CHANGES IN VIABILITY OF RAT ADIPOSE-DERIVED STEM CELLS ISOLATED
FROM ABDOMINAL/PERINUCLEAR ADIPOSE TISSUE STIMULATED
WITH
PULSED ELECTROMAGNETIC FIELD
INTRODUCTION
Low-frequency electromagnetic field (LF-EMF) was shown to modulate several cellular processes, including proliferation, differentiation and viability of many cell types in an intensity- and field type-dependent manner. Due to its documented beneficial biological effects, LF-EM is commonly used in medicine for magnetotherapy, and recently also as tumor treating field (TTF) therapy, a novel antimitotic electric field-based treatment for glioblastoma (1).
Biological effects of EMF documented in living organisms, tissues and cell lines are heterogeneous and seem to depend on cell type, electromagnetic field characteristics, such as range, intensity, waveform and time of exposure. Nevertheless, many experiments demonstrated that EMF may induce changes in various metabolic pathways (2, 3). Previous studies involving variable cell types showed that EMF may interfere with nearly all cellular processes, affecting proliferation, inducing changes in signal transduction pathways, causing DNA damage, modulating function and viability of immune cells, and exerting tumoricidal/anti-tumoricidal effects. The most extensively studied of these processes was cell proliferation; electromagnetic stimulation was shown to impair cell division via activation of cell death pathways (4-13).
Apoptosis, i.e. programmed controlled cell death, plays a key role in tissue and organ development, as well as during cell turnover within mature tissues. Apoptosis is distinct from necrosis which is an uncontrolled cell death resulting from acute injury, lysis, inflammation, tissue damage or carcinogenesis (14, 15).
Stem cells can be found virtually in whole body, including bones, cartilage, fat, tendons, muscles and bone marrow. They control tissue regeneration and healing (16, 17). Adipose tissue (AT) is a heterogeneous body compartment with endocrine properties, playing complex role in maintenance of homeostasis. Aside from being energy resource, AT interferes with various metabolic pathways, as well as with the endocrine and immune system (18). Mature adipocytes represent approximately 30% of body fat. Aside from adipocytes, adipose tissue contains also multipotent stem cells, nerve fibers, small blood vessels, fibroblasts and preadipocytes at various stages of differentiation (19).
Obesity is classified as a civilization disease; its etiopathogenesis is presumed to include both genetic predisposition and influence of modified environmental factors, such as unbalanced diet with excess calories and/or too low physical activity. Obesity may lead to a number of metabolic disorders, including type 2 diabetes mellitus, cardiovascular diseases (associated with atherosclerosis) related to primary hypertension and leading to ischemic heart disease, myocardial infarction and other complications (20).
In this study, we used animal model for obesity, namely Wistar rats of various age (both pups and adults), exposed to high- (HF) or low-fat (LF) diet. ADSCs were isolated from two different compartments, subcutaneous adipose tissue in females and perinuclear fat in males, to reflect sex-specific differences in body fat distribution. ADSCs were cultured in vitro; cells from the first passage were exposed to PEMF (7 Hz, 30 mT) for 4 hours at 24-h intervals, which corresponded to a total of three exposures during a 3-day period. 24 hours after the last exposure, PEMF-induced changes in ADSC viability were studied by means of flow cytometry (FC).
The aim of this study was to analyze PEMF-induced changes in viability parameters of in vitro cultured ADSCs from female and male rats of various age.
MATERIALS AND METHODS
Animals
The studies included Wistar rats (WistarKrf: (Wi) Wu) because of the physiological parameters analysis of the particular researched groups. The animals were obtained from the central animal house at the Faculty of Pharmacy, Jagiellonian University in Cracow (breeder registration no. 0056). Upon admission to the local animal house at the Department of Pathophysiology, the animals were allowed to adapt to new living conditions for seven days (as recommended by Szarek et al. adaptation period should last 5 – 15 days). During this period, female rats with pups were housed under specific hygienic conditions: in an air-conditioned room with air temperature maintained at 21 – 25°C, 50 – 60% humidity, with 12-h day-night cycle and unlimited access to water and food. Protocol of the study was approved by the Local Bioethics Committee at the Jagiellonian University in Cracow, Poland (decision no. 84/2014).
Study groups and experimental procedures
The study included the following eight groups of rats, identified on the basis of sex, age (pups kept with their mothers and adult animals) and diet (LF and HF diet):
Groups 1 and 3 - female and male pups kept with their mothers and receiving LF diet for 21 days of the experiment;
Groups 2 and 4 - female and male pups kept with their mothers and receiving HF diet for 21 days of the experiment;
Groups 5 and 7 - adult female and male rats receiving LF diet for 21 days of the experiment;
Groups 6 and 8 - adult female and male rats receiving HF diet for 21 days of the experiment.
Animals from all experimental groups underwent the following procedures:
Procedure 1. Animal weighing. Body weight was measured twice a week using electronic scales (Scale OHAUS NAWIGATOR 2100/0.1 g)
Procedure 2. Temperature measurements. Body temperature was measured twice a week. To minimize stress and pain associated with the procedure, the measurements were taken with an infrared thermometer (Anima Vivari). The data on body weight and body temperature obtained within the framework of this project have already been published elsewhere (21).
