It has been generally accepted that regular and intense exercises lead to reactive oxygen and nitrogen species (RONS) generation and changes in pro-antioxidant parameters such as glutathione, glutathione enzymes, lipid peroxidation products etc. Therefore the measurement of various pro-antioxidant parameters can be used in determining risk of oxidative stress or effectiveness of antioxidant supplementation.

-Lipoic acid (ALA)
as a pro-glutathione dietary supplement has been the focus of intensive research
in nutrition in the last few years. ALA and its reduced form dihydrolipoate
(DHLA) have been suggested to function as powerful antioxidant. ALA and DHLA
couple has shown the ability to react with reactive and oxygen species (RONS)
such as hydroxyl radical, hypochlorous acid and singlet oxygen and reduce glutathione
disulfide, tocopherol radicals and ascorbate. Moreover, DHLA and ALA can work
as a redox regulator of myoglobin, prolactin, thioredoxin, glucose transporter
protein (GLUT4) and NF-
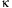
B
transcription factor. ALA, as lipoamid, has functioned as a cofactor in the
multienzyme complexes that catalyse the oxidative decarboxylation of

-keto
acids such as pyruvate,

-ketoglutarate,
and branched chain

-keto
acids (1-3).
Even though, ALA has been applied in sport as a dietary supplement, its use
by the athletes gives rise to controversy. It has been reported that ALA supplementation
prevented the decline of others antioxidants, improved glucose metabolism and
attenuated exercise-induced oxidative damage in various tissues (1). On the
other hand, it has been shown that long-term ALA administration led to enhancement
of lipid peroxidation, mitochondrial damage and inhibition of glycogen synthesis
(1, 2). The majority results concerning the effects of ALA on cell metabolism
have been obtained from
in vitro studies or animal models (4-9). There
has been lack of studies in physically active men.
Thus, the aim of the present study was 1) to compare the parameters of pro-antioxidant
status in untrained and trained healthy men, and 2) to assess the antioxidant
effectiveness of oral

-lipoic
acid administration in trained men performed muscle-damaging exercise.
MATERIALS AND METHODS
Thirteen healthy and weight trained males with resistance training experience of at least 3 years (T group: age 25.5 ± 6.0 yr, height 180.5 ± 5.9 cm, body mass 86.4 ± 8.1 kg, body fat 14.1 ± 3.9%) participated in this randomised, double-blind, placebo-controlled and cross-over study. Twenty healthy, non-smoking and untrained males (NT group: age 21.8 ± 1.1 yr, height 180.3 ± 7.8 cm, body mass 82.2 ± 8.7 kg, body fat 12.5 ± 4.4%) made a reference group. Subjects had not taken any supplements, particularly antioxidant vitamins or minerals, for 4 weeks prior to the study. All the subjects were informed of the aim of the study and were given their written consent for participation in the project. The protocol of the study was approved by the local ethics committee in accordance with the Helsinki Declaration.
Trained (T) subjects were administered with 600 mg of

