Cell lines and reagents
Neonatal HMVECs (Neo-HMVECs) were obtained from Cambrex Biosciences (Walkersville, MD). Aged HMVECs from 66 and 90 years old donors (66-HMVECs and 90-HMVECs) were obtained from PromoCell (Heidelberg, Germany) and Cambrex Biosciences (Walkersville, MD), respectively. Cells were maintained in culture as an adherent monolayer in EGM-2 MV endothelial cell growth media (Cambrex Biosciences). Recombinant human ghrelin was purchased from Phoenix Pharmaceutical (Belmont, CA). Anti-phospho ERK (SC-7383) and anti-ERK (SC-154) were obtained from Santa Cruz (Santa Cruz, CA) and used at a dilution of 1:500. MEK inhibitor PD98059 was purchased from BIOMOL (Plymouth Meeting, PA). VEGFR2 inhibitor 1 (Z)-3-((2,4-Dimethyl-3-(ethoxycarbonyl)pyrrol-5-yl)methylidenyl)indolin-2-one) was purchased from Calbiochem/EMD Biosciences (#676480, San Diego, CA).
ELISA measurement of ghrelin levels
We examined ghrelin levels in cell culture supernatants and in cell lysates of HMVECs using the human Bioassay ELISA kit (US Biological, Swampscott, MA) according to the manufacturer's protocol. Cells were grown in 6-well plate for 48 hrs and the cell culture supernatant and cell lysate were collected. Fifty Ál of either: culture supernataunt, cell lysates or recombinant human ghrelin protein at different concentrations, and 25 Ál of ghrelin polyclonal antibody and 25 Ál biotinylated ghrelin standard peptide were added to each well. After 2 h incubation, the plates were washed, and the immunoreactivity was determined using the avidin-HRP-TMB detection system. The reactions were stopped by adding 100 Ál of 0.18 N H2SO4 and absorbance was determined using an ELISA microtiter plate reader (DYNEX Technologies, Inc. Chantilly, Virginia) at 450 nm. A standard curve of the absorbance and ghrelin concentration was plotted and the ghrelin levels in the samples determined from this standard curve.
Immunocytochemical (ICC) staining for ghrelin receptor (GHSR1)
Ghrelin receptor (GHSR1) expression and localization in HMVECs were analyzed by ICC staining as described in our previous study (14). Briefly, endothelial cells were plated on 24-well coverslips coated with rat type I collagen gel, cultured for 48 h and used for immunostaining. Samples were washed twice with PBS and fixed in 4% paraformaldehyde (Sigma-Aldrich) in PBS for 10 min and permeabilized with acetone for 5 min. After washing twice with PBS, cells were incubated with a blocking solution (DAKO, Carpentaria, CA) for 7 min at room temperature. Excess blocking solution was drained and samples were incubated with anti-GHSR1 antibodies for 2 h at room temperature. The samples were then rinsed with PBS and incubated with Alexa-conjugated secondary antibody (Molecular Probes, Eugene, CA). Cells were then washed, mounted using anti-fade mounting media (Molecular Probes, Eugene, CA) and examined under a Nikon epifluorescence microscope. As a negative control we performed staining using all reagents except the primary antibody.
in vitro angiogenesis on matrigel
First, we examined angiogenic ability of neo- and aged-HMVECs using in vitro capillary tube formation assay performed as described in our previous studies (14, 24). Briefly, 2 x 104 HMVECs were plated on a Matrigel (BD Biosciences, Mountain View, CA) coated 48-well plate with either medium alone or medium containing ghrelin 0.1, 1 and 10 nM. To determine the mechanism of ghrelin-induced angiogenesis, cells were treated with PD98059 (ERK inhibitor, 10 ÁM) for 30 min prior to treatment with ghrelin. After 6 hours, the plates were examined for capillary tube formation under an inverted Nikon microscope and photographed. Each assay was done in triplicate and each experiment was repeated three times. The total length of capillary tube in control and each of treatment groups was measured in 5 random fields on coded samples by two independent observers. The tube formation was examined and quantified using the image analytical software - Metamorph version 7.0 (Universal Imaging Corporation, West Chester, PA)
Effect of exogenous ghrelin on endothelial cell proliferation
We examined endothelial cell proliferation using bromodeoxyuridine (BrdU) ELISA assay (Chemicon, Temecula, CA) and BrdU immunostaining. Briefly, endothelial cells were seeded into 96-well culture plates in triplicate and allowed to adhere overnight. The cultures were then washed and cultured with medium alone (control) or medium containing different concentrations of ghrelin. Following 24 hr incubation, cell proliferation was determined by BrdU ELISA following the manufacturer's instruction.