Procedure 3. Diet. Pups and adult rats maintained on HF diet received chow with higher contents of fat and protein (32% and 22%, respectively) and less carbohydrates (40%) (VERSELE-LAGA Opti Life Adult Active) than in the LF chow (protein 25%, fat 8%, carbohydrates 67%; Labofeed B, Pasze Kcynia).
Procedure 4. Euthanasia and tissue specimen collection. On the 21st day of the experiment, all animals were euthanized by an overdose of anesthetic (Pentobarbital, Morbital, Pulawy, 200 mg per kg body weight intraperitoneally), and adipose tissue specimens, subcutaneous tissue in females and periepididymal fat in males, were harvested. The specimens were collected in sterile conditions, under a laminar flow, using sterile surgical instruments (ASHE/A, mp type steam sterilizer, 15 min at 121°C).
Isolation of adipose-derived stem cells and cell culture
Adipose tissue specimens were collected aseptically as described previously in adult BalbC mice (22). The specimens were washed with phosphate-buffered saline (PBS, Sigma-Aldrich, Germany) containing 1% penicillin/streptomycin solution (Sigma-Aldrich, Germany), homogenized and digested with me type collagenase (1 mg/mL; Gibco by Life Technologies, USA) at 37°C for 1 hour. Enzymatic activity was neutralized with a Dulbecco's modified eagle's medium (DMEM Sigma-Aldrich, Germany) containing 10% fetal bovine serum (FBS; Gibco by Life Technologies, USA) and 1% penicillin/streptomycin solution. Subsequently, the reaction cocktail obtained from disintegrated tissues was filtered (filters with 100-µm pore diameter, Fisher Scientific, USA) and centrifuged at 300 × g for 10 min to obtain a high-density cell pellet. The pellet was suspended in DMEM supplemented with 10% FBS (Gibco by Life Technologies, USA) and 1% penicillin/streptomycin solution (Sigma-Aldrich, Germany), placed in T75 flasks (Corning, Sigma-Aldrich, Germany) and incubated overnight at 37°C in a 5% CO2 incubator with 90% humidity. After the 24-h incubation, non-attached cells and non-adherent erythrocytes were washed with antibiotic-supplemented PBS. Adherent cells (ADCSs) were suspended in DMEM with 10% FBS and 1% penicillin/streptomycin solution. The medium was changed every 72 h until the cells became confluent. After reaching an 80 – 90% confluence, the cells were placed in trypsin-EDTA solution (0.25% weight/volume; Sigma-Aldrich, Germany), incubated at 37°C for 10 – 15 min, and dissociated by trituration. The trypsin reaction was blocked by addition of DMEM with 10% FBS and 1% penicillin/streptomycin solution, and the cell suspension was centrifuged at 416 × g for 10 min. ADSCs were suspended in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin solution, counted with a hemocytometer, seeded on a 96-well plate (Corning, Sigma-Aldrich, Germany) in triplicate at density of 0.25 × 106 cells/ml per sample, and cultured at 37°C at humidified atmosphere of 5% CO2.
Magnetic stimulation of adipose-derived stem cells cultures
PEMF stimulation was started after 24 hours of ADSC culture. A generator produced and kindly provided by the Institute of Electron Technology (Cracow, Poland) generated pulsed electromagnetic field with a frequency 7 Hz at a flux density of 30 mT inside the cell culture incubator. The reason behind choosing this particular PEMF frequency was its minimal heating effect and the fact that similar frequency EMF could be generated by household power devices. Furthermore, electromagnetic fields with similar parameters are used for medical purposes, e.g. magnetotherapy. A 96-well plate with ADSC culture was placed in the generator's pocket and exposed to EMF for 4 hours daily at 24-h intervals, during three consecutive days; this corresponded to a total of three PEMF exposures over the 3-day period. Control samples were placed in the same incubator but at a 35-cm distance from the generator, additionally protected from PEMF exposure with an aluminum foil shield.
Cell viability assessment by means of flow cytometric analysis
Twenty-four hours after the last exposure to PEMF, ADSCs were detached from culture plates by trypsinization, washed three times with cold PBS (Sigma-Aldrich, Germany) and then stained exactly as described in FCM manufacturer's procedure. The proportion of apoptotic cells was determined with allophycocyanin (APC) conjugated annexin V (AnV-APC, BD, Pharmingen TM, USA). Propidium iodide (PI, BD, Pharmingen TM, USA) was used as a standard flow cytometric viability probe to distinguish necrotic cells from viable ones (23). AnV-APC-positive, PI-negative cells (AnV+) were classified as early apoptotic, AnV-APC- and PI-positive cells (AnV+PI+) as late apoptotic, and AnV-APC-negative, PI-positive cells (PI+) as necrotic. For staining, ADSCs were washed twice with cold PBS and resuspended in 1 × binding buffer (BD, Pharmingen TM, USA) at a concentration of 1 × 106 cells/ml. Then, the solution (100 µl) was transferred to a 5-ml culture tube, and AnV-APC and PI were added, 5 µl each. The cells were vortexed gently and incubated in darkness at room temperature for 15 min. After adding 1 × binding buffer (400 µl), the cells were analyzed on a FACS CALIBUR flow cytometer (Becton Dickinson, San Jose, CA) using Cell-Quest software to calculate the proportion of ADSCs representing various types. Unstained cells, cells stained with AnV-APC alone (for FL-4 fluorescence) and cells stained with PI alone (detected in FL-3) were used as the controls to set up compensation and appropriate quadrants. At least 10,000 events were acquired for each sample (16).
