NEW INSIGHT INTO THE DIRECT ANTI-INFLAMMATORY ACTIVITY OF A MYOKINE IRISIN AGAINST PROINFLAMMATORY ACTIVATION OF ADIPOCYTES. IMPLICATION FOR EXERCISE IN OBESITY
2Department of Glycoconjugate Biochemistry, Institute of Zoology, Jagiellonian University, Cracow, Poland;
3Department of Physiology, Faculty of Medicine, Jagiellonian University Medical College, Cracow, Poland
INTRODUCTION
The prevalence of obesity has reached pandemic proportions and is a major risk for several metabolic and cardiovascular diseases. It is believed that this phenomenon is related to an increased energy intake and reduction of physical activity, leading to diminished insulin sensitivity, the reduced skeletal muscle mass and an increase of visceral adipose tissue and epididymal fat (1-4). Chronic low-grade inflammation, accompanied by proinflammatory macrophages infiltration in white adipose tissue (WAT), has been closely correlated with obesity and its comorbidities (5-8). Adipose tissue, though historically considered as inert energy storage, has been now recognized as an endocrine organ due to secretion of an array of signaling molecules called adipokines (9, 10). Adipokines are also involved in the regulation of inflammatory responses and the dysregulation of adipokines secretion might lead to the development of an excess of adiposity and the decrease of insulin sensitivity (9-12). On the other hand, it is generally accepted that regular, moderate physical activity is inversely correlated with systemic low-grade inflammation supporting the notion that protective effects of exercise in patients with chronic diseases could be attributed to the exercise-induced an anti-inflammatory action (13, 14).
These protective effects of exercise could be due to the secretion of muscle-derived peptides, so-called 'myokines' released by contracting skeletal muscles, and exerting a direct anti-inflammatory effects, and/or specific effects on adipose tissue (3, 15, 16). Of particular interest is this myokine ability to modify adipose tissue metabolism and its contribution to a cross-talk of signals from skeletal muscle and adipose tissue. It was suggested that skeletal muscle myokines could be of therapeutic value in chronic diseases in which the exercise has been demonstrated to exhibit a beneficial effect like obesity, type 2 diabetes, hypertension and others (3, 15). The particularly promising therapeutic target seems the pathways activated by irisin, the myokine identified by Bostrom et al. (17). Irisin is a peptide released into the circulation by cleavage of fibronectin type III domain containing protein 5 (FNDC5), a membrane-bound protein in skeletal muscle and released in response to exercise or muscle shivering, to cause browning and to regulate the thermogenesis within white adipose tissue (17-19).
In skeletal muscle FNDC5 expression strictly depends upon an increased level of PGC-1 peroxisome proliferator-activated receptor gamma (PPARγ) which is known to regulates genes in response to nutritional and physiological cues especially to a physical exercise (20).
It is of the interest that the WAT is not only a target for irisin action, but also it can be a source of FNDC5 which is upregulated after exercise (21-24). Recent studies questioned the existence of irisin in humans (25) but Jedrychowski et al. (26) using quantitative mass spectrometry confirmed the presence of human irisin and its release by exercise.
Since irisin exerts a beneficial protective effect in several tissues, it is believed that irisin possesses also anti-inflammatory properties, however anti-inflammatory activities of this peptide are relatively little studied. In some studies anti-inflammatory action of exercise was associated with increase of plasma irisin and suggesting that this peptide might be implicated in this action (27-30). In recent study irisin was shown to decrease the expression of inflammatory markers by peritoneal macrophages and stimulate macrophage shift from M1 to M2 types (31). Irisin was also reported to suppress proinflammatory activation of macrophages (32, 33) as well as NFκB activation in malignant breast epithelial cells (34).
While irisin metabolic effect on white adipose tissue has been well studied and some anti-inflammatory actions have been shown in other tissues, to our best knowledge this is the first attempt to characterize the anti-inflammatory effects of irisin in adipocytes, the main non immunocompetent components of adipose tissue, which plays a crucial role in pro-inflammatory activation of macrophages in the obesity state.