Western blotting
Cell lysates were subjected to SDS-PAGE and transferred onto a nitrocellulose membrane. The membranes were incubated with primary antibody against p-ERKs or total ERKs followed by peroxidase-conjugated secondary antibody. Immunoreactive proteins were visualized using ECL detection system (Amersham, Arlington Heights, IL), similarly as in our previous study (24).
Statistical analysis
Values are expressed as means ▒ SD. Student's t test was used to compare data between two groups. One-way ANOVA followed by Bonferroni correction was used to compare data between three or more groups. A P value < 0.05 was considered statistically significant.
Aged HMVECs exhibit decreased ghrelin expression
Our previous study using neonatal HMVECs was the first to demonstrate the expression of ghrelin and its receptor - GHSR1 (14) in human endothelial cells. In the present study, we examined the levels of ghrelin and its receptor (GHSR1) in neonatal and aged HMVECs. Using ELISA we measured the levels of ghrelin in HMVECs. Compared to Neo-HMVECs, the aged 90- HMVECs had significantly 3.2-fold (p<0.05) reduced ghrelin level (Fig. 1). In a previous study we found GSHR1 mRNA and protein expression in neonatal HMVECs. In the present study immunofluorescence staining demonstrated no significant difference in expression of ghrelin receptor (GSHR1) between Neo-HMVECs and aged 90- HMVECs (data not shown).
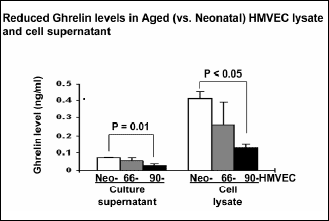 |
Fig. 1. Reduced ghrelin levels in aged HMVECs. Ghrelin levels were determined in cell culture supernatants and cell lysates of Neo- and aged HMVECs by ELISA. The values are mean ghrelin concentration ▒ S.D. This is representative of two experiments done in duplicate (n=4). |
Aged HMVECs exhibit decreased in vitro angiogenesis
Using an in vitro angiogenesis assay on matrigel, we compared the angiogenic ability of Neo- and aged HMVECs. In aged 66- and 90-HMVECs, in vitro angiogenesis was significantly decreased by 39.7% (p=0.003) and 62.4%-fold (p=0.003) respectively, compared to Neo-HMVECs (Fig. 2).
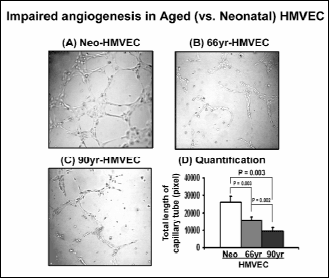 |
Fig. 2. Impaired angiogenesis in aged HMVECs. Cells were grown on matrigel for 6 hrs under normal growth condition (a) Neo-HMVECs, (b) 66-HMVECs, (c) 90-HMVECs, (d) quantification of capillary tube formation in Neo- and aged HMVECs. The values are mean capillary tube length ▒ S.D. This is representative of three experiments done in triplicate (n=9). |
Ghrelin increases in vitro angiogenesis in aged HMVECs
We next examined if exogenous ghrelin could reverse impaired angiogenesis in aged HMVECs using an in vitro angiogenesis assay on matrigel. Ghrelin at 0.1 nM significantly increased in vitro angiogenesis in Neo-HMVECs by 36.5%-fold (p<0.01) (Fig. 3). In aged HMVECs, 10 nM ghrelin significantly increased by 36.7% (p<0.05) in vitro angiogenesis to levels similar seen in untreated Neo-HMVECs. These data demonstrate that ghrelin can partly reverse impaired angiogenesis in aged HMVECs (Fig. 3).