Statistical analysis
The results were presented as means (±) their standard deviations (S.D.). Intergroup comparisons were conducted with Student's t-test. The differences were considered statistically significant at P < 0.05.
RESULTS
The study included eight groups of rats, differing in terms of age, sex and diet. Animals maintained on fat- and carbohydrate-rich diet, both pups and adults, presented with obesity and lipid disorders (21). ADSCs isolated from various anatomical regions were cultured in vitro and exposed to PEMF, and then their viability parameters were determined.
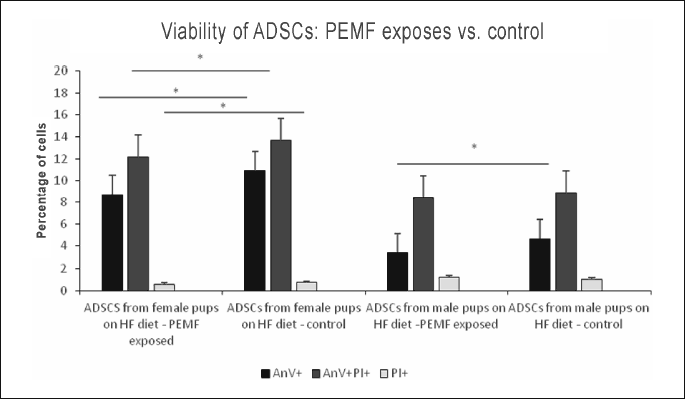
Pulsed electromagnetic field-induced changes in viability of adipose-derived stem cells from female and male pups maintained on low fat diet
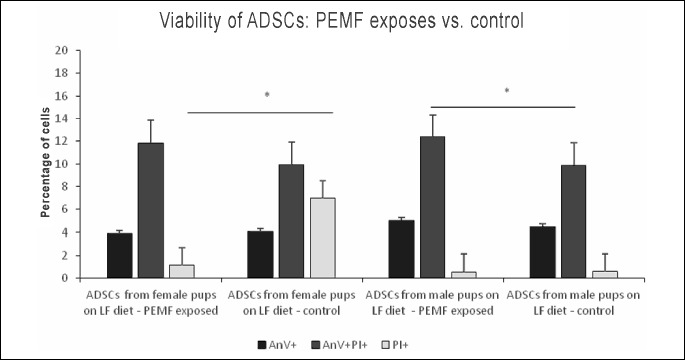
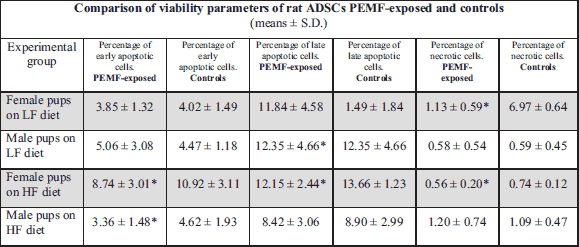
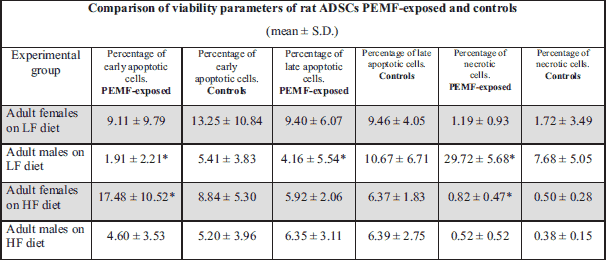
While PEMF-exposed ADSC cultures from female pups contained a larger proportion of late apoptotic (AnV+PI+) cells and a significantly lower percentage of necrotic cells (PI+) than the non-exposed control cultures, virtually no intergroup differences were found in the percentages of early apoptotic cells (AnV+). In ADSC cultures from male pups, exposure to PEMF resulted in a significant increase in the proportion of late apoptotic cells (AnV+PI+), as well as in a mild, statistically insignificant, increase in the percentage of early apoptotic cells (AnV+); virtually no changes were observed in the case of necrotic cells (PI+) (Figs. 1, 2, 3); Table 1.