MATERIALS AND METHODS
Chemicals and materials
DMEM medium, antibiotics (streptomycin and penicillin), fetal bovine serum (FBS), and accutase were from PAA (Pasching, Austria). Lipopolysaccharide (LPS), calf serum, 2',7'-dichlorodihydrofluorescein diacetate (DCFDA), β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). Annexin V kit was purchased from BD Biosciences Pharmingen (San Diego, CA, USA). RNeasy Plus Mini Kit (74134) for RNA isolation was obtained from Qiagen (Hilden, Germany). High Capacity RNA-to-cDNA Kit (4387406) for reverse transcription and TaqMan Gene Expresson Master Mix (4369026) for real time PCR were provided by Applied Biosystems (ThermoFisher; Foster City, CA, USA). Phospho-NFκBp65 and total NFκBp65 and secondary antibodies were from Cell Signaling Technology (Beverly, MA, USA). ELISA kits for TNF-α, IL-6 and leptin were supplied by IBL International (Hamburg, Germany). ELISA kits for mouse MCP-1 was purchased from R&D BioVendor.
Cell culture
The study was conducted in the Mycoplasma-free mouse preadipocytes 3T3 L1 cell line differentiated to adipocytes. Cell line was kindly provided by Professor Alicja Jozkowicz from the Department of Medical Biology, Jagiellonian University. For the differentiation assessment cells were grown until confluence in DMEM medium supplemented with 1% antibiotic solution and 10% calf serum. After additional 2 days of culture the growth medium was replaced with differentiation medium for next 2 days (DMEM medium with 10% fetal bovine serum, 0.5 mM isobutylmethylxanthine - IBMX, 0.25 µM dexamethasone). After that period of time the cells were incubated in a DMEM medium containing 10% FBS and 1 µg/ml insulin until the end of 3-week differentiation process. Oil-Red-O staining was used for evaluation of adipocyte differentiation. Then adipocytes were co-cultured with graded concentrations of irisin (0, 10, 50 or 100 nM) for 3 weeks labeled as IR0, IR10, IR50 and IR100 groups, respectively. In order to induce a state of mild inflammation at day 21 cells were stimulated with LPS (100 ng/ml; Escherichia coli, serotype 0111: B4) or vehicle (medium). During incubation 3T3 L1 cells were cultured under standard condition (37°C, 5% CO2). Fresh cells were used for cytometric assessment and RNA isolation. Supernatants and cell pellets were frozen (–60°C) for future quantification of protein levels.
Cell activity and viability
Overall cell viability and activity was measured using a commercial CellTiter kit (Promega). Moreover, the percent of apoptotic and necrotic cells were quantified cytometrically using an Annexin V-PE Apoptosis Detection Kit I (BD Pharmingen). Both tests were performed according to the manufacturer's instruction using a spectrophotometer (Expert Plus, ASYS/Hitech; for CellTiter), or a FACScan cytometer (BD Biosciences; for apoptosis test).
Real-time PCR analysis of gene expression
Total RNA was purified from the 3T3 L1 adipocytes cultured in four separate repetitions for each variant of the experiment. RNeasy Plus Mini Kit with elimination of genomic DNA was used to RNA extraction. The RNA concentration and quality was assessed using NanoDrop 2000 spectrophotometer (Thermo Scientific). Reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit. Real-time PCR gene expression analysis for 7 genes (TNF-α, IL-6, adiponectin, leptin, PPARγ, NFκB, MCP-1) was performed using Applied Biosystems StepOne system and Taq Man primers. Glucose-6-phosphate dehydrogenase (GAPDH) was used for normalizing the amounts of cDNA. Gene expression changes in the presence of three different doses of irisin were calculated based on 2-ΔΔCt algorithm (35).
Western blotting assay
To the analysis of total pool of NFκB and its phosphorylated form as well as PPARγ proteins were extracted from adipocyte pellets according to the manufacturer's protocol. The protein concentration was quantified using the BCA protein assay kit. Then the samples were subjected to the SDS-PAGE gels (10 – 15%), and after electroforetical separation were transferred onto the PVDF membrane. The nonspecific binding sites were blocked with 5% dried milk in TBSS for 1 h (37°C) and the membranes were incubated overnight with primary antibodies (4°C; 1:1000), and then with secondary antibodies conjugated with horseradish peroxidase (HRP; 1:5000) for 2 hours at room temperature. After reaction with DAB, membranes were imaged and the relative protein expression was normalized to b-actin expression used as the internal reference.
