THE SHORT CHAIN FATTY ACIDS AND LIPOPOLYSACCHARIDES STATUS IN SPRAGUE-DAWLEY RATS FED WITH HIGH-FAT AND HIGH-CHOLESTEROL DIET
INTRODUCTION
Short chain fatty acids (SCFA) are volatile fatty acids produced by anaerobic microbiota during the fermentation of non-digestible carbohydrates and proteins in the large intestine (1). 95% of all produced SCFA are linear-chain fatty acids. The main representatives of this group are the following acids: acetate (C2:0), propionate (C3:0), and butyrate (C4:0). Branched-chain fatty acids produced from dietary proteins which are unabsorbed in the small intestine represent only 5% of total SCFA production (2). SCFA are produced by colonic microbiota through several metabolic pathways. Therefore, SCFA levels may be considered as a biomarker of a health. The proper level and proportion of SCFA has been linked to lower risk of inflammatory diseases, diabetes, and cardiovascular disease and liver steatosis (3).
Diet significantly impacts gut microbiota composition and affects different SCFA production which has been confirmed by both epidemiological and interventional studies (4). One of the main dietary and health-promoting factors connected with changes in the SCFA profile is fiber. The levels of SCFA are strongly associated with the quantity of consumed vegetables, fruits, and legumes consumed, irrespective of the type of diet normally eaten. Incorrect nutrition may cause numerous negative changes in the composition of the microbiota and bacterial activity. Recently, the evidence has mount that western-style diet, rich in carbohydrates and saturated fats, has a big impact on human gut microbiome (5). It has been observed that high fat diet (HFD) induced the decrease of Bacteroidetes and promoted the growth of Protebacteria (6, 7). Similar observation was reported during HFD and high-sucrose feedings (8). There is strong evidence that HFD and high-sugar diet cause alterations of gut microbiota (refered also as dysbiosis) despite the differences in host genotype. Carmody et al. examined around 200 mice strains to determine whether dysbiosis is primarily caused by host genotypes or dietary factors (9).
The interactions of SCFA with their specific receptors have a big impact on host energy homeostasis regulation. Metabolic syndrome, obesity or type 2 diabetes are metabolic disorders characterized by disrupted dynamics of energy homeostasis, which depends on the balanced dietary intakes and expenditure (10). The exposure to intestinal bacterial products, such as specific SCFA, has also been implicated in the pathogenesis of obesity and related to metabolic conditions (11). There is still not enough information about bacterial activity during long-lasting Western-style diet, which is very popular in many countries.
The aim of our study was to examine how a fat-rich and cholesterol-rich diet, which consumption has been linked to variety of metabolic disorders, affects the SCFA profile and lipopolysaccharide (LPS) concentration.
MATERIALS AND METHODS
Animals and the high-fat high-cholesterol diet
The experiment was carried out on male, 8-weeks-old Sprague-Dawley rats. The rats were randomly assigned to study arms and separated into plastic cages (3 rats per cage). The rats were kept in 12 hour light/darkness cycles in rooms with heating and temperature control, and they had ad libitum access to food and water. The study group (n = 30, 5 groups - 6 rats each) received high-fat and high cholesterol diet (HFHCh) previously described by Xu et al. (12). In order to maintain the same level of fiber, vitamins, and minerals as the control group, the diet consisted of 88 g of standard food (Rodent Lab Chow, PURINA), 10 g of lard oil, 2 g of cholesterol, and additional micro and makroelements (22.5% of protein, 13.78% of fat, 39% of carbohydrates 3.5% of fiber and 2% of cholesterol in dry mass). The control group (n = 30, 5 groups - 6 rats each) received standard food for laboratory rats (Rodent Lab Chow, PURINA) which contain: 25.6% of protein, 4.3% of fat, 44.3% carbohydrates, 3.5% of fiber in dry mass. The diet in the control and the study groups included 3.5% of fiber obtained from oily wheat and oily oats. The rats from study and control groups were sacrificed after 4, 8, 12, 16 and 20 weeks of diet exposure. At each time point, 12 animals were sacrificed - 6 from the control group and 6 from the study group. The animals were sacrificed by injection of ketamine intraperitoneally. Subsequently, blood was collected from the heart, and the animals were bled via cardiac puncture. Procedures involving animals were carried out in strict accordance with international standards of animal care guidelines and every effort was made to minimize suffering and the number of animals used.