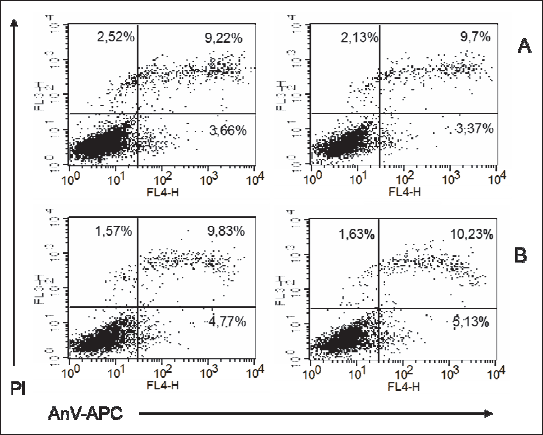 |
Fig. 2. Representatives log fluorescence dot plots of ADSCs originated from female pups grown on low fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence ( FL3) versus log annexin V-APC ( AnV-APC) fluorescence (FL4) dot plots of ADSCs. |
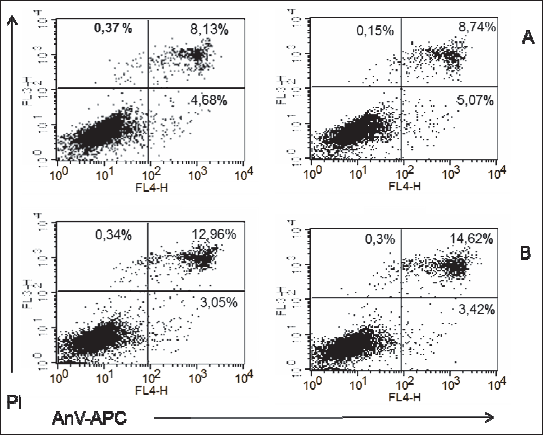 |
Fig. 3. Representatives log fluorescence dot plots of ADSCs originated from male pups grown on low fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence ( FL3) versus log annexin V-APC ( AnV-APC) fluorescence (FL4) dot plots of ADSCs. |
Pulsed electromagnetic field-induced changes in viability of adipose-derived stem cells from female and male pups maintained on high fat diet
PEMF-exposed ADSC cultures from female pups contained significantly smaller proportions of early apoptotic (AnV+), late apoptotic (AnV+PI+) and necrotic cells (PI+) than the non-exposed control cultures. Similar pattern of PEMF-induced viability changes was observed in the case of ADSC cultures from male pups, except from an increase in the percentage of necrotic cells. However, only the difference in the proportion of early apoptotic cells among ADSCs from male pups turned out to be statistically significant (Figs. 4, 5, 6); Table 1.
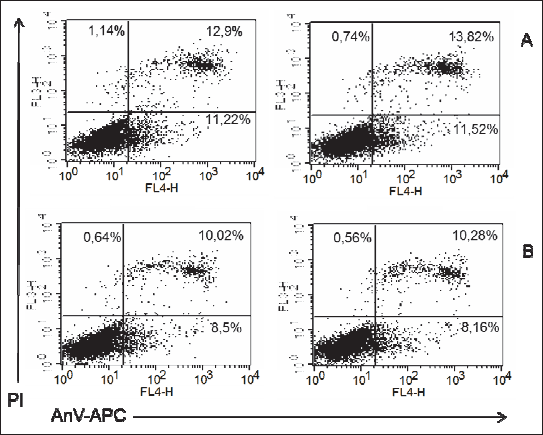 |
Fig. 5. Representatives log fluorescence dot plots of ADSCs originated from female pups grown on high fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence ( FL3) versus log annexin V-APC ( AnV-APC) fluorescence (FL4) dot plots of ADSCs. |
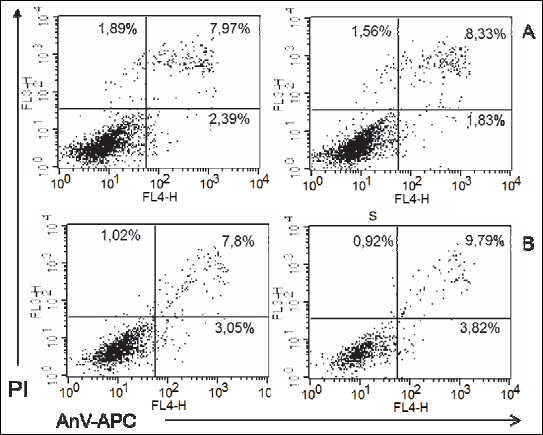 |
Fig. 6. Representatives log fluorescence dot plots of ADSCs originated from male pups grown on high fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence ( FL3) versus log annexin V-APC ( AnV-APC) fluorescence (FL4) dot plots of ADSCs. |
Pulsed electromagnetic field-induced changes in viability of adipose-derived stem cells from adult female and male rats maintained on low fat diet
PEMF exposure of ADSCs from adult female rats maintained on LF diet resulted in a statistically insignificant decrease in the proportions of early apoptotic (AnV+) and necrotic (PI+) cells. In the case of ADSC cultures from adult male rats maintained on LF diet, exposure to PEMF contributed to a significant decrease in the percentage of early and late apoptotic cells (AnV+) and (AnV+PI+), respectively), as well as to a significant increase in the proportion of necrotic cells (PI+) (Figs. 7, 8, 9); Table 2.