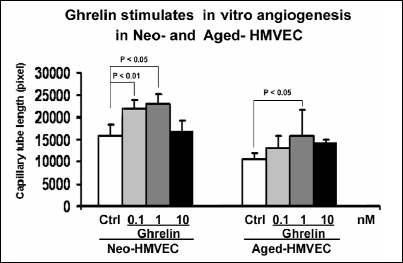 |
Fig. 3. Ghrelin increases in vitro angiogenesis in aged HMVECs. Cells were grown on matrigel for 6 hrs under normal growth condition in the presence or absence of ghrelin. The values are mean capillary tube length ▒ S.D. This is representative of three experiments done in triplicate (n=9). |
Ghrelin stimulates proliferation of HMVECs
One of the essential components of angiogenesis is endothelial cell proliferation. Since aged HMVECs exhibit decreased ghrelin expression, next we determined if exogenous ghrelin can increase cell proliferation in HMVECs. Ghrelin added to culture medium at 0.1 nM significantly increased endothelial cell proliferation in Neo-HMVECs by 2.3-fold (p<0.05) compared to untreated cells (Fig. 4). However in aged HMVECs, a substantially higher doses of ghrelin, 1 nM was required to increase cell proliferation by a significant 2.2-fold (p<0.05) compared to untreated cells (Fig. 4). These data indicate that aged HMVECs have reduced ghrelin levels are also less responsive to ghrelin compared to Neo-HMVECs.
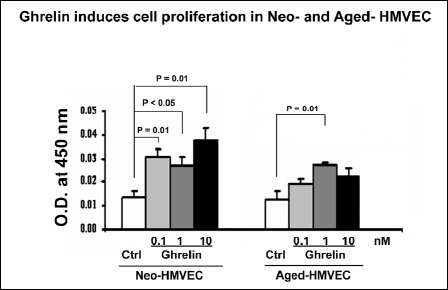 |
Fig. 4. Ghrelin stimulates proliferation of HMVECs. Cell proliferation was assessed by BrdU labeling in Neo- and aged HMVECs. The values are mean viable cell O.D. ▒ S.D. of triplicate culture. This is representative of three experiments done in triplicate (n=9). |
Ghrelin induces phosphorylation of ERKs
Since MAPK/ERK2 pathway is the mitogenic signaling pathway, the most likely mechanism for ghrelin mediated cell proliferation and angiogenesis is through the MAPK/ERK2. To examine whether the mechanism of ghrelin action involves MAPK/ERK2, we next examined using Western blotting the phosphorylation of ERKs in Neo- and aged HMVECs in response to ghrelin treatment. Ghrelin stimulates the phosphorylation of ERKs in Neo- and aged HMVECs by 2.4-fold or 139% (p<0.05) and 57.4% (p<0.05) respectively (Fig. 5).
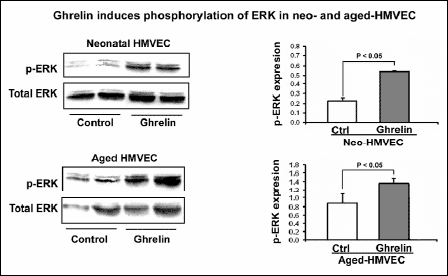 |
Fig. 5. Ghrelin induces phosphorylation of ERKs. Effect of 1 nM ghrelin on phosphorylation of ERKs in Neo- and aged HMVECs was examined using Western blotting. The values shown are mean intensity of bands ▒ S.D. This is representative of two experiments done in duplicate (n=4). |
ERK phosphorylation is required for angiogenesis
To further determine whether ghrelin-induced angiogenesis is mediated through ERK2 activation, Neo-HMVECs were treated with the MEK/ERK2 inhibitor PD98059 prior to ghrelin treatment and then subjected to in vitro angiogenesis assay on matrigel. Inhibition of ERK2 phosphorylation by PD98059 abolished ghrelin-induced in vitro angiogenesis (Fig. 6). These data demonstrate that ERK phosphorylation is required for angiogenesis in HMVECs.