Experiments were approved by the Local Ethical Committee on Animal Testing in Poznan, Poland (approval No 76/2016, 16.12.2016).
Fecal sample collection
The feces were collected at the time rats’ defecation from the lower part of the large intestine. The samples were immediately protected in liquid nitrogen and stored at –80°C.
Short chain fatty acid analysis
1. The isolation of short chain fatty acids
Fecal sample 0.5 g was suspended in 5 ml of water and homogenized for 5 min. Subsequently, the pH in suspension was adjusted to 2 – 3 by adding 5M HCl. The samples were shaken for 10 min and centrifuged for 20 min at 5.000 rpm, and next transferred to a chromatographic vial followed by analysis in GC-FID (13).
2. Chromatographic analysis
The analysis of SCFA in feces included the following fatty acids: acetic acid (C 2:0), propionic acid (C 3:0), isobutyric acid (C 4:0 i), butyric acid (C 4:0 n), isovaleric acid (C 5:0 i) valeric acid (C 5:0 n), isocaproic acid (C 6:0 i), caproic acid (C 6:0 n) heptanoic acid (C 7:0). Chromatographic analysis was carried out using the Agilent Technologies 1260 A GC system with a flame ionization detector (FID). Fused-silica capillary column with a free fatty acid phase (DB-FFAP, 30 m × 0.53 mm × 0.5 um) was used. Hydrogen was supplied as the carrier gas at the flow rate of 14.4 ml/min. The initial temperature was 100°C. It was maintained for 0.5 min, then raised to 180°C at 8°C/min and was held for 1 min. Next, the temperature was increased to 200°C at 20°C/min, and finally was held at 200°C for 5 min. The injection volume was 1 µl and the run time for each analysis was 17.5 min.
Lipopolysaccharides measurements
In rat serum, lipopolysaccharide concentration was measured by means of the double antibody sandwich technique. (Rat Lipopolysaccharides ELISA Kit, MyBioSource, Cat No MBS268498).
Statistical analysis
The statistical analysis was performed using the Statistica 12.0 Software. Shapiro-Wilk test was used to inspect the normal distribution. Since the distribution did not deviate from the norm, parametric tests were used. The results are presented as mean values and standard deviation (SD). In order to check the differences between the studied parameters the student test (t.test) was used both for the paired and unpaired data. In order to estimate the correlation, the Pearson’s correlation test was used. The values of P < 0.05 were considered as statistically important. In reference to the results which were not statistically significant, the abbreviation NS (not significant) was used.
RESULTS
The differences between the concentration of short chain fatty acids
There were no statically significant differences in the concentration of SCFA between HFHCh and the control group. Moreover, we did not see any significant differences in the concentration of SCFA between the sub-groups. The amounts of SCFA are shown in Table 1.
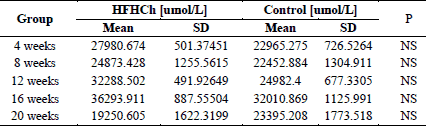
The differences between the content of particular fatty acids
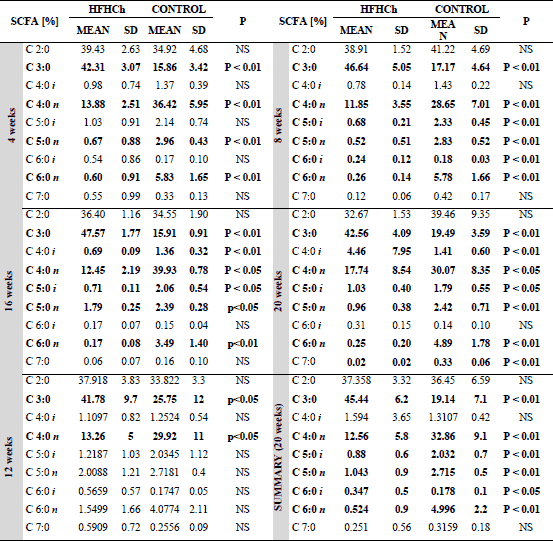
The exposure to high-fat and high-cholesterol diet was associated with significant changes in SCFA level. Relative to the control, each HFHCh subgroup revealed a statistically significant decrease in butanoic acid and an increase in propionic acid level. Most subgroups also showed reduced amounts of pentanoic acid and hexanoic acid. These results concerned both branched and linear acid forms. The levels of acetic acid were at a similar level in the control and the study groups. However, there were no significant changes between particular subgroups in the study group. The same observation was for the control group. The significant differences between the HFHCh and the control groups are presented in Table 2.