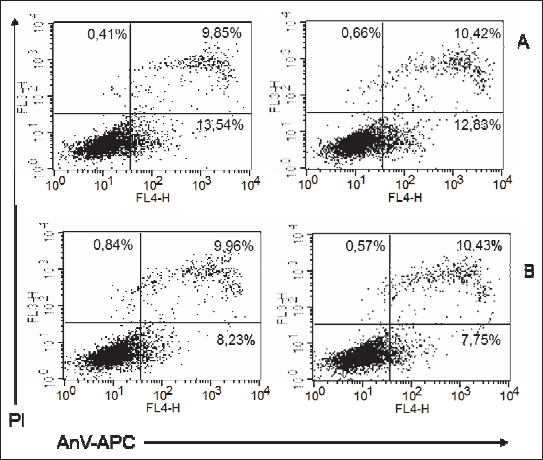 |
Fig. 8. Representatives log fluorescence dot plots of ADSCs originated from female adult grown on low fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence (FL3) vs. log annexin V-APC ( Anv-APC) fluorescence (FL4) dot plots of ADSCs. |
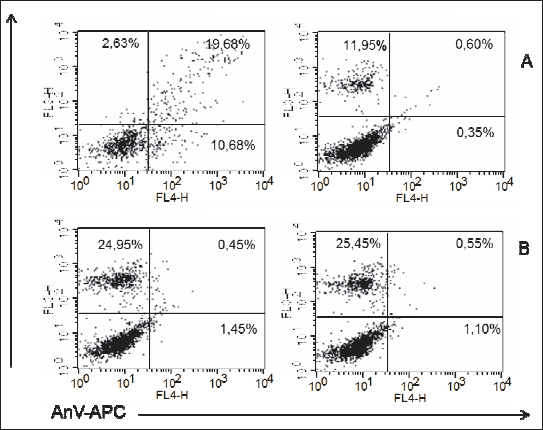 |
Fig. 9. Representatives log fluorescence dot plots of ADSCs originated from male adult grown on low fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence (FL3) versus log annexin V-APC (Anv-APC) fluorescence (FL4) dot plots of ADSCs. |
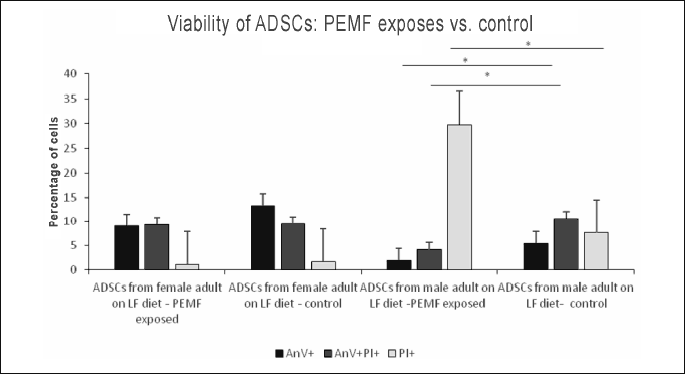
Pulsed electromagnetic field-induced changes in viability of adipose-derived stem cells from adult female and male rats maintained on high fat diet
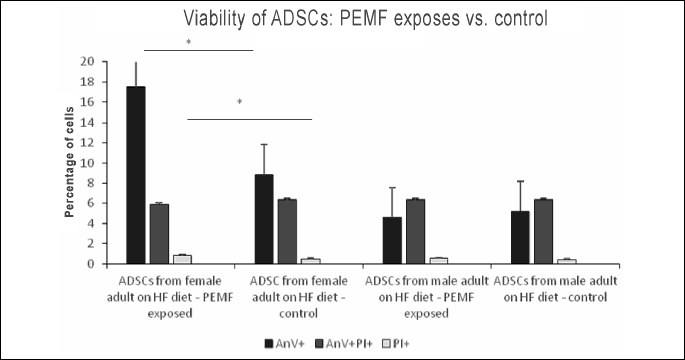
In ADSC cultures from adult female rats maintained on HF diet, exposure to PEMF was reflected by a significant increase in the proportion of early apoptotic cells (AnV+), as well as by a mild albeit still significant increase in the percentage of necrotic cells (PI+). No statistically significant PEMF-induced changes were observed in the viability parameters of ADSCs from adult male rats maintained on HF diet (Figs. 10, 11, 12); Table 2.