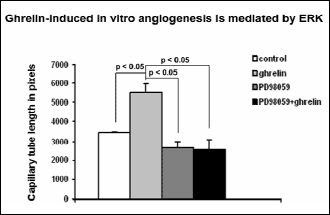 |
Fig. 6. ERK phosphorylation is required for angiogenesis. Capillary tube formation in ghrelin treated cells in the presence or absence of the Erk inhibitor PD98059 was measured on matrigel. Photograph was taken after 6 hrs. The values are mean capillary tube length ▒ S.D. This is representative of three experiments done in triplicate (n=9). |
Aging is associated with impaired angiogenesis - formation of new capillary blood vessels. Healing of tissue injury including gastric lesions and ulcers requires angiogenesis (13). Recent studies have demonstrated that ghrelin stimulates gastric growth, exerts a potent protective action on the gastric mucosa and accelerates the healing of alcohol- induced and ischemia/reperfusion-induced gastric lesions (11, 12, 25, 26). Ghrelin has also been implicated in regulation of cardiovascular functions including vasodilation, increased contractility and cardioprotection (5-8), inhibiting proinflammatory response in human umbilical vein endothelial cells (9) and reversing endothelial dysfunction in patients with metabolic syndrome (10). These studies taken together suggest a pro-angiogenic role for ghrelin. However, there have been no studies exploring the role and mechanism of ghrelin in promoting angiogenesis and also its regulation during aging. Also, the cellular and molecular mechanisms of impaired angiogenesis in aging remain not fully explained. The aims of this study were to determine the expression of ghrelin during aging, the role of ghrelin in angiogenesis and the mechanism of ghrelin-induced angiogenesis in aged HMVECs.
Our previous study using neonatal HMVECs was the first to demonstrate the expression of ghrelin and its receptor (GHSR1) and that exogenous ghrelin promotes angiogenesis in neonatal HMVECs (14). Our present study reveals the presence of ghrelin and ghrelin receptor in both neonatal and aged HMVECs and further demonstrates reduced ghrelin in aged HMVECs. Reduced ghrelin is responsible, at least in part for impaired angiogenesis in aged HMVECs since exogenous ghrelin can significantly restore angiogenesis in aged HMVECs. We also demonstrated that aged HMVECs have reduced phosphorylation of ERKs.
This is the first demonstration that reduced angiogenesis in aging HMVECs is due to reduced ghrelin. Previous studies on angiogenesis using pathological models such as ischemia in the rabbit hind limb have demonstrated impaired angiogenesis in aging tissues and have implicated reduced angiogenesis in delayed wound healing (21). Recent studies have suggested use of ghrelin and its receptor (GHS-R1A) antagonists as novel therapeutic approaches for age-related metabolic and physiological changes (27, 28). However, the role and regulation of angiogenesis by ghrelin during aging has not been examined in endothelial cells. Our study showed for the first time that ghrelin levels are significantly reduced in aged HMVECs. Moreover, treatment with ghrelin partly restores angiogenesis in aged HMVECs demonstrating that the reduction in ghrelin levels accounts in part for the significantly reduced angiogenic capability of aged HMVECs. Regarding the relevance of in vitro angiogenesis to in vivo condition, a recent study demonstrated that during in vivo angiogenesis, endothelial cells form capillary like endothelial tubes and develop lumina, very similar to those seen during in vitro angiogenesis models (29). Therefore, in vitro angiogenesis process closely resembles in vivo angiogenesis and likely represents a relevant model (29).
Cell proliferation significantly contributed to the process of in vitro angiogenesis, but this process in addition also requires, as demonstrated in previous publication, G-actin polymerization, cell migration and assembly of capillary tubes (30).
We have demonstrated that the mechanism of ghrelin action involves phosphorylation and thus activation of ERKs and that this process is reduced in aged HMVECs. Activation of MAPK pathway and phosphorylation of ERKs by ghrelin was reported previously in hepatoma cells and neonatal HMVECs (31, 14). However, these studies did not examine ghrelin's action on ERK activation in endothelial cells during aging.
In summary, our present study has identified reduced ghrelin levels in aged endothelial cells as one of the mechanisms that mediate the impairment of angiogenesis. Ghrelin plays a key role in angiogenesis by promoting endothelial cell proliferation through activation of the MAPK/ERK2 mitogenic signaling pathway. Our findings provide a rationale for use of ghrelin as a novel therapy for improving aging related impairment of angiogenesis.
Grant Support: Supported by the VA Merit Review (to AST) and AHA Grant (to AA)
Conflict of interests: None declared.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-release acylated peptide from stomach. Nature 1999; 402: 656-660.
- Date Y, Kojima M, Hosoda H, Sawaguchi A et al. Ghrelin, a novel growth hormone releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and human. Endocrinology 2000; 141: 4255-4256.
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 2001; 12: 118-122.
