This paired group of light-responsive neurons is located in the mediobasal preoptic area at the diencephalic-telencephalic junction just anterior to the hypothalamus. Since these neurons lie immediately above the decussating axons of the optic nerve, i.e., the optic chiasma, they are named the suprachiasmatic nuclei (SCN) (3, 4). The SCN orchestrate all known circadian rhythms in vertebrates and are referred to as the master biological clock or the central rhythm generator.
The paired set of neurons that constitute the SCN exhibits an intrinsic cycle of electrical activity that is not precisely 24 hours in duration (Fig. 1). Rather the period of this rhythm is, in fact, closer to 25 hours (5). Thus, the neural "clock" runs slow. If this rhythm would not be adjusted nearer to a 24-hour cycle, the physiology of the organism would quickly be out of phase with the appropriate environmental time, i.e., the organism would be desynchronized or chronodisrupted (6-8). This assertion is supported by studies of completely blind people, who naturally develop desynchronized - 'free-running' - biological rhythms, which can be entrained by administration of exogenous melatonin (9, 10). This is reminiscent of an individual being in the state of "jet lag." Moreover, this would compromise physiological performance and could have negative health consequences (11-14).
The purpose of the regular light:dark cycle is to more precisely adjust the activity of the central circadian pacemaker, i.e., the SCN, to 24 hours (15, 16). This synchronization requires light perception by highly specialized intrinsically photosensitive retinal ganglion cells (ipRGC) (17-19). Thus, the regulation of the SCN does not rely on the conventional photoreceptor cells of the retina, i.e., the rods and cones.
The ipRGC that are involved in synchronizing the neural clock make up only a small percentage (1-2%) of the total ganglion cell population and they contain their own specialized photopigment, melanopsin (20-22). Melanopsin is further specialized in that it does not respond to all visible wavelengths equally, but rather especially those in the blue range, roughly 460-480 µm wavelength of light (23-25).
The axons of the ip RGC, rather than centrally projecting to the lateral geniculate nuclei and other visual relay centers, convey their information primarily, but not exclusively, to the SCN (26-28). The axons of these neurons travel in the optic nerve to the level of the optic chiasm where they then diverge to penetrate the SCN where they make synaptic contact with clock neurons. It is via this neural pathway, referred to as the retinohypothalamic tract (RHT), that the light:dark cycle adjusts the activity of the clock to near 24 hours, rather than letting it run with a 25-hour period.
The molecular aspects of the circadian clock have been, in part, elucidated. Each neuron in the SCN has the necessary molecular machinery for generating circadian rhythmicity (29, 30). The neuronal clock includes CLOCK and BMAL1 which dimerize and then bind to the specific promoter region of the Per and Cry genes leading to their rhythmic transcription (31-33). Once translation occurs, the PER and CRY proteins also form dimers that translocate to the nucleus where they suppress the coupling of CLOCK and BMAL1; this reduces the further transcription of Per and Cry genes.
Other clock-regulated genes are also influenced by the intrinsic molecular machinery which contributes to the generation of circadian rhythmicity. The fluctuations in central clock neurons are neural or humorally communicated to all peripheral tissues whose cells also possess molecular clocks (35-37). In this way the central circadian pacemaker, i.e., the SCN, regulates circadian rhythmicity in all peripheral tissues. When the peripheral oscillators become disordered from the central clock, circadian disruption (chronodisruption) results.
To keep cellular rhythms in the neural clock in synchrony, the system requires regular input from the ipRGC. Thus, a major, albeit not the only synchronizer (zeitgeber) of the neurons in the SCN, is the regular alternating intervals of light and darkness (38, 39). When the photoperiodic environment is artificially perturbed, e.g., with light exposure during the normal dark period, the central circadian pacemaker receives inappropriate information for that time, and melatonin suppression and circadian disruption results (5, 11, 36).
The pineal gland is one of the organs that receives a neuronal input from the SCN. Thus, axons of the pacemaker neurons in the central clock project, among other places, to the paraventricular nuclei of the hypothalamus. Fibers from these neurons descend through the brain stem presumably without making synaptic contact with other cells until their arrival at the intermediolateral cell column of the upper thoracic cord (40); these are preganglionic sympathetic neurons. After synapsing with these cells, the preganglionic axons exit the spinal cord and pass up the sympathetic chain to the superior cervical ganglia where the final synapse is made. Axons of the postganglionic neurons accompany blood vessels to the pineal gland (41).
