 4 – 9 healthy volunteers (experiments done in triplicate). The effect of five different concentrations of the tested fraction (0.5, 1, 5, 10 and 50 µg/ml; for inhibition of platelet adhesion) was statistically significant: P < 0.05 (vs. control platelets); *P > 0.05 (vs. control platelets). Microscopic examination of adherent platelets (treated with tested plant fraction at the highest concentration of 5 and 50 µg/ml, for 30 min at 37°C) to microplate wells coated with collagen (C). |
The next part of this paper included determination of abilities of the H. rhamnoides fraction (5, 10 and 50 µg/ml) to reduce blood platelet aggregation (Fig. 2). Platelet aggregation stimulated by ADP was assessed in PRP. In these experiments, the H. rhamnoides fraction had no anti-aggregatory actions (Fig. 2).
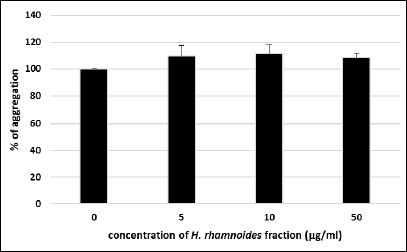 |
Fig. 2. Effects of the phenolic fraction from fruits of H. rhamnoides (5 – 50 µg/ml; 30 min) on blood platelet aggregation stimulated by 10 µM ADP. Data represent mean ± S.E.M. of 4 healthy volunteers (experiments done in triplicate). The effect of three different concentrations of the tested fraction (5, 10 and 50 µg/ml; for platelet aggregation) was not statistically significant: P > 0.05 (vs. control platelets). |
Blood platelets exposure to the phenolic fraction from H. rhamnoides fruit (at concentrations between 0.5 – 50 µg/ml) did not result in the change of GSH concentration (Fig. 3). However, after 30 min incubation of blood platelets with tested phenolic fraction at low concentrations (0.5 and 1 µg/ml), the amount of thiol groups in platelet proteins increased (Fig. 3). In control sample of blood platelets, the level of thiol groups in platelet proteins was about 140 nmol thiol groups/ml platelets, compared to increase to level of approximately 160 nmol thiol groups per 1 ml of platelets in the presence of the fraction from H. rhamnoides fruits at low concentrations of 0.5 and 1 µg/ml (Fig. 3). The tested fraction also changed the level of thiol groups in platelet proteins when used at higher concentrations (respectively 5, 10 and 50 µg/ml), however this process was not statistically significant (Fig. 3).
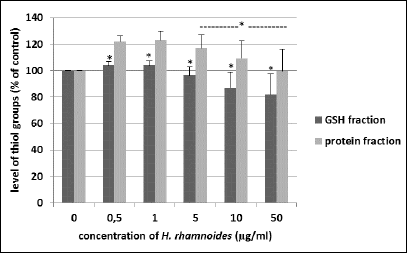 |
Fig. 3. Effects of the phenolic fraction from fruits of H. rhamnoides (0.5 – 50 µg/ml; 30 min) on the level of thiol groups in GSH fraction and protein fraction isolated from blood platelets. Data represent mean ± S.E.M. of 8 healthy volunteers (experiments done in triplicate), *P > 0.05 (vs. control platelets; for GSH fraction). The effect of two different concentrations of the tested plant fraction (0.5 and 1 µg/ml) was statistically significant, P < 0.02 (vs. control platelets; for protein fraction). The effect of three different concentrations of the tested fraction (5, 10 and 50 µg/ml) was not statistically significant, *P > 0.05 (vs. control platelets; for protein fraction). |
DISCUSSION
Plants are not only good source compounds (i.e. phenolic compounds) with antioxidant activity, but they are also good source of compounds that may be useful in the reduction of blood platelet activation. Blood platelets and their activation are very important elements of hemostasis, pathomechanisms of various cardiovascular disorders and complications of other diseases. They participate in inflammatory process and metastasis (18). Antiplatelet activity of the phenolic fraction from the fruit of H. rhamnoides and its biological significance remain unclear. Only a few experiments on humans which examined the effects of H. rhamnoides on blood platelet activation were described (6, 8). Moreover, most of experiments were carried out using different kinds of H. rhamnoides extracts which composition was not always determined. Our previous LC-MS analyses (6) demonstrated that flavonoids were the dominant compounds in the phenolic fraction of sea buckthorn fruits tested presently. Isorhamnetin 3-O-rhamnosyl(1 → 6)glucoside and isorhamnetin 3-O-glucoside were present in the highest concentrations (6). Luo et al. (19) observe that isorhamnetin inhibited atherosclerotic plaque development. It is also an important that the concentration of phenolics (242.7 mg/g of tested extract of H. rhamnoides) is similar to the concentrations of these compounds in a commercial extract of berries of Aronia melanocarpa (309.6 mg/g of extract) (20, 21). Moreover, H. rhamnoides fruits are also better source of phenolics than other berries, including raspberry, black currant and strawberry (20, 21). In addition, different in vivo and in vitro experiments have indicated that diets rich in berries, i.e. grapes and aronia berries are correlated with the inhibition of blood platelet activation and decreased risk of cardiovascular diseases (21). Su et al. (22) observed that ethanol extract of Rubus chingii has cardioprotective effects in rats. It induced vasorelaxation via two-step signaling. It is very important that there are no data in the literature indicating toxic properties of berries, extracts or other berry products. The results of Golynski et al. (23) also showed a high level of safety of other fruit administration - habanero fruits in rats, i.e. there were no biochemical and hematological changes in tested