- Kotunia A, Zabielski R. Ghrelin in the postnatal development of the gastrointestinal tract. J Physiol Pharmacol 2006; 57 (Suppl 5): 97-111.
- Nagaya N, Miyatake K, Uematsu M et al. Hemodynamic, renal and hormonal effects of ghrelin infusion in patients with chronic heart failure. Clin Endocrinol Metab 2001; 86: 5854-5859.
- Wiley KE, Davenport AP. Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol 2002; 136: 1146-1142.
- Kleinz M J, Maguire JJ, Skepper JN, Davenport A P. Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovasc Res 2006; 69: 227-235.
- King MK, Gay DM, Pan LC et al. Treatment with a growth hormone secretagogue in a model of developing heart failure: effects on ventricular and myocyte function. Circulation 2003; 103: 308-313.
- Li WG, Gavrila D, Liu X et al. Ghrelin inhibits proinflammatory response and nuclear factor- B activation in human endothelial cells. Circulation 2004; 109: 2221-2226.
- Tesauro M, Schinzari F, Iantorno M et al. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 2005; 112: 2986-2992.
- Konturek PC, Brzozowski T, Walter B et al. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. Eur J Pharmacol 2006; 536: 171-181.
- Brzozowski T, Konturek PC, Sliwowski Z et al. Prostaglandin/cyclooxygenase pathway in ghrelin-induced gastroprotection against ischemia-reperfusion injury. J Pharmacol Exp Ther 2006; 319: 477-478.
- Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci 2005; 50: S24-S33.
- Li A, Cheng G, Zhu G, Tarnawski AS. Ghrelin stimulates angiogenesis in human microvascular endothelial cells: Implications beyond GH release. BBRC 2007; 353: 238-243.
- Malara B, Josko J, Tyrpien M, Malara P, Steplewska K. Dynamics of changes in vascular endothelial growth factor (VEGF) expression and angiogenesis in stress-induced gastric ulceration in rats. J Physiol Pharmacol. 2005; 56: 259-271.
- Tanigawa T, Pai R, Arakawa T, Higuchi K, Tarnawski AS. TGF-beta signaling pathway: its role in gastrointestinal pathophysiology and modulation of ulcer healing. J Physiol Pharmacol 2005; 56: 3-13
- Swift ME, Kleinman H, DiPetro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 1999; 79: 1479-1487.
- Wu L, Xia YP, Roth SI, Gruski E, Mustoe TA. Transforming growth factor-beta1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age. Am J Pathol 1999; 154: 301-309.
- Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004; 28: 321-326.
- Edelberg JM, Reed MJ. Aging and angiogenesis. Front Biosci 2003; 8: s1199-s1209.
- Rivard A, Fabre JE, Silver M et al. Age-dependent impairment of angiogenesis. Circulation 1999; 99: 111-120.
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem 2003; 51: 1119-1130.
- Tarnawski A, Hollander, D, Stachura J, Gergely H, Krause WJ, Sarfeh IJ. Role of angiogenesis in healing of experimental gastric ulcer. In Mechanisms of Peptic Ulcer Healing. F Halter, A Garner, GNJ Tytgat (eds). Dordrecht/Boston/London, Kluwer Acad. Publ., 1991, pp.165-171.
- Jones MK, Wang H, Peskar BM et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med 1999; 5: 1418-1423.
- Brzozowski T, Konturek PC, Sliwowski Z et al. Neural aspects of ghrelin-induced gastroprotection against mucosal injury induced by noxious agents. J Physiol Pharmacol 2006; 57 (Suppl 6): 63-76.
- Warzecha Z, Dembinski A, Ceranowicz P et al. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J Physiol Pharmacol. 2006; 57(3): 425-437.
- Ariyasu H, Iwakura H, Yamada G, Nakao K, Kangawa K, Akamizu T. Efficacy of ghrelin as a therapeutic approach for age-related physiological changes. Endocrinology 2008; 149: 3722-3728.
- Smith RG, Sun Y, Jiang H, Albarran-Zeckler R, Timchenko N. Ghrelin receptor (GHS-R1A) agonists show potential as interventive agents during aging. Ann N Y Acad Sci 2007; 1119: 147-164.
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006; 442: 453-456.
- Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J 2004; 18: 1264-1266.
- Murata M, Okimura Y, Iida K et al. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem 2002; 277: 5667-5674.