Within the pineal gland, the postganglionic fibers end in close proximity to the pinealocytes, the melatonin-producing cells of the gland (42). During darkness, the SCN sends a neural impulse which causes the discharge of norepinephrine from the postganglionic terminals near the pinealocytes. The catecholamine acts primarily on conventional ß-adrenergic receptors on the pinealocyte membranes; this action culminates in a series of molecular events that induce the night-related rise in pineal melatonin synthesis and release (43, 44). The association of the ipRGCs, the SCN, the peripheral nervous system, and the pineal gland are diagrammatically depicted in Fig. 1.
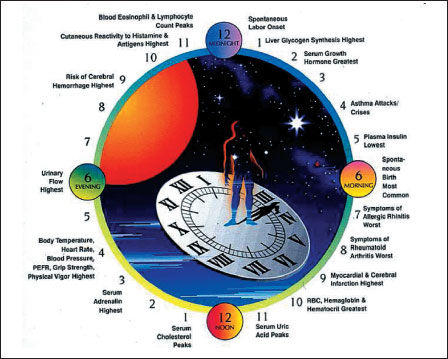 |
Fig. 1. Various physiological states and pathological events occur most frequently at particular times of day, demonstrating a biological rhythmicity. Melatonin is the major regulatory hormone of circadian rhythm. |
The induction of nocturnal pineal melatonin synthesis is accompanied by a rapid discharge of the indoleamine from the pinealocytes into the capillaries that perfuse the gland as well as into the cerebrospinal fluid of the third ventricle the brain. As a consequence, as pineal melatonin production increases likewise the levels of this indoleamine rise in these fluids (45-47). Thus, the circadian rhythm of melatonin is inextricably linked to the prevailing light: dark environment. Interruption of the neural pathway bilaterally at any point between the SCN and the pineal gland destroys the circadian melatonin rhythm and renders the pineal gland physiologically inept (48).
As mentioned above, light pulses during darkness cause misinformation to be sent to the SCN; in turn, the clock neurons pass this information to the pinealocytes via the complex pathway described above, and pineal melatonin synthesis and secretion cease (49). This results in a rapid drop in circulating melatonin levels at a time they should be elevated. As a result, this disturbance of the circadian melatonin cycle is provided to all cells in the periphery giving these cells the wrong time of day information and contributing to chronodisruption and pathophysiology (51-54).
It is only after the invention of the artificial light that the regularly alternating periods of light and darkness could be seriously disturbed. The common use of artificial light after darkness onset, a situation that is especially prominent in the well-developed countries but that is also becoming usual in virtually all societies, provides the circadian system with a great deal of misinformation to which it responds.
In reference to the nocturnal rise in melatonin, light during the normal dark period has two major consequences. When the daily light period is extended into the night or when the period of darkness is prematurely terminated by early light exposure in the morning, the nocturnal period of melatonin production is abbreviated. Likewise, if during the night individuals are acutely exposed to light, these photons signal the ipRGCs to shut down pineal melatonin production. In both of these scenarios, i.e., extension of light into the dark period or acute light exposure at night, the circadian system is upset and melatonin production and secretion is compromised. As a consequence, what is referred to as the misuse of light has rendered humans relatively melatonin deficient (when light is extended into the night) or it causes the perturbed melatonin rhythm (when light occurs acutely at night) to provide incorrect circadian information to all cells capable of "reading" the melatonin message, and many (possibly all) cells do.
As noted above, the artificial extension of the daily light period into the night with the use of manufactured light or, conversely, being exposed to light in the early morning before sunrise, shortens the dark interval and thereby reduces the total amount of melatonin produced without actually changing its rhythm (55), i.e., low blood levels during the day and elevated values at night. Thus, the circadian message that the melatonin rhythm imparts to the cells throughout the organism under these conditions is not lost. Nevertheless, there is a reduction in the total amount of melatonin generated (a relative melatonin deficiency) during each 24-hour interval, at least based on blood melatonin levels. In this case, chronodisruption is presumably not a consideration but the reduced amount of melatonin likely is.