Macrophage migration
To assess the effect of irisin on a release of chemotactic factors by activated adipocytes, a 48-well microchemotaxis chamber (Neuro Probe) and RAW 264.7 macrophage cell line were used. The test was performed according to the manufacturer's protocol. Briefly, the lower wells of chamber were filled with the supernatants collected from 3T3 L1 adipocytes (cultured in the presence of various irisin concentrations: 0; 10; 50 or 100 nM) 24 hours after LPS stimulation, control supernatants, fMLP as a positive control, or DMEM medium as a negative control. The lower wells of chamber were covered with polycarbonate membrane (3 µm pore size; Neuro Probe). The upper wells of chamber were filled with 50 µl of RAW 264.7 macrophages suspension (2 mln/ml) and incubated for 45 min in standard culture condition. After that, the membrane was washed in PBS and stained with Diff-Quick solution (Medion Diagnostic). The macrophages that had migrated to the lower side of membrane were counted in four microscopic fields in each well. The results are expressed as the mean numbers of migratory macrophages per well.
Determination of cytokine/chemokine release
After 24-hours incubation with or without LPS the supernatants were collected and the levels of cytokine were quantified using commercial Elisa Kit. The concentration level of TNF-α, IL-6, MCP-1, adiponectin and leptin was determined according to the manufacturer's instructions and analyzed using Expert Plus spectrophotometer (ASYS/Hitech, Austria).
Statistical analysis
Data were tested for normality of the distribution, and differences among groups were determined using the Duncan's new multiple range test 3.1. All data were expressed as means ± standard deviation (S.D.) with the level of statistical significance (P) set at 0.05.
RESULTS
The influence of irisin on the inflammatory activity of adipocytes was evaluated in LPS-activated 3T3 L1 cells cultured in the presence of three different concentrations of irisin (10 nM, 50 nM and 100 nM) in relation to the fat cells not-treated by irisin (IR0). As the control to adipocyte activation, the 3T3 L1 cells were treated with medium instead of LPS.
The effects of irisin on adipocyte viability and activity
To assess whether irisin affects the overall activity of the adipocytes as well as their viability first we performed the study using a CellTiter test. As shown in Fig. 1A, the incubation of adipocytes with irisin induced an intensification of general cell activity in a dose dependent manner. The adipocyte activity was significantly elevated in both IR50 and IR100 groups (P > 0.05) as compared to IR0 control cells. This effect was seen in both LPS-stimulated and non-stimulated adipocytes. Furthermore, to assess whether the increased activity is related to cell viability, we performed an examination of irisin impact on apoptosis and necrosis induction. As shown in Fig. 1B, the highest irisin concentration (IR100) efficiently inhibited LPS-induced apoptosis resulting in an increase of lipid droplets accumulation in these adipocytes (P > 0.05).
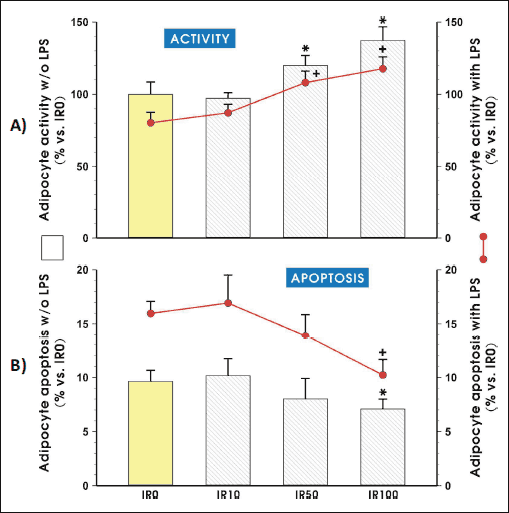 |
Fig. 1. The adipocyte activity (A) and apoptosis (B) after pretreatment with irisin. Adipocytes were differentiated from 3T3 L1 cell line as described in the Section of Materials and Methods and then cultured in the presence of 10 nM (IR10), 50 nM (IR50) or 100 nM (IR100) of irisin for 3 weeks. After additional 24 hours of LPS-stimulation (with LPS groups) or without stimulation (w/o LPS groups), CellTiter test (A) or annexin-V staining (B) were performed according to a manufacturer's instruction. Values are means ± S.D. of 4 – 5 independent experiments. Asterisk (*; with LPS groups) and dash (#; w/o LPS groups) indicate a significant change as compared with non-treated (IR0) and irisin (IR10) -treated cells at P < 0.05 by Duncan's test. |
Irisin reduces expression and secretion of pro-inflammatory cytokines
The expression and secretion of two pro-inflammatory cytokines (TNF-α and IL-6) was assessed in irisin-treated with two adipocytes compared to irisin-free culture of adipocytes by the real time PCR and cytokine protein secretion to the culture medium using enzyme-linked immunosorbent assay (ELISA).