-lipoic
acid (T
ALA group) or 700 mg lactose (placebo,
T
CON group) for eight days before the muscle-damaging
effort (isometric/isokinetic exercise). The participants took the a-lipoic acid
or placebo in the morning in a fasted state. The last dose of ALA was taken
24 h before exercise. The wash-out period between the trials with a-lipoic acid
and placebo was three weeks.
Blood collection
Blood samples were obtained from a cubital vein with an anticoagulant (EDTAK
2)
in the morning in NT group. In trained men (T
CON
and T
ALA), the blood samples were collected
before exercise, immediately after completing exercise and after 24 h of the
recovery period. The samples were immediately placed in 4°C temperature after
collection. Within 10 min, the blood samples were centrifuged (2500 g, 10 min,
4°C). Aliquots of plasma were stored at -20°C. Erythrocyte fraction was resuspended
three times in cold isotonic saline solution and centrifuged (2500 g, 10 min,
4°C). Washed erythrocytes were stored at -20°C until analysis. All the samples
were analysed within 7 days.
Isometric/isokinetic exercise
The exercise protocol consisted of three 10-second maximal voluntary (the highest efficiency) isometric contractions of the quadriceps muscles in 30° and 75° of knee flexion on Biodex System 3 dynamometer (Biodex Medical Systems, Shirley, NY). Subjects finished the isometric sequence with peak torque for right limb on the level of 195.1 ± 28.6 Nm (extensor m.) and 154.9 ± 26.3 Nm (flexor m.) at 30° knee flexion, 357.4 ± 46.2 Nm (extensor m.) and 127.6 ± 28.4 Nm (flexor m.) at 75° knee flexion, and for left limb on the level of 198.7 ± 33.8 Nm (extensor m.) and 138.9 ± 27.1 Nm (flexor m.) at 30° knee flexion, 335.1 ± 55.0 Nm (extensor m.) and 123.7 ± 24.0 Nm (flexor m.) at 75° knee flexion. Then the isokinetic sequence was carried out at angular velocities of 60, 120, 180, 210 and 450°.s-1. Isokinetic sequence was performed in seated position with the knee in 90° of flexion and repeated until exhaustion. Peak torque, time to reach peak torque, total work, average power and max average peak torque were collected from the Biodex during muscle performance measures. Subjects were familiarized with the both exercise protocols before the start of data collection.
Biochemical analysis
The plasma total thiol (TT) concentration was estimated by the method of Hebeeb (10) using 5,5’-dithiobis-2-nitrobenzoic acid (DTNB). The samples were added to a denaturing solution containing sodium dodecyl sulfate in order to ionise the sulfhydryl groups and make them more reactive to DTNB. The samples were measured at 410 nm against control samples (minus DTNB). The intra-assay coefficient of variation (CV) for thiol procedure was <7%.
Blood reduced glutathione (GSH) concentration was estimated by the method of
Beutler
et al. (11) using 5,5’-dithiobis-2-nitrobenzoic acid (DTNB).
GSH detection limit for the procedure was 2.5 mg
.
ml
-1 and the intra-assay coefficient of variation
(CV) was <10%.
Erythrocyte glutathione reductase (GR) and glutathione peroxidase (GPx) activities
were evaluated using Randox assay (UK). GR and GPx detection limits for Randox
kits were 11 U
. l
-1
and 8.86 U
. l
-1
, respectively. The intra-assay coefficient of variation (CV) for the GR kit
was 4.63% and for the GPx kit it was 4.20%.
The enzymatic activities and glutathione concentration were expressed relatively to Hb concentration measured by the Drabkin method (12) using Drabkin’s reagent (POCH Poland).
Plasma lipid peroxidation products were estimated using the measurement of thiobarbituric
acid-reactive substance (TBARS) level according to the method of Buege and Aust
(13). To avoid further peroxidation, plasma samples were treated with 15% TCA
containing 0.25M HCl immediately after separation of plasma. The TBARS level
was expressed as nmol of malondialdehyde using 1,1,3,3- tetraethoxypropane as
a standard. TBARS detection limit was 0.13 nmol
.
ml
-1.
Plasma protein carbonyls (PC) were measured by the method of Levine
et al.
(14) using 2,4-dinitrophenyl hydrazine. The carbonyl content was calculated
using an extinction coefficient of 22000 M
-1 .
l
-1. cm
-1 and expressed
as nmol PC per mg of plasma protein. Protein concentration was determined by
the method of Bradford (15). The intra-assay coefficient of variation (CV) for
TBARS and PC procedures were <10%.
Plasma creatine kinase (CK) and lactate dehydrogenase (LDH) activities were
evaluated using the diagnostic assays for the kinetic enzyme analyser Konelap
60 BioMerieux (France). CK and LDH detection limits for the applied kits were
6 U
. l
-1 and 18
U
. l
-1 respectively.
The intra-assay coefficient of variation (CV) for the CK kit was 1.85% and the
LDH kit was 2.61%.
Statistics
Statistical analysis was carried out using Statistica 6.0. All the data were
tested for their normal distribution. For the comparison of untrained (NT) and
trained subjects (T
CON, T
ALA)
one-way ANOVA was chosen. To determine the effects of exercise and