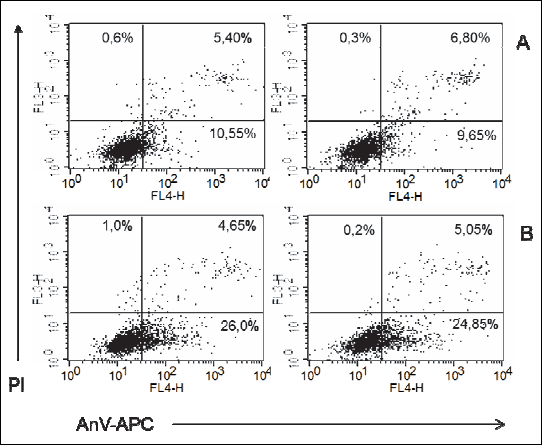 |
Fig. 11. Representatives log fluorescence dot plots of ADSCs originated from female adult grown on high fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence ( FL3) versus log annexin V-APC (AnV-APC) fluorescence (FL4) dot plots of ADSCs. |
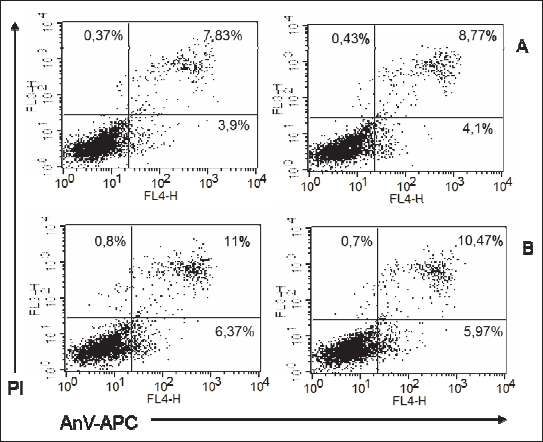 |
Fig. 12. Representatives log fluorescence dot plots of ADSCs originated from male adult grown on high fat diet in control (A) and PEMF exposed cells (B). Log propidium iodide (PI) fluorescence (FL3) versus log annexin V-APC (AnV-APC) fluorescence (FL4) dot plots of ADSCs. |
DISCUSSION
Proportion of apoptotic and necrotic cells among adipose-derived stem cells from female and male pups maintained on low fat and high fat diet
To the best of our knowledge, none of the previously published experiments analyzed the issue of obesity starting from in vitro ADSC cultures and changes in their viability in response to an external stimulus, such as exposure to electromagnetic field. Typical frequencies of magnetic fields used for medical purposes range between 10 Hz and 20 Hz, and never exceed 100 Hz (24). In our present study, exposure to PEMF (frequency 7 Hz, magnetic induction 30 mT, 3 daily sessions, each lasting 4 hours) contributed to sex-specific changes in the viability of in vitro cultured ADSCs. Whereas exposure to PEMF seemed to improve the viability of ADSCs from female pups maintained on LF diet, as shown by a decrease in the percentage of necrotic cells, an opposite effect, namely an increase in the proportion of late apoptotic cells, was observed in cultured cells from male pups. Meng et al. (25) investigated effects of high-intensity pulsed electromagnetic field (HI-PEMF, 0.1 Hz, 0.5 – 10 T, five exposures) on growing neural stem cells from neonatal rats. The treatment resulted in an increase in proliferative activity, particularly evident after exposure to HI-PEMF with magnetic intensity of 4.0 T. However, no statistically significant differences were found in the viability of HI-PEMF-exposed and non-exposed neural stem cells (25). Perhaps, discrepancies between the abovementioned findings and our hereby presented results reflected different parameters of electromagnetic fields. In another published study, the effects PEMF exposure on the activity and viability of neonatal rat osteoblast-like cells were similar to those documented in our present experiment. However, the authors of this study used PEMF with markedly lower magnetic induction values, 0.06 mT to 0.2 mT. An increase in cell number and proliferative activity was observed solely after exposure to PEMF with 0.2-mT value. The viability of cells exposed to 0.06-mT PEMF was lower than in the case of control cells, and even more pronounced intergroup differences were observed following exposure to 0.2-mT PEMF (26).
Stem cell-based therapies can be used to treat many diseases, including several types of cancer, musculoskeletal, cardiovascular, metabolic, autoimmune diseases as well as in the treatment of wounds, ulcers, and burns. However, before clinical applications of stem cells, it is essential to isolate and multiply them in vitro to obtain a sufficient quantity.
Long term cell culture studies investigating changes in the biological and morphological properties of in vitro expanded human adipose tissue-derived stem cells (hADSCs) over 30 passages, showed that long-term expansion leads to significant changes in morphology and affects proliferation kinetics, but hADSCs maintained a prototypical immunophenotype, normal cell cycle, apoptosis regulator function, and possessed a low level of telomerase activity during later passages, what stays in agreement with findings of our studies (27)
Irrespective of sex, maintenance of rat pups on HF diet resulted in excess weight gain and development of lipid disorders (21). In the case of ADSCs from female pups maintained on HF diet, stimulation with low-frequency PEMF (7 Hz, 30 mT) contributed to a decrease in the proportion of early apoptotic, late apoptotic and necrotic cells; in contrast, only a decrease in the percentage of early apoptotic cells was observed in male pups. Quite opposite findings were reported by other authors, according to whom exposure to high-frequency electromagnetic field (900 Hz) enhanced apoptosis in differently localized cells obtained from rat pups. Specifically, Odaci et al. analyzed the effects of 60-min in utero exposure to high-frequency electromagnetic field (HF-EMF, 900 Hz) on brain granule cells. They showed that treatment with EMF contributed to a decrease in the proportion of viable cells, inducing apoptosis thereof (28). Similar effects were also reported by Hanci et al., according to whom exposure to EMF enhanced apoptosis of rat testicular cells (29). The observation that exposure to electromagnetic field exerts sex-specific effects on viability of cells from rat adipose tissue seems to be the principal finding of our study. Furthermore, the hereby presented results imply that the effects of PEMF exposure on viability of ADSCs from adipose tissue of obese pups are different from those observed in normal-weight rats.