The significant reduction of TNF-α and IL-6 mRNA expression was observed in 3T3 L1 cells treated by the highest concentrations of irisin (50 nM and 100 nM) in comparison to adipocytes treated with vehicle (saline). The concentration of TNF-α and IL-6 quantified in supernatants were also significantly reduced when adipocytes were growing in the presence of 50 nM and 100 nM concentrations of irisin (P < 0.05) (Fig. 2A and 2B).
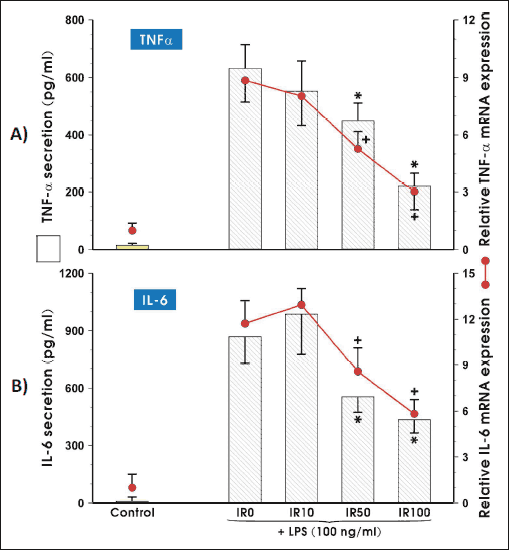 |
Fig. 2.The mRNA and protein expression of proinflammatory cytokines: TNF-α (A) and IL-6 (B). Adipocytes were differentiated from 3T3 L1 cell line as described in the Section of Materials and Methods and then co-cultured in the presence of 10 nM (IR10), 50 nM (IR50) or 100 nM (IR100) of irisin for 3 weeks. After LPS stimulation, adipocytes were harvested to evaluate the amount of mRNA transcript (6 h, real time PCR) or each cytokine concentration was measured in cell culture medium (24 hours, ELISA). Values are means ± S.D. of 4 – 5 independent experiments. Asterisk (*, protein) and cross (+, mRNA) indicate a significant change in mRNA and protein concentration as compared with non-treated (IR0) and irisin-treated cells at P < 0.05 by Duncan's test. CTR, control (non-activated adipocytes); IL-6, interleukin 6; IR, irisin; LPS, lipopoly-saccharide; TNF-α, tumor necrosis factor-α |
Effects of irisin on adiponectin and leptin mRNA expression and protein secretion
Figs. 3A and 3B shows the expression of two major adipokines: adiponectin and leptin in adipocytes cultured for 3 weeks in the presence of the three different doses of irisin. The activation of adipocytes by LPS significantly reduced adiponectin mRNA and protein expression compared to CTR but irisin significantly enhanced these expressions nearly to the level observed in vehicle - control adipocytes (Fig. 3A). In the case of leptin expression, we have observed an opposite effect because the activation of adipocytes by LPS significantly increased mRNA and protein for leptin but irisin administration markedly decreased its expression both, at mRNA as well as protein levels resulting comparable value in the presence of 100 nM irisin as in control (CTR) LPS-untreated cells (Fig. 3B).