-lipoic
acid as well as the interaction between exercise and supplementation, statistical
analysis was performed using two-way ANOVA and post-hoc Tukey’s test. Correlations
were calculated by the Pearson correlation coefficients. The accepted level
of significance was defined as P< 0.05. Data are presented as mean ± SD.
RESULTS
The comparison of pro-antioxidant parameters has indicated significant differences
between untrained and trained subjects concerning blood GSH, erythrocyte GR
and GPx, plasma TT but no differences in plasma TBARS and PC levels. The resting
plasma TT concentration was almost two-fold higher in NT (167.10 ± 14.58 mg
. l
-1) than in T
CON
(83.26 ± 10.35 mg
. l
-1).
Eight-day ALA administration significantly elevated the resting TT level in
T
ALA to 112.60 ± 10.82 mg
.
l
-1 which remained on high level at 48 h after
the last dose of ALA (
Fig. 1). In T
ALA,
TT did not reach the level found in NT group. This could indicate the high thiol
consumption during resistance training. The resting GSH concentration was significantly
lower in NT (1.35 ± 0.13 mg
. gHb
-1)
than in trained group (T
CON: 1.94 ± 0.18 mg
. gHb
-1). ALA administration
did not affect the resting GSH concentration (
Fig. 2). The resting activities
of glutathione-related enzymes
i.e. GR (NT: 14.27 ± 2.75 U
.
gHb
-1) and GPx (NT: 39.51 ± 12.97 U
.
gHb
-1) were considerably lower in NT than in trained
subjects (
Fig. 3, 4). ALA intake caused increase in resting GR thus elevating
the difference in enzyme activities by 30% between NT and trained subjects.
The resting GPx activity decreased following

-lipoic
acid but did not reach the level observed in NT group. TBARS and PC concentrations
in NT were similar those in T
CON group, however,
ALA significantly reduced the resting plasma lipid peroxidation and protein
carbonylation (
Fig. 5, 6).
 |
Fig. 1. Changes in plasma
total thiols (TT) concentration; ** P<0.01 indicates TCON
vs. TALA; ab means significant
(P<0.05) difference between NT and trained subjects (TCON
and TALA). |
 |
Fig. 2. Changes in blood reduced
glutathione (GSH) concentration; ** P<0.01 indicates TCON
vs. TALA; # P<0.05 indicates post-exercise
vs. pre-exercise values; E x ALA indicates a significant interaction between
exercise and supplementation (ANOVA, P<0.05); ab means significant (P<0.05)
difference between NT and trained subjects (TCON
and TALA). |
 |
Fig. 3. Changes in erythrocyte
glutathione reductase (GR) activity; ** P<0.01 indicates TCON
vs. TALA; # P<0.05 and ## P<0.01 indicate
post-exercise vs. pre-exercise values; E x ALA indicates a significant
interaction between exercise and supplementation (ANOVA, P<0.05); ab means
significant (P<0.05) difference between NT and trained subjects (TCON
and TALA). |
The applied isometric/isokinetic effort induced muscle damage in trained group.
This was demonstrated by statistically significant changes in plasma activities
of muscle damage markers. Plasma CK activity increased from resting values of
149.40 ± 35.57 U
. l
-1
(T
CON) and 150.00 ± 42.26 U
.
l
-1 (T
ALA) to
418.50 ± 161.04 U
. l
-1
(T
CON) and 534.25 ± 160.06 U
.
l
-1 (T
ALA) at
24 h of the post-exercise recovery period. LDH activity increased from 303.91
± 56.73 U
. l
-1
(T
CON) and 297.90 ± 68.20 U
.
l
-1 (T
ALA) to
418.45 ± 65.81 U
. l
-1
(T
CON) and 381.00 ± 77.81 U
.
l
-1 (T
ALA) at
24 h of the post-exercise period. The changes in CK and LDH activities were
independent of

-lipoic
acid supplementation and did not correlate with oxidative damage markers.
As it was showed in
Fig. 1-6, even though the isometric/isokinetic exercise
did not trigger off visible changes in pro-antioxidant parameters, its application
has enhanced