The ratio of acetic, propionic, and butyric acid
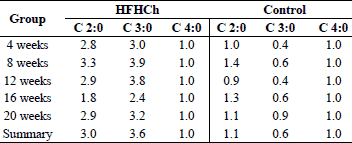
High fat and high cholesterol diet changed the ratio of produced fatty acids. The main short chain fatty acid produced in the control group was acetic acid, but the amount of butyric acid was at a similar level. HFHCh diet increased propionic acid level and changed propionic acid/acetic acid ratio as well as propionic acid/butyric acid ratio (Table 3).
The differences between lipopolysaccharides concentration
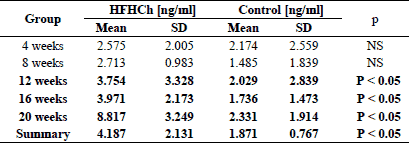
Exposure to a high-fat and high-cholesterol diet was associated with significant differences in LPS concentration (Table 4). After 12 weeks of HFD exposure, LPS concentration was significantly higher compared to control groups (Table 4). A significant difference between particular subgroups in the study group was also identified. LPS concentrations in the second part of the study (16 weeks and 20 weeks) were significantly (P < 0.05) higher than in the first part of the experiment (4 weeks and 8 weeks). There were significant differences between particular subgroups in the study group during the whole experiment.
The correlation between short chain fatty acids and lipopolysaccharides
The relationship between LPS concentration and the level of particular fatty acids showed a statistically significant correlation (P < 0.05) with the following fatty acids: propionate (R = 0.38), butyrate (R = (–)0.35), caproate acid (R = (–)0.36).
DISCUSSSION
The gut microbiome of healthy individuals is different in comparison to individuals with obesity and type 2 diabetes mellitus. These observations suggest a possible link between the composition and activity of gut microbiota and the pathology of metabolic disorders (14-16). The most important phyla which have the biggest impact on the pathophysiology of metabolic disorders are: Bacteroidetes (Gram negative), Actinobacteria (Gram positive) and Firmicutes (Gram positive). Other phyla also contribute to the pathogenesis of obesity, but their role seems to be less significant (17).
Bacterial fermentation of nondigestible carbohydrates results in the formation of SCFA (1). It has been estimated that acetate and propionate are mainly produced by the phylum Bacteroidetes, whereas butyrate is the main product of the phylum Firmicutes (17). The imbalance between SCFA-producing bacteria are linked to metabolic disorders, obesity and nonalcoholic steatohepatitis (18). In our study high fat and high cholesterol diet changed the SCFA production. The composition of gut microbiota varies between each individuals and undergoes constant changes related to lifestyle, medications (especially antibiotics) and illnesses. One of the most important factors that determines microbiota composition and activity is the diet (6, 7).
The amount and type of fiber in the diet was the same in each group. The total amount of SCFA between the test group and the control group also remained at a similar level. Based on our observations, we can assume that the HFHCh diet affects intestinal milieu and growth of selected bacteria. We noticed changes in propionate and butyrate production but not the level of acetate. Acetate is produced by many types of bacteria while propionate and butyrate are produced by more limited phyla. It seems that the similar level of acetate is determined by the same amount of fiber despite the difference in the composition of microbiota.
The dominant butyrate producers are Lachnospiraceae and Faecalibaxterium prausnitzii, but large screening of bacterial genomes identified many other species that can produce butyrate, with no specification in families. Propionate is mainly produced by Bacteroides, Negativivutes and some Clostridium species (19). Regular activity of appropriate microbiota should produce the following order of SCFA: acetate > propionate > butyrate, with ratio ranging from 3:1:1 to 10:2:1 (20). The dominant short chain acid in every study sub-group was priopionic acid instead of acetic acid (which is dominant in the control sub-groups). Significant decrease in butyrate production was observed. We noticed the differences in SCFA profiles associated with significant increase in LPS concentration. Relative to the control, the elevated concentration was observed after 12 weeks of dietary regime. Moreover the LPS secretion was positively correlated with propionate and negatively with butyrate. The summary of our findings is graphically represented in Fig. 1.