animals. Our findings demonstrate for the antiadhesive properties of the phenolic fraction from H. rhamnoides fruits, in an experimental system of isolated washed platelets for the first time. The tested fraction significantly inhibited platelet adhesion (induced by various agonists: thrombin and ADP) to two adhesive proteins - collagen and fibrinogen, but the inhibitory action of the tested fraction is weaker when ADP. It has known that reactive oxygen species (ROS) may enhance different step of platelet activation stimulated by thrombin. This process has not been observed in platelets activated by ADP. Therefore, we may suggest that the antiadhesive action of the tested fraction was weaker when ADP was used as the agonist than when we used thrombin, because tested fraction decreases the level of ROS (which may act as secondary messengers) in platelets activated by thrombin (6). In addition, our present and earlier (6) results may suggest that the phenolic fraction from H. rhamnoides fruits may partly inhibit the proteolytic properties of thrombin. Moreover, it may also inhibit the activation cascade downstream of G-protein in platelets.
The effects of the tested plant fraction on hemostatic properties of platelets were also assessed by measurements of platelet aggregation in more physiological environment - in platelet-rich plasma. Blood platelet aggregation was performed using ADP as a blood platelet activator. However, the effects on the platelet aggregation stimulated by ADP caused by three different concentrations (5, 10 and 50 µg/ml) of the phenolic fraction from H. rhamnoides fruits were not statistically significant. These findings are in accordance with results of other Authors. Results obtained by Eccleston et al. (9) demonstrated that there were no significant changes in platelet aggregation between treatment groups (twenty healthy male volunteers were given either a placebo or H. rhamnoides juice for 8 weeks). However, H. rhamnoides wine has protective effects against hypercholesterolemia (24). Chong et al. (25) noted that the concentration of the phenolic compounds in the berries is not only factor influencing their effect on blood platelet aggregation, but also the class of phenolic compounds plays an important role. Moreover, the inhibitory action of berry phenolic compounds can be influenced by the type and concentration of platelet agonist used to stimulate platelet aggregation (21).
Both our previous (6) and present experiments suggest that the tested fraction has a multiple action on platelets and it may play a role in modulating blood platelet activation, especially platelet adhesion, by interfering with the metabolism of arachidonic acid and the generation of reactive oxygen species in these cells. Yet, the tested fraction may reveal that other mechanisms of action exist. It is known that the reaction in blood platelets involving thiol groups play an important role in their biological functions. Protein disulfide isomerase was found on blood platelet surface. This enzyme is involved in different steps of platelet activation: platelet adhesion, aggregation and secretion (10, 26). Our results on thiol groups in platelet proteins may suggest that changes in the level of these groups induced by the phenolic fraction from H. rhamnoides fruit at low tested concentrations (0.5 and 1 µg/ml) may be involved in modulation of platelet activation - platelet adhesion by studied fraction. In addition, we may suppose that the phenolic fraction from H. rhamnoides (at low concentrations) may modulate the activity of protein disulfide isomerase, because Lin et al. (27) observed that flavonoids (i.e. quercetin) inhibit protein disulfide isomerase by binding to its b’x domain. Other authors (28) demonstrated this process in blood platelets. Moreover, it is very important that the phenolic fraction from H. rhamnoides fruits has not changed the level of physiological antioxidant - GSH in blood platelets.
Our results may come in useful in prevention of cardiovascular events, because H. rhamnoides fruits contain compounds with potential anti-platelet activities. Moreover, H. rhamnoides fruits may be a good source of nutraceuticals or medicinal compounds, not only in cardiovascular diseases, but also in other disorders associated with changes in hemostasis, including cancer. We may suppose that the high content of phenolic compounds seem to be responsible for the observed anti-adhesive properties. However, the effect of pure compounds (which are presented at the highest concentrations, i.e. flavonol glycosides - isorhamnetin 3-O-rhamnosyl(1 → 6)glucoside and isorhamnetin 3-O-glucoside in tested plant fraction) still remain to be examined.
Abbreviations: ADP, adenosine diphosphate; BCA, bicinchoninic acid; BSA, bovine serum albumin; DMSO, dimethylsulfoxide; GSH, glutathione; PBS, phosphate-buffered saline; PDA, photodiode array; PRP, platelet-rich plasma; ROS, reactive oxygen species.
Acknowledgements: This work was supported by National Science Centre, Poland 2015/19/B/NZ9/03164. Special thanks go to A. Rogalska, Ph.D. (Department of Thermobiology, Institute of Biophysics, University of Lodz) for helpful technical assistance in microscopic experiments.