-lipoic
acid action particularly in relation to glutathione and glutathione-related
enzymes. The changes in GSH, GR and GPx were demonstrated 24 h after exercise.
Blood GSH concentration significantly increased from 1.62 ± 0.17 mg
.
gHb
-1 in T
CON
to 2.26 ± 0.28 mg
. gHb
-1
in T
ALA (
Fig. 2). GR activity decreased
from 24.27 ± 3.13 U
. gHb
-1
(T
CON) to 18.54 ± 2.15 U
.
gHb
-1 in T
ALA
whereas GPx activity increased from 43.03 ± 7.65 U
.
gHb
-1 (T
CON) to
55.30 ± 9.99 U
. gHb
-1
in T
ALA group (
Fig. 3, 4). ANOVA showed
a significant interaction between ALA and exercise (
Fig. 2, 4).
 |
Fig. 4. Changes in erythrocyte
glutathione peroxidase (GPx) activity; * P<0.05 indicates TCON
vs. TALA; #P<0.05 and ## P<0.01
indicate post-exercise vs. pre-exercise values; E x ALA indicates
a significant interaction between exercise and supplementation (ANOVA,
P<0.05); ab means significant (P<0.05) difference between NT and trained
subjects (TCON and TALA). |
 |
Fig. 5. Changes in plasma
lipid peroxidation (TBARS) concentration; ** P<0.01 indicates TCON
vs. TALA; b means significant
(P<0.05) difference between NT and TALA
groups. |
 |
Fig. 6. Changes in plasma
protein carbonylation (PC) concentration; ** P<0.01 indicates TCON
vs. TALA. #P<0.05 indicates post-exercise
vs. pre-exercise values. |
The levels of TT, TBARS and PC changed in TALA group independently of applied
isometric/isokinetic exercise
i.e. ANOVA did not show any interactions
between ALA and exercise. In T
ALA, TT significantly
increased whereas peroxidation and carbonylation products decreased before and
after exercise compared with T
CON. The high
concentration of TT and low of TBARS and PC remained to 48 h after the last
dose of ALA (
Fig. 1, 5, 6).
Exercise parameters measured by Biodex system
i.e. peak torque, time
to reach peak torque, total work, average power and max average peak torque
were not statistically changed in subjects after ALA supplementation. However,
it was observed tendency to increase in measured parameters at lower angular
velocities in isometric trial
e.g. the mean total work was by 5%-17%
higher in T
ALA than T
CON
group at angular velocity of 60°
.s
-1
(
Fig. 7).
 |
Fig. 7. Changes in total work
[J] during isokinetic trial in TCON (black
marks) and TALA (grey marks) groups. |
DISCUSSION
Plasma total thiols have been an integral and important part of antioxidant
mechanism which regulates RONS production. The thiol level has to depend on
concentration of sulphur containing compounds such as glutathione,

-lipoic
acid, cysteinylglycine, homocysteine and cysteine releasing from liver and muscle.
The present study has shown that the level of TT in trained subjects was two-fold
lower than in sedentaries. Training, particularly the resistance training, can
induce a decline in plasma TT due to the high absorption of thiol compounds
by active muscles (16-18). Eight-day ALA supplementation elevated the resting
plasma TT concentration by 35% but did not eliminate the differences between
trained and untrained subjects. This confirms that plasma TT level is determined
by different sulphur-containing compounds, not only by a-lipoic acid. Even though
the isometric/isokinetic exercise induced muscle damage, it did not provoke
any changes in plasma TT concentration in T
CON
and T
ALA groups.
Our study has shown that resistance training enhanced the glutathione antioxidant
system. The erythrocyte level of reduced glutathione and glutathione-related
enzymes was markedly higher in trained than untrained subjects. The previous
studies have demonstrated contradicting changes in GSH concentration and GR
and GPx activities after physical training. For example, Kretschmar
et al.
(19) observed the higher level GSH in runners compared with sedentaries whereas
Balakrishnan
et al. (20) found the low concentration of GSH and low activity
of GPx in athletes (soccers, hockey players, runners). Even though ALA has been
pro-glutathione supplement, its administration did not influence the resting
GSH concentration in trained subjects. However, the application of isometric/isokinetic
exercise revealed the ALA action and led to changes in erythrocyte GSH, GR and
GPx that were found at 24 h after exercise. In trained group, eight-day oral
ALA supplementation elevated the GSH concentration by 40% compared with TCON
group. It may suggest that