Proportion of apoptotic and necrotic cells among adipose-derived stem cells from adult female and male rats maintained on low fat and high fat diet
In this study, exposure to PEMF was reflected by a decrease in the proportion of early and late apoptotic ADSCs from male adult rats kept on LF diet, as well as by a concomitant increase in the percentage of necrotic cells. Our findings are consistent with the results published by Xie W. (30), according to whom 2-week PEMF therapy reduced apoptosis in rabbit chondrocytes. Recently, Lou F et al. reported the effects of PEMF on mesenchymal cells. According to these authors, PEMF induces differentiation of human mesenchymal stem cells (MSCs) (31), widely used in medicine and tissue engineering. However, in another study, exposure to low-frequency EMF (75 ± 2 Hz, 2 ± 0.2 mT, duration 3 – 40 min) exerted no effects on proliferation and differentiation of subcutaneous fat cells (ASCs) and bone marrow stem cells (BM-MSCs), except from an increase in the proliferation rate of the latter (32). Similar findings were also reported by Lu et al., who analyzed BM-MSCs from adult male and female rats; exposure to PEMF (20 Hz, 2 mT, 6 hours per day for 12 days) promoted osteogenesis but not adipogenesis (33). These findings imply that PEMF exposure exerts an unfavorable effect on AT formation.
In our study, exposure to PEMF altered the proportion of apoptotic and necrotic cells among ADSCs from adult female rats maintained on HF diet. The unfavorable effects of PEMF on viability of ADSCs from obese female rats were particularly evident, as shown by both a substantial increase in the proportion of early apoptotic cells and somehow less spectacular increase in the percentage of necrotic cells. Surprisingly, similar effects were not observed in ADSC cultures from male rats maintained on high fat diet.
Associations resulting from high fat diet during pregnancy in rats and adipokines level in obese humans
There are research data related to our findings in term of used diet in rats and its influence on hormones release, which show that, feeding a HF diet to dams during pregnancy and lactation disturbs plasma adiponectin levels and protein expression, both in female and male offspring; it is lowered adiponectin secretion and protein expression in the female whereas in male it is increased. Observed consequence is disruption of steroid secretion in offspring, towards testosterone and in females, and oestradiol in males (34).
Obesity in term of pathogenesis represents disease with participation of several adipokines such as leptin, adiponectin, resistin, tumor necrosis factor-α (TNF-α) support hepatic and peripheral carbohydrate homeostasis, insulin sensitivity, lipid metabolism, energy expenditure, hematopoiesis, wound healing, fertility and vascular hemodynamics in the pathomechanism.
As it regards obese patients with high BMI (BMI ≥ 40 kg/m2) the study has shown that adiponectin has a positive effect on platelets activation through a possible reduction in sP-selectin and an elevated sE-selectin what revealed perspective about the endothelium stimulation, a higher risk of endothelial damage in morbidly obese patients.
The higher leptin level, leptin-to-adiponectin ratio and simultaneously lower concentration of leptin receptor were associated with leptin resistance, possible future risk of insulin resistance and development of diabetes type 2 in humans (35).
Our observation that PEMF induces apoptosis in cultured ADSCs from obese rats (especially in the cells from subcutaneous adipose tissue of females maintained on HF diet), implies that low-frequency electromagnetic field may be considered as an option of non-invasive anti-obesity treatment, free from adverse effects inherent to pharmacotherapy, e.g. drug interactions. However, this attractive hypothesis needs to be verified in future studies; specifically, future research should focus on the influence of PEMF on the differentiation of ADSCs to adipocytes.
Acknowledgements: The study was conducted at the Department of Pathophysiology, Jagiellonian University Medical College, and supported from the grant no. K/ZDS/ 006681 from the Jagiellonian University Medical College in Cracow, Poland.
Conflict of interest: None declared.
REFERENCES
- Gutin PH, Wong ET. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book 2012; 126-131. doi: 10.14694/EdBook_AM.2012.32.126
- World Health Organization, International Agency for Research on Cancer. Non-Ionizing Radiation, Part 1, Static and 4 Extremely Low Frequency (ELF) Electric and Magnetic Fields. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, France 2005.
- Kaszuba-Zwoinska J, Chorobik P, Juszczak K, Zaraska W, Thor PJ. Pulsed electromagnetic field affects intrinsic and endoplasmatic reticulum apoptosis induction pathways in MonoMac6 cell line culture. J Physiol Pharmacol 2012; 63: 537-545.
- Wang X, Zhou A, Liu M, et al. Effects of ELF capacitively coupled weak electric fields on metabolism of 6B1 cells. Bioelectrochem Bioenerg 1999; 61: 201-205.
- Aldinucci C, Garcia JB, Palmi M, et al. The effect of strong static magnetic field on lymphocytes. Bioelectromagnetics 2003; 24: 109-117.
- Wetzel BJ, Nindl G, Vesper DN, Swez JA, Jasti AC, Johnson MT. Electromagnetic field effects: changes in protein phosphorylation in the Jurkat E6.1 cell line. Biomed Sci Instrum 2001; 37: 203-208.
- Gluck B, Guntzschel V, Berg H. Inhibition of proliferation of human lymphoma cells U937 by a 50 Hz electromagnetic field. Cell Mol Biol (Noisy-le-grand) 2001; 47. Online Pub:OL115-7.