Conflict of interests: None declared.
REFERENCES
- Pang X, Zhao J, Zhang W, et al. Antihypertensive effect of total flavones extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J Ethnopharmacol 2008; 117: 325-331.
- Cheng T, Li T. Protective action of seed oil of Hippophae rhamnoides L. (HR) against experimental liver injury in mice. Zhonghua Yufang Yixue Zazhi 1992; 26: 227-229.
- Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides) during maturation. J Agric Food Chem 2000; 48: 1485-1490.
- Suleyman H, Demirezer LO, Buyukokuroglu ME, et al. Anti-ulcerogenic effect of Hippophae rhamnoides L. Phytother Res 2001; 15: 625-627.
- Malinowska P, Olas B. Sea buckthorn - valuable plant for health. Kosmos 2016; 2: 288-292.
- Olas B, Kontek B, Malinowska P, Zuchowski J, Stochmal A. Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxid Med Cell Longev 2016;2016: 1-8. doi 10.1155/2016/4692486
- Basu M, Prasad R, Jayamurthy P, Pal K, Arumughan C, Sawhney RC. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007; 14: 770-777.
- Sayegh M, Miglio C, Ray S. Potential cardiovascular implications of sea buckthorn berry consumption in humans. Inter J Food Sci Nutr 2014; 65: 521-528.
- Eccleston C, Baoru Y, Tahvonen R, Kallio H, Rimbach GH, Minihane AM. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J Nutr Biochem 2002; 13: 346-354.
- Karolczak K, Olas B, Kolodziejczyk J. The role of thiols in blood platelet activation. Adv Cell Biol 2009; 1: 101-120.
- Doolittle RF, Schubert D, Schwartz SA. Amino acid sequence studies on artiodactyl fibrinopeptides I Dromedary camel, mule deer, and cape buffalo. Arch Biochem Biophys 1967; 118: 456-467.
- Walkowiak B, Michalak E, Koziolkiewicz W, Cierniewski CS. Rapid photometric method for estimation of platelet count in blood plasma or platelet suspension. Thromb Res 1989; 56: 763-766.
- Tuszynski GP, Murphy A. Spectrophotometric quantitation of anchorage - dependent cell numbers using the bicinchoninic acid protein assay reagent. Anal Biochem 1990; 184: 189-191.
- Olas B, Wachowicz B, Tomczak A, Erler J, Stochmal A, Oleszek W. Comparative anti-platelet and antioxidant properties of polyphenol-rich extracts from: berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets 2008; 19: 70-77.
- Walkowiak B, Kesy A, Michalec L. Microplate leader - a conventient tool in studies of blood coagulation. Thromb Res 1997; 87: 95-103.
- Ando Y, Steiner M. Sulphydryl and disulphide groups of platelet membranes: determination of disulphide groups. Biochim Biophys Acta 1973; 311: 26-37.
- Ando Y, Steiner M. Sulphydryl and disulphide groups of platelet membranes: determination of sulphydryl groups. Biochim Biophys Acta 1973; 311: 38-44.
- Blockmans D, Deckmyn H, Vermylen J. Platelet activation. Blood Rev 1995; 9: 143-156.
- Luo Y, Sun G, Dong X, Wang M, Qin M, Yu Y, Sun X. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS One 2015; 10: e0120259. doi: 10.1371/journal.pone.0120259
- Olas B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem Toxicol 2016; 97: 199-204.
- Olas B. The multifunctionality of berries toward blood platelet and the role of berry phenolics in cardiovascular disorders. Platelets 2016; Oct 25: 1-10. doi: 10.1080/09537104.2016.1235689
- Su XH, Duan R, Sun YY, et al. Cardiovascular effects of ethanol extract of Rubus chingii hu (Rosaceae) in rats: an in vivo and in vitro approach. J Physiol Pharmacol 2014; 65: 417-424.
- Golynski M, Balicki J, Lutnicki K, et al. Systemic and local effects of intragastric administration of the habanero fruit (Capsicum chinense jacquin c.v.) in rats. J Physiol Pharmacol 2015; 66: 259-265.
- Negi B, Kaur R, Dey G. Protective effects of a novel sea buckthorn wine on oxidative stress and hipercholesterolemia. Food Funct 2013; 4: 240-248.
- Chong MFF, Macdonald R, Lovegrove JA. Fruit polyphenols and CDV risk: a review of human intervention studies. Br J Nutr 2010; 104: S28-S39.
- Essex DW, Li N. Redox control of platelet aggregation. Biochemistry 2003; 42: 129-136.
- Lin L, Gopal S, Sharda A, et al. Quercetin-3-rutinoside inhibits protein disulfide isomerase by binding to its b’x domain. J Biol Chem 2015; 290: 23543-23552.
- Flaumenhalt R, Furie B, Zwicker JI. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arterioscler Thromb Vasc Biol 2015; 35: 16-23.
A c c e p t e d : April 19, 2017