-lipoic
acid have been used only when the stress factor occurred
i.e. physical
exercise. The similar results were previously observed by Khanna
et al.
(4). The authors found the high level of blood and liver GSH after intragastric

-lipoic acid supplementation
and exhaustive treadmill exercise in rats. Busse
et al. (21) observed
in mouse cell lines that GSH content depends on dose of

-lipoic
acid. According Han
et al. (22) and Moini
et al. (1) the high
level of GSH in response to ALA supplementation has been associated with the
reduction of disulfide glutathione and de novo synthesis of glutathione by improving
cysteine transport.
In our study, increase in blood GSH concentration also resulted in activities of glutathione-related enzymes during recovery. The kinetic studies have demonstrated that the elevation of GSH can affect the activity of GR and GPx (23, 24).
The study has demonstrated two markers of oxidative damage, the thiobarbituric
acid reactive substances (TBARS) and carbonyl groups (PC). Although the plasma
TBARS and PC have been non-specific techniques, using them can offer an empirical
view on the complex process of lipid peroxidation and protein carbonylation.
The enhancement of peroxidation and carbonylation, followed by training or single
exercise, was observed by many authors (25-28). However, our study failed to
show the significant differences between trained and untrained subjects, and
no changes in TBARS and PC after isometric/isokinetic exercise. We conclude
that intensity of the applied isometric/isokinetic exercise was not enough to
promote oxidative damage. Yet, ALA administration caused significant decrease
in TBARS and PC in trained subjects. It has been very interesting that

-lipoic
acid maintained its antioxidant action for 48 h after the last dose of ALA.
The recent studies have proved that thiol compounds can induce some ergogenic effects. Reid
et al. (29) were the first who have shown that thiols improved muscle performance. Then, Medved
et al. (30) and Matuszczak
et al. (31) have revealed that cysteine derivatives application during incremental or isometric exercises delayed muscle fatigue.
The present study has not confirmed an ergogenic action of ALA in athletes performed
isometric/isokinetic exercise. The applied dose of ALA (600 mg
.
d
-1 for 8 days) has shown a tendency to elevate
the level of parameters measured at lower angular velocities by Biodex system.
It is likely that higher value of ALA could induce the significant ergogenic
effects. However, a high content of cell

-lipoic
acid has been related with a risk of pro-oxidant reaction (2). According to
Cakatay (2) a high dose of ALA could cause the removing of iron from the ferritin
and stimulate the auto-oxidation of thiols. This reaction has been additionally
enhanced by ascorbate with ensuing RONS production. Therefore, we have stated
that long-term or high-dose application of ALA should not be recommended for
athletes until the full explanation of the pro-oxidant role of