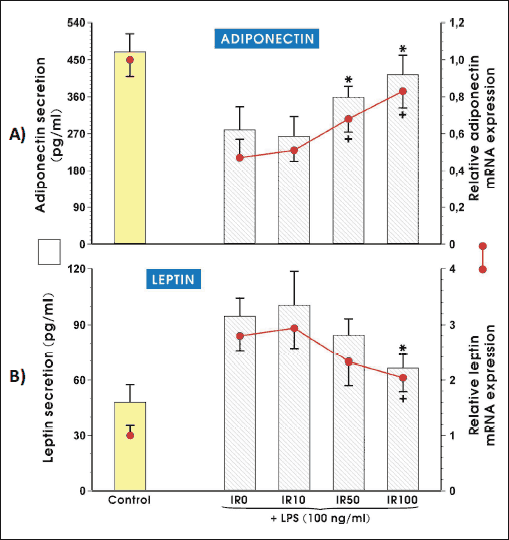 |
Fig. 3.The expression of mRNA and secreted protein levels for adiponectin (A) and leptin (B). Adipocytes were differentiated from 3T3 L1 cell line as described in the Section of Materials and Methods and then cultured for 3 weeks in the presence of 10 nM (IR10), 50 nM (IR50) or 100 nM (IR100) irisin. After LPS stimulation, adipocytes were harvested to evaluate the amount of adiponectin and leptin mRNA transcripts (6 h, real time PCR) and both adipokines concentration was measured in cell culture medium (24 h, ELISA). Values are means ± S.D. of 4 – 5 independent experiments. Asterisk (*, protein) and cross (+, mRNA) indicate a significant change in mRNA and protein concentration as compared with non-treated (IR0) and irisin (IR10) -treated cells at P < 0.05 by Duncan's test. CTR, control (non-activated adipocytes); IR, irisin; LPS, lipopolysaccharide. |
PPARγ synthesis is elevated in irisin-treated adipocytes
The co-culture of adipocytes with irisin in a presence of LPS dose-dependently increased the PPARγ mRNA and protein expression. The statistically significant differences in expression of PPARγ mRNA and protein were observed when irisin was applied in concentration of 50 nM and 100 nM (Fig. 4A). Fig. 4B shows the representative Western blot of PPARγ protein expression in response to graded concentrations of irisin in the presence of LPS. As shown in Fig. 4B the expression of PPARγ protein was increased in a concentration-dependent manner in adipocytes co-incubated with irisin.
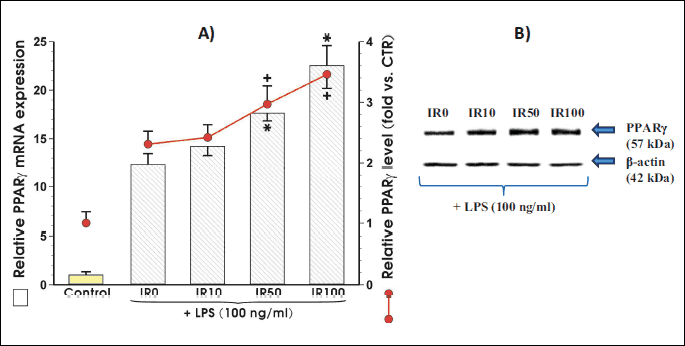
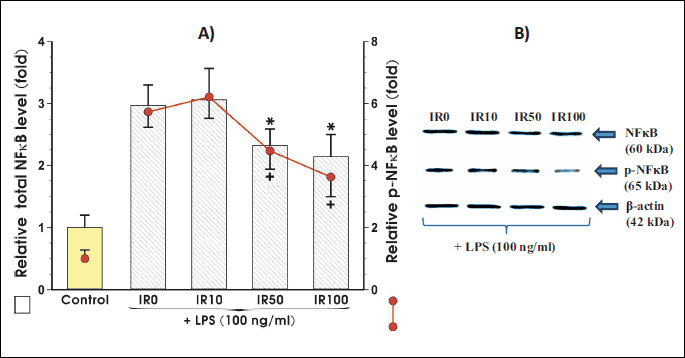
Irisin attenuates the expression and phosphorylation of NFκB in activated adipocytes
Fig. 5 shows that NFκB expression was significantly reduced when adipocytes were co-incubated with irisin in the concentrations of 50 nM and 100 nM (Fig. 5). When irisin was co-incubated with adipocytes a significant decrease in the expression of NFκB was observed at 50 nM and 10 nM of this peptide (Fig. 5A). The NFκB phosphorylation was significantly inhibited at the concentration of 100 nM of this peptide.
Macrophage chemotaxis driven by adipocytes is suppressed in the presence of irisin
In the final step of our present study we assess the irisin ability to modify macrophage migration towards chemoattractants secreted by adipocytes. We found the altered monocyte chemotactic protein 1 (MCP-1) expression on mRNA and protein levels in irisin-treated adipocytes in two the highest doses of irisin but only the highest concentration of irisin namely 100 nM significantly reduced the MCP-1 mRNA and protein expression (P < 0.05) (Fig. 6A). The irisin-caused changes in MCP-1 expression were reflected by significantly suppressed macrophage migration but only in the presence of 100 nM concentration of irisin (Fig. 6B).