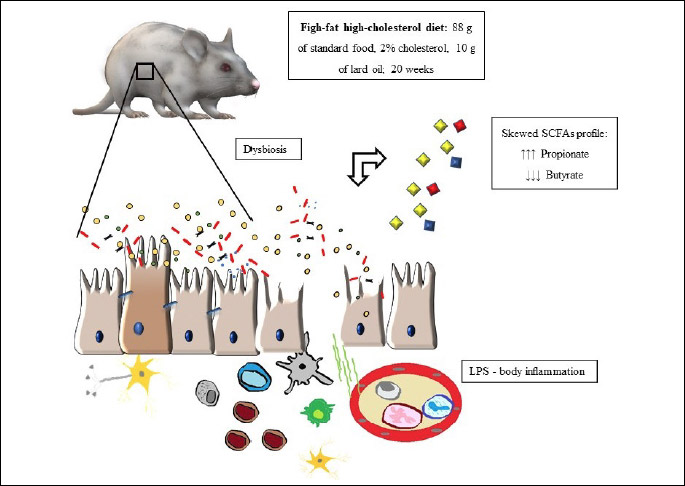
Our study showed that the HFHCh diet significantly decreased the amount of butyric acid, which could potentially affect intestinal metabolism and increase the rate of inflammation. SCFA, especially butyric acid, play an important role in maintaining proper condition of the colonic epithelium. Butyric acid is the most important SCFA, which is utilized by colonocytes as an energy substrate. The lower level of butyrate is linked to severe metabolic dysfunctions. Butyrate shows a dual-positive mechanism of action, often referred to as ‘the butyrate paradox’. It stimulates proliferation in healthy colonocytes, but also promotes apoptosis in transformed cells (21). Butyrate plays a key role in the regulation of epithelial barrier and tight junction proteins (TJP) integrity. TJP are a group of integral membrane proteins involved in cell-to-cell contacts, barrier function, and they are responsible for the intracellular pathway between the lumen and hepatic molecular system. The imbalance of barrier function can lead to increased permeability which is associated with the translocation of bacteria and their cell wall components. Cell wall products of luminal commensal microbiota that cross the gastrointestinal barrier can induce endotoxemia and lipopolysaccharide (LPS) translocation. The translocation can trigger an inflammatory cascade that has been linked to insulin resistance, obesity and liver steatosis (22). The increased release of bacterial lipopolysaccharide (LPS) affects the activation of toll-like receptor 4 (TLR4) mediated proinflammatory pathways in macrophages, Kupffer cells, and monocytes. Induced immune cells can activate several signaling pathways and promote the production of proinflammatory cytokines, such as TNF-α and IL-6 (23). Butyric acid plays a major role in the regulation of TJP and has been shown to maintain proper intestinal barrier functioning through the increased production of claudin-1, zonula occludens-1 (ZO-1), and occludin proteins which are critical components of the tight junction assembly (24). It has been estimated that butyrate can reverse the improper expression of ZO-1 and decrease LPS dislocation, leading to the inhibition of immune cells activation and cytokine production (25). Our study showed that chronic decrease in butyrate was associated with the increase in LPS secretion. The motion that these changes are result of the imbalance of barrier function and exacerbation of inflammatory cascade activation required validation in further studies. The current study showed that significant differences in bacterial SCFA production appeared in a relatively short period of time (4 weeks). However, the significant increase in the secretion of LPS was noticed after 12 weeks of HFHCh exposure. Our observation in rats differs from that in humans. Pendtala et al. reported 71% increase in plasma level of ebdotoxin activity afer 1 month of placing healthy individuals on ‘Western style’ diet (26). It is likely, that other environmental factors induce changes in gut barrier integrity. The exposure to a pathological factor in rats, such as HFHCh, need to last longer to evoke similar effect. This hypothesis seems to be supported by the fact that LPS secretion showed a significant positive correlation (R = 0.38) with propionate and a negative correlation with butyrate (R = (–)0.35).
SCFA seem to play an important role in hepatic glucose homeostasis regulation which has been well described in animal model. However, the role of SCFA has not been fully evaluated in humans. Acetic, propionic, and butyric acids take part in lipid and glucose homeostasis (27). It has been estimated that a part of SCFA is used as a source of energy and that it can provide nearly 10% of daily caloric requirements. Recent studies have shown that 62% of infused propionate was used as gluconeogenic substrate (28). However, majority of studies on propionate as a gluconeogenic substrate were conducted before the intestine was described as a gluconeogenic organ.