-lipoic
acid.
In summary, the present investigation has shown that resistance training induced a significant increase in the resting level in glutathione antioxidant system and decrease in total thiols, and no change in lipid peroxidation and protein carbonylation. The eight–day administration with 600 mg a-lipoic acid reduced resting and post-exercise level of oxidative damage but revealed the significant effect on antioxidant glutathione system only after isometric/isokinetic exercise.
Acknowledgments:
We thank Boguslawa Wisniewska and Grzegorz Sniegula for technical and analytical
assistance.
The laboratory where the work was performed: Department of Biochemistry and
Sports Medicine, Faculty of Physical Culture Gorzow Wlkp.
Conflict of interests: None declared.
REFERENCES
- Moini H, Packer L, Saris NEL. Antioxidant and prooxidant of a-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol 2002; 182: 84-90.
- Cakatay U. Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med Hypotheses 2006; 66: 110-117.
- Bilska A. Kwas liponowy. Udzial w metabolizmie oraz mozliwosci farmakologicznego dzialania. In Biotiole w warunkach fizjologicznych, patologicznych i w terapii, L. Wlodek (ed). Wydawnictwo Uniwersytetu Jagiellonskiego, Krakow 2003, pp. 215-240.
- Khanna S, Atalay M, Laaksonen DE, Gul M, Roy S, Sen CK. Lipoic acid supplementation: tissue glutathione homeostasis at rest and after exercise. J Appl Physiol 1999; 86: 1191-1196.
- Williams CA, Hoffman RM, Kronfeld DS, Hess TM, Saker KE, Harris PA. Lipoic acid as an antioxidant in mature thoroughbred geldings: a preliminary study. J Nutr 2002; 132: 1628S-1631S.
- Marsh SA, Laursen PB, Coombes JS. Effects of antioxidant supplementation and exercise training on erythrocyte antioxidant enzymes. Int J Vitamin Nutr Res 2006; 76: 324-331.
- Palaniappan AR, Dai A. Mitochondrial ageing and the beneficial role of a-lipoic acid. Neurochem Res 2007; 32: 1552- 1558.
- Goraca A, Jozefowicz-Okonkwo G. Protective effects of early treatment with lipoic acid in LPS-induced lung injury in rats. J Physiol Pharmacol 2007; 58: 541-549.
- Goraca A, Skibska B. Beneficial effect of a-lipoic acid on lipolysaccharide-induced oxidative stress in bronchoalveolar lavage fluid. J Physiol Pharmacol 2008; 59: 379-386.
- Hebeeb AFSA. Reaction of protein sulfhydryl groups with Ellman’s reagent. Methods Enzymol 1972; 25: 457-464.
- Beutler E, Duron O, Mikus KB. Improved method for determination of blood glutathione. J Lab Clin Med 1963; 61: 882-888.
- Drabkin DL. Spectrophotometric studies. XIV. The crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. J Biol Chem 1946; 164: 703-723.
- Buege J, Aust SD. The thiobarbituric acid assay. In Techniques in Free Radical Research. CA. Rice-Evans, AT. Diplock, MCR. Symons (eds). Elsevier 1991, pp. 147-148.
- Levine RL, Garland D, Oliver CN. Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol 1990; 86: 464-479.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1972; 76: 248-254.
- Gaume V, Mougin F, Figard H et al. Physical training decreases total plasma homocysteine and cysteine in middle-aged subjects. Ann Nutr Metab 2005; 49: 125-231.
- Gaume V, Figard H, Mougin F et al. Effect of a swim training on homocysteine and cysteine levels in rats. Amino Acids 2005; 28: 337-342.
- Tsakiris S, Parthimos T, Parthimos T, Tsakiris T, Schulpis KH. The beneficial effect of L-cysteine supplementation on DNA oxidation induced by forced training. Pharmacol Res 2006; 53: 386-390.
- Kretzschmar M, Muller D. Aging, training and exercise. A review of effect on plasma glutathione and lipid peroxides. Sports Med 1993; 15: 196-209.
- Balakrishnan SD, Anuradha CV. Exercise, depletion of antioxidants and antioxidant manipulation. Cell Biochem Funct 1998; 16: 269-275.
- Busse E, Zimmer G, Schopohl B, Kornhuber B. Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittelforschung 1992; 42: 829-831.
- Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H et al. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors 1997; 6: 321-338.
- Flohe L. Glutathione peroxidase brought to focus. In Free Radical in Biology and Medicine. W. Pryor (ed). New York, Academic Press, vol. 5, 1982, pp. 223-253.
- Sexton DJ, Mutus B. Glutathione reductases from a variety of sources are inhibited by physiological levels of glutathione. Comp Biochem Physiol 1992; 103: 897-901.
- Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc 2000; 32: 1576-1581.
- Cavas L. Does underwater rugby stimulate the over-production of reactive oxygen species? Cell Biochem Funct 2004; 23: 59-63.
- Jammes Y, Steinberg JG, Bregeon F, Delliaux S. The oxidative stress in response to routine incremental cycling exercise in healthy sedentary subjects. Resp Physiol Neurobiol 2004; 144: 81-90.
- McBride JM, Kraemer WJ, Triplet-McBride T, Sebastianelli W. Effect of resistance exercise on free radical production. Med Sci Sports Exerc 1998; 30: 67-72.
- Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 1994; 94: 2468-2474.
- Medved I, Brown MJ, Bjorksten AR, McKenna MJ. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J Appl Physiol 2004; 96: 211-217.
- Matuszczak Y, Farid M, Jones J et al. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve 2005; 32: 633-638.