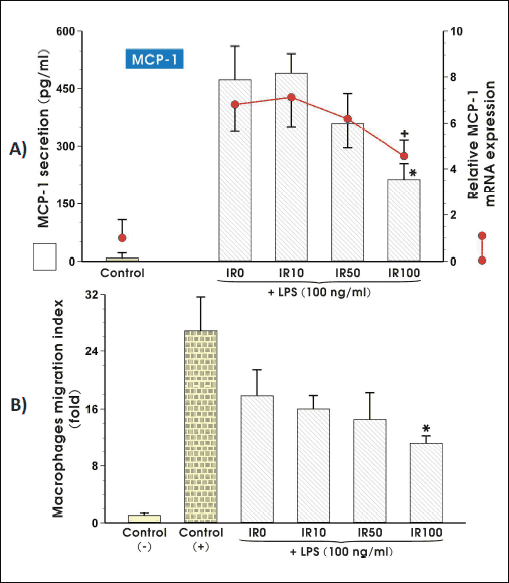 |
Fig. 6. The mRNA and protein expression of MCP-1 in adipocytes exposed to LPS (100 ng/ml) (A) and the macrophage migration ability (B), are modified by irisin action. Adipocytes were differentiated from 3T3 L1 cell line as described in the section Materials and Methods and then co-cultured in the presence of 10 nM (IR10), 50 nM (IR50) or 100 nM (IR100) irisin for 3 weeks. After LPS stimulation, adipocytes were harvested to evaluate the amount of mRNA transcript (6 h, real time PCR) or cytokine concentration was measured in cell culture medium (24 h, ELISA). Macrophages RAW 264.7 migration to conditioned medium collected from adipocytes cultured in the presence of various concentrations of irisin for 24 hours. Normal medium was used as a negative control (CTR-), fMLP was used as a positive control (CTR+). Values are means ± S.D. of 4 – 5 independent experiments. Asterisk (*, protein and migration) and cross (+, mRNA) indicate a significant change as compared with non-treated (IR0) and irisin-treated cells (IR10) at P < 0.05 by Duncan's test. CTR, control (non-activated adipocytes); IR, irisin; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein 1. |
DISCUSSION
In this study, we demonstrated that irisin exerts the marked anti-inflammatory activity in the lipopolysaccharide-activated adipocytes.
Regular physical activity has a significant role in the modulation of immune and anti-infiammation functions presumably due to myokines released from contracting skeletal muscle (3). Nowadays, irisin is considered as myokine of particular interest, released in response to exercise and muscle shivering. Irisin exerts its action on white adipose, cells causing their transformation into so called 'bright' cells - white fat cells with a phenotype similar to that of brown fat cells. Moreover, irisin has been implicated in process of induction of thermogenesis and increased energy expenditure (18). Huh et al. (36) demonstrated that, irisin induced UCP1 and consequently increased adipocyte energy expenditure, the expression of metabolic enzymes and metabolite intermediates, caused an inhibition of lipid accumulation in human adipocytes. They also observed that irisin and FNDC5 reduced preadipocyte differentiation, which can be an additional mechanism in decreasing adiposity (36). Irisin has been shown to reduce high-fat-diet induced insulin resistance through its action on WAT and increased energy expenditure (37, 38). Liu et al. (39) observed that T2D patients had lower plasma irisin level compared to non-diabetic controls. The possible therapeutic role has been suggested for irisin in human metabolic disease, obesity and other disorders to which the exercise seems to be beneficial (40, 41).
The results of recent study (42) have suggested that obesity represents a low-grade chronic inflammation in WAT manifested by hypertrophied adipocytes and increased number of adipose tissue macrophages (ATM). This inflammation state is characterized by the activation of pro-inflammatory signaling pathways and the increased pro-inflammatory cytokine production (42). Thus, adipocytes are considered since now as members of the immune system, because they were shown to produce and release of several pro-inflammatory cytokines and chemokines such as IL-6, TNF-α, and MCP-1 in addition to adipokines (43, 44). In obesity, the inflammatory molecules may originate not only from adipocytes but also from infiltrated macrophages. This proinflammatory mediators can exhibit local effect on adipose tissue and also systemic effects on other organs as well as they contribute to the insulin resistance. Macrophages' infiltration in white adipose tissue is associated with obesity causing a phenotypic switch in these cells from an anti-inflammatory M2 to a pro-inflammatory M1 state (45).