- Arafa HM, Abd-Allah AR, El-Mahdy MA, Ramadan LA, Hamada FM. Immunomodulatory effects of L-carnitine and q10 in mouse spleen exposed to low-frequency high intensity magnetic field. Toxicology 2003; 187: 171-181.
- Johnson MT, Vanscoy-Cornett A, Vesper DN, et al. Electromagnetic fields used clinically to improve bone healing also impact lymphocyte proliferation in vitro. Biomed Sci Instrum 2001; 37: 215-220.
- Onodera H, Jin Z, Chida S, Suzuki Y, Tago H, Itoyama Y. Effects of 10-T static magnetic field on human peripheral blood immune cells. Radiat Res 2003; 159: 775-779.
- Jankowska A, Wrzesinski M, Laubitz D, et al. Intestinal MMC-related electric fields and pancreatic juice control the adhesion of Gram-positive and Gram-negative bacteria to the gut epithelium - in vitro study. J Physiol Pharmacol 2008; 59: 795-810.
- Ahlbom A, Feychting M, Koskenvuo M, et al. Electromagnetic fields and childhood cancer. Lancet 1993; 342: 1295-1296.
- Taubes G. EMF-cancer links: yes, no, and may be. Science 1993; 262: 649.
- Narita K, Hanakawa K, Kasahara T, Hisamitsu T, Asano K. Induction of apoptotic cell death in human leukemic cel line, HL-60, by extremely low frequency electric magnetic fields: analysis of the possible mechanisms in vitro. in vivo 1997; 11: 329-335.
- Cruchten SV, Broeck WV. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 2002; 31: 214-223.
- Maziarz A, Kocan B, Bester M, et al. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther 2016; 7: 54. doi: 10.1186/s13287-016-0312-5
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143-147.
- Booth A, Magnuson A, Fouts J, Foster MT. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig 2016; 26: 25-42.
- Mammi C, Calanchini M, Antelmi A, et al. Androgens and adipose tissue in males: a complex and reciprocal interplay. Int J Endocrinol 2012, 2012: 789653. doi: 10.1155/2012/789653
- Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010; 18 : 1283-1288.
- Baranowska A, Skowron B, Ciesielczyk K, Domagala J, Thor PJ. Experimental gender related obesity effect of diet. Folia Medica Cracoviensia 2016; 56: 49-60.
- Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 2002; 294: 371-379.
- Smolewski P, Darzynkiewicz Z. Contemporary methods of apoptosis testing. [in Polish] Acta Hematologica Polonica 2003; 34: 35-47.
- Ciesla A, Kraszewski W, Skowron M, Syrek P. New concept of magnetic field-inducing winding for magnetotherapy. [in Polish]. Agrolaser 2006; 248: 213-228.
- Meng D, Xu T, Guo F, Yin W, Peng T. The effects of high-intensity pulsed electromagnetic field on proliferation and differentiation of neural stem cells of neonatal rats in vitro. J Huazhong Univ Sci Technolog Med Sci 2009; 29: 732-726.
- Yusuf E, Kivanc A, Buket A, et al. Low-level laser therapy vs. pulsed electromagnetic field on neonatal rat calvarial osteoblast-like cells. Lasers Med Sci 2013; 28: 901-909.
- Danisovic L, Oravcova L, Krajciova L, et al. Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem cells. J Physiol Pharmacol 2017; 68:149-158.
- Odacia E, Basb O, Kaplanc S. Effects of prenatal exposure to a 900 MHz electromagnetic field ( EMF) on the dentate gyrus of rats: a stereological and histopathological study. Brain Res 2008; 1238: 224-229.
- Hanci H, Odaci E, Kaya H, et al. The effect of prenatal exposure to 900-MHz electromagnetic field on the 21-old-day rat testicle. Reprod Toxicol 2013; 42: 203-209.
- Xie W, Zhou J, Luo QL, Liu HF, He CQ. Pulsed electromagnetic field therapy inhibits chondrocyte apoptosis in rabbits with osteoarthritis. [in Chinese] Sichuan Da Xue Xue Bao Yi Xue Ban 2014; 45: 107-110.
- Luo F, Hou T, Zhang Z, Xie Z, Wu X, Xu J. Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics 2012; 35: 526-531.
- Ceccarelli G, Bloise N, Mantelli M, Gastaldi G, Fassina L, Cusella MG. A comparative analysis of the in vitro effects of pulsed electromagnetic field treatment on osteogenic differentiation of two different mesenchymal cell lineages. BioRes Open Access 2013; 2: 283-294.
- Lu T, Huang YX, Zhang C, Chai MX, Zhang J. Effect of pulsed electromagnetic field therapy on the osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Genet Mol Res 2015; 14: 11535-11542.
- Gregoraszczuk E, Slupecka M, Wolinski J, et al. Maternal high-fat diet during pregnancy and lactation had gender difference effect on adiponectin in rat offspring. J Physiol Pharmacol 2016; 67: 543-553.
- Iwan-Zietek I, Ruszkowska-Ciastek B, Michalska M, et al. Association of adiponectin and leptin-to-adiponectin ratio with the function of platelets in morbidly obese patients. J Physiol Pharmacol 2016; 67: 555-561.
A c c e p t e d : April 24, 2017