The current data emerge that the intestine is able to efficiently convert propionate to glucose (before it reaches the liver) and the conversion guarantees significant amount of monosacharide (27). This promotes proper energy homeostasis, better glucose control, the decrease of hepatic glucose production, lowered adiposity and insulin resistance (27).
The availability of propionate for the gluconeogenesis pathways is dependent on butyric acid. Butyrate, not propionate, directly activates intestinal gluconeogenesis gene expression in enterocytes (27, 29). The increased level of propionate and decreased level of butyrate caused by the HFHCh diet can affect the imbalance of the intestinal gluconeogenesis homeostasis. It is estimated that propionic acid plays an important role in improving insulin resistance, weight gain, and obesity prevention. It is also well described that the intake of propionic acid can be beneficial. Studies that confirm these effects are mainly related to the supplementation of propionic acid with butyrate or a mixture of the most important SCFAs: butyrate, propionate, and acetate (30). It seems that the proper ratio between particular acids has the most significant effect in the prevention of metabolic diseases.
In summary, the HFHCh diet increased the level of priopionate with parallel decrease of butyrate. The ratio between SCFA was also changed (from 1.1: 0.6: 1 for control groups to 3: 3.6: 1 for HFHCh groups). The main SCFA in the HFHCh group was propionate instead of acetate (which is considered physiological). These changes can potentially affect the imbalance of intestinal barrier function and intestinal glucose homeostatis. Chronic decrease in butyrate production was associated with increased release of LPS. The hypothesis that observed changes could be the consequence of microbiota and intestinal barrier alterations requires further studies.
Acknowledgements: Supported by National Science Centre, Poland, No. 2016/21/N/NZ5/02584.
Conflicts of interests: None declared.
REFERENCES
- Den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013; 54: 2325-2340.
- Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016; 7: 185. doi: 10.3389/fmicb.2016.00185
- De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2015; 65: 1812-1821. doi: 10.1136/gutjnl-2015-309957
- Brussow H, Parkinson SJ. You are what you eat. Nat Biotechnol 2014; 32: 243-245.
- Murphy EA, Velazquez KT, Herbert KM. Influence of high-fat-diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care 2015; 18: 515-520.
- Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J 2012; 6: 1848-1857.
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137: 1716-1724.
- Parks BW, Nam E, Org E, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab 2013; 17: 141-152.
- Carmody RN, Gerber GK, Luevano JM, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015; 17: 72-84.
- Gregoraszczuk E, Slupecka M, Wolinski J, et al. Maternal high-fat diet during pregnancy and lactation had gender difference effect on adiponectin in rat offspring. J Physiol Pharmacol 2016; 67: 543-553.
- Li X, Shimizu Y, Kimura I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci Microbiota Food Health 2017; 36: 135-140.
- Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci 2010; 55: 931-940.
- Zhao G, Nyman M, Jonsson JA. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr 2006; 20: 674-682.
- Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol 2015; 6: 110-119.
- Konturek PC, Haziri D, Brzozowski T, et al. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol 2015; 66: 483-491.
- Bosscher D, Breynaert A, Pieters L, Hermans N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J Physiol Pharmacol 2009; 60 (Suppl. 6): 5-11.
- Abdallah Ismail N, Ragab SH, Abd Elbaky A, Shoeib AR, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci 2011; 7: 501-507.
- Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy 2007; 108: 354-358.
- Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014; 5: e00889. doi: 10.1128/mBio.00889-14
- Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 1992; 72: 57-64.
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016; 7: 189-200.
- Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470-1481.
- Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010; 31: 817-844.
- Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci 2012; 57: 3126-3135.
- Liu B, Qian J, Wang Q, Wang F, Ma Z, Qiao Y. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS One 2014; 9: e106184. doi: 10.1371/journal.pone.0106184
- Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 2012; 142: 1100-1101.
- De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014; 156: 84-96.
- LeBlanc JG, Chain F, Martin R, Bermudez-Humaran LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 2017; 16: 79. doi: 10.1186/s12934-017-0691-z
- Wang A, Si H, Liu D, Jiang H. Butyrate activates the cAMPprotein kinase A-cAMP response element-binding protein signaling pathway in Caco-2 cells. J Nutr 2012; 142: 1-6.
- Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 2010; 1801: 1175-1183.
A c c e p t e d : April 24, 2018