Our results show that irisin reduced expression of pro-inflammatory cytokines, TNF-α and IL-6 in LPS-activated adipocytes and this effect depend upon concentration of this peptide. The similar effect was observed in LPS-activated peritoneal macrophages (32, 33) because of irisin not only decreased the expression of TNF-α and IL-6 but also stimulated macrophage polarization from M1 into M2 response (31).
In line with these inhibitory effects of irisin on pro-inflammatory cytokines, this peptide has been shown to exert an opposite effect on adiponectin and leptin expression in LPS-treated adipocytes. While LPS-activation leads to reduced adiponectin and increased leptin expression in adipocytes, irisin was shown in our study to reverse this effect resulting in an increase in adiponectin and an apparent fall in leptin expression. Leptin, one of the first proteins shown to be secreted from adipose tissue, exhibits pro-inflammatory properties and the concentration of this adipokine is increased in obese subjects (46). Adiponectin is believed to act as the main anti-inflammatory mediator produced in and released by adipose tissue. In contrast to leptin, the expression and plasma levels of adiponectin, the anti-inflammatory adipokine, are down-regulated in obesity (46).
Recently, TNF-α has been shown to reciprocally increase the expression of TNF-α and IL-6 and inhibited the expression of adiponectin in adipocytes, these effects mediated by the activation of the cellular transcription factor NFκB (47). It is interesting to note, that in our present study, irisin exerted a suppressive effect on expression of NFκB in LPS-activated adipocytes. This observation strongly supports the notion that irisin exerts an anti-inflammatory action, potentially counteracting inflammatory cytokines including TNF-α induced activation of NFκB pathway. Our findings are consistent with previous studies showing that irisin suppressed NFκB activation in macrophages (33) and in malignant breast epithelial cells (34).
Our present study provides an evidence that MCP-1 expression was downregulated in irisin-treated adipocytes followed by the reduced macrophage migration in the presence of irisin. Adipocytes were shown to release MCP-1, chemoattractant molecule which is implicated in macrophage infiltration of WAT (48). Previous studies revealed that when overexpressing of MCP-1, the WAT leads to an accumulation of macrophages in adipose tissue, an event associated with a development of metabolic disorders (6, 49, 50). The MCP-1 protein levels were significantly higher in the obese mice than those in the lean controls and the concentration of MCP-1 in these mice far exceeded that determined in mesenteric adipose tissue of lean mice (51). This reduction in MCP-1 expression in adipocytes co-incubated with irisin observed in our study can significantly contribute to the anti-inflammatory action of irisin and supports the protective activity of this peptide released from skeletal muscle during exercise especially in obese individuals. This local anti-inflammatory activity of irisin is in keeping with recent observation that this peptide is not associated with depressiveness, anxiety and perceived stress in female obese patients ruling out the assumption of irisin being involved in mental functions and psychoendocrine pathways (52).
In conclusion, we confirmed the anti-inflammatory activity of irisin in adipocytes. We demonstrated for the first time that irisin can directly attenuate the inflammation process in cultured adipocytes, in addition to inhibition of migratory activity of macrophages the action to this myozine that has been shown before.
Acknowledgements: This study was financially supported by the research project no. K/DSC/002108 (Agnieszka I. Mazur-Bialy) and by the grant no. UMO-2013/09/B/NZ4/01566 from National Centre of Science in Poland (Tomasz Brzozowski).
Conflict of interest: None declared.
REFERENCES
- Ertek S, Cicero A. Impact of physical activity on inflammation: effects on cardiovascular disease risk and other inflammatory conditions. Arch Med Sci 2012; 8: 794-804.
- Hamer M, Sabia S, Batty GD, et al. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation 2012; 126: 928-933.
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457-465.
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012; 2: 1143-1211.
- Eagle TF, Gurm R, Goldberg CS, et al. Health status and behavior among middle-school children in a midwest community: what are the underpinnings of childhood obesity? Am Heart J 2010; 160: 1185-1189.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796-1808.
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014; 105: 141-150.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860-867.
- Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 2015; 64: 131-145.
- Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci 2015; 36: 461-470.
- Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology 2015; 96: 124-134.
- Bilski J, Jaworek J, Pokorski J, et al. Effects of time of day and the wingate test on appetite perceptions, food intake and plasma levels of adipokines. J Physiol Pharmacol 2016; 67: 667-676.
- Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985) 2007; 103: 693-699.
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011; 11: 607-615.
- Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1alpha, myokines and exercise. Bone 2015; 80: 115-125.
- Bilski J, Mazur-Bialy AI, Wierdak M, Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol 2013; 64: 143-155.
- Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463-468.
- Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 2014; 19: 302-309.
- Murawska-Cialowicz E, Wojna J, Zuwala-Jagiello J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J Physiol Pharmacol 2015; 66: 811-821.
- Castillo-Quan JI. From white to brown fat through the PGC-1alpha-dependent myokine irisin: implications for diabetes and obesity. Dis Model Mech 2012; 5: 293-295.
- Rocha-Rodrigues S, Rodriguez A, Gouveia AM, et al. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci 2016; 165: 100-108.
- Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem 2014; 289: 34129-34140.
- Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 2013; 98: E769-E778.
- Roca-Rivada A, Castelao C, Senin LL, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One 2013; 8: e60563. doi: 10.1371/journal.pone.0060563
- Albrecht E, Norheim F, Thiede B, et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep 2015; 5: 8889. doi: 10.1038/srep08889
- Jedrychowski MP, Wrann CD, Paulo JA, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 2015; 22: 734-740.
- Wang C, Wang L, Liu J, et al. Irisin modulates the association of interleukin-17A with the presence of non-proliferative diabetic retinopathy in patients with type 2 diabetes. Endocrine 2016; 53: 459-464.
- Bluher S, Panagiotou G, Petroff D, et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring) 2014; 22: 1701-1708.
- Bilski J, Mazur-Bialy AI, Brzozowski B, et al. Moderate exercise training attenuates the severity of experimental rodent colitis: the importance of crosstalk between adipose tissue and skeletal muscles. Mediators Inflamm 2015; 2015: 605071. doi: 10.1155/2015/605071
- Mazur-Bialy AI, Bilski J, Wojcik D, et al. Beneficial effect of voluntary exercise on experimental colitis in mice fed a high-fat diet: the role of irisin, adiponectin and proinflammatory biomarkers. Nutrients 2017; 9: 410. doi: 10.3390/nu9040410
- Dong J, Dong Y, Chen F, Mitch W, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (London) 2016; 40: 434-442.
- Mazur-Bialy AI. Irisin acts as a regulator of macrophages host defense. Life Sci 2017; 176: 21-25.
- Mazur-Bialy AI, Pochec E, Zarawski M. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci 2017; 18: E701. doi: 10.3390/ijms18040701
- Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer 2015; 136: E197-E202.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001; 25: 402-408.
- Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014; 38: 1538-1544.
- Sesti G, Andreozzi F, Fiorentino TV, et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol 2014; 51: 705-713.
- Sanchis-Gomar F, Perez-Quilis C. The p38-PGC-1alpha-irisin-betatrophin axis: exploring new pathways in insulin resistance. Adipocyte 2014; 3: 67-68.
- Liu JJ, Wong MD, Toy WC, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications 2013; 27: 365-369.
- Cereijo R, Giralt M, Villarroya F. Thermogenic brown and beige/brite adipogenesis in humans. Ann Med 2015; 47: 169-177.
- Chen JQ, Huang YY, Gusdon AM, Qu S. Irisin: a new molecular marker and target in metabolic disorder. Lipids Health Dis 2015; 14: 2. doi: 10.1186/1476-511X-14-2
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972-978.
- Mazur-Bialy A, Pochec E, Plytycz B. Immunomodulatory effect of riboflavin deficiency and enrichment-reversible pathological response versus silencing of inflammatory activation. J Physiol Pharmacol 2015; 66: 793-802.
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 2007; 3: 716-724.
- Jiao P, Chen Q, Shah S, et al. Obesity-466 related upregulation of monocyte chemotactic factors in adipocytes: involvement of 467 nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 2009; 58: 104-115.
- Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013; 2013: 139239. doi: 10.1155/2013/139239
- Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Arch Pharm Res 2013; 36: 208-222.
- Christiansen T, Richelsen B, Bruun J. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obesity (Lond) 2005; 29: 146-150.
- Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006; 281: 26602-26614.
- Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494-1505.
- Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006; 14: 1353-1362.
- Hofmann T, Elbelt U, Ahnis A, et al. The exercise-induced myokine irisin does not show an association with depressiveness, anxiety and perceived stress in obese women. J Physiol Pharmacol 2016; 67: 195-203.
A c c e p t e d : April 24, 2017