Even independent of the relative melatonin deficiency that occurs when the night is shortened is the abbreviated duration of sleep. Ample sleep is an important feature of optimal physiology during the waking hours (56). In the US, the duration of sleep (and the associated period of daily dark exposure) has decreased steadily over the last five decades from roughly 8 hours in the 1960s to 6.5 hours in the early 21st century (57, 58). The resulting sleepiness that is a consequence of the sleep debt these individuals experience is estimated to have a significant negative impact on work efficiency and productivity and, as a result, the cost to industry is surely in the range of millions of dollars annually. Additionally, the health effects of inadequate sleep are also a major concern. It seems intuitive that the failure to get adequate sleep, especially when extended over time, may well lead to altered physiology and comprised health which, of course, impacts life quality (59).
In addition to any pathophysiologies that may develop as a result of reduced daily sleep intervals is the associated deficiency of melatonin that occurs as a consequence of extended daily light periods. Given the multiple functions of melatonin (60, 61) and the fact that it and its metabolites fight against free radicals and the molecular damage that they mete out (62-65), the reduction in melatonin associated with artificially-induced long days would surely enhance the degree of oxidative damage that cells incur (66). Conquering free radicals, which are produced as a consequence of normal cellular metabolism especially at the mitochondrial level, is essential for maintaining good health (63, 67).
Melatonin is a multifacted antioxidant which, even at physiological levels, engages free radicals and related toxic species, i.e., reactive oxygen and reactive nitrogen intermediates, to detoxify them before they inflict damage. By reducing the length of the daily dark period and consequently simultaneously limiting the amount of pineal melatonin produced on a daily basis is, in a sense, reminiscent of what also occurs in advanced age. As organisms grow old, endogenous melatonin production wanes judging from the reduced nocturnal melatonin rise (68-70). The loss of this protective molecule allows free radicals, which are believed to be produced at an increasingly more rapid rate in aged and less optimally functioning cells (71), to go unchallenged thereby elevating the damage they are capable of inflicting. Certainly, supplementing aged animals with extra melatonin readily reduces the accumulated free radical-damaged cellular debris (72-74). Predictably, this would also curtail the development of disease states. The parallels between aging and prematurely reducing total melatonin production by excessive light exposure would be expected to cause similarly elevated free radical-mediated cellular damage. One implication of this is that disease processes related to oxidative stress which normally are most common in advanced age may in fact develop earlier in life. Experimentally, giving animals extra melatonin after middle age has been shown to defer the development or severity of age-related diseases (72, 75).
Interrupting the dark period with acute light exposure not only rapidly reduces pineal melatonin synthesis as well as lowering its levels in the blood, this abnormal light exposure also leads to severe chronodisruption (6). When nighttime melatonin levels are high, exposure of humans or animals to bright and wave length appropriate light (460-480 mm light) causes a rapid drop in melatonin (23, 29). Given that the regular circadian production of pineal melatonin is a reflection of the activity of the SCN, then it is obvious that light during the normal dark period imposes a marked misalignment of the central circadian pacemaker and the peripheral cellular clocks. This creates a situation that surely must lead to malfunctions at the cellular and tissue levels (76-79). Certainly, chronodisruption in terms of the potential negative consequences it has for optimal health has attracted a great deal of investigative interest within the last decade. It seems likely that disease states as diverse of metabolic syndrome and psychological depression may be consequences of circadian misalignment (80-83).
One particular disease that has frequently been discussed relative to excessive or abnormal light exposure is cancer (51, 84-87). There is a vast amount of literature suggesting that the photoperiodic environment influences the incidence of cancer. While an association of chronodisruption was initially proposed only for breast cancer, we recently suggested a more general theory, i.e., chronodisruption may aggravate the development of many cancer types (52). Melatonin demonstrates dose-dependent inhibition on the growth of MCF-7 breast cancer cells. In fact, the International Agency for Research on Cancer has classified light at night as a Group 2A carcinogen, i.e., a probable carcinogen in humans (51). The elevated cancer incidence that is believed to be a result of light exposure at night could be a consequence of, i), a depression in melatonin, ii), chronodisruption or, iii), both of these. Certainly, many scientific publications have documented that melatonin is oncostatic and, likewise, perturbations of circadian rhythmicity also lead to accelerated tumor growth (51, 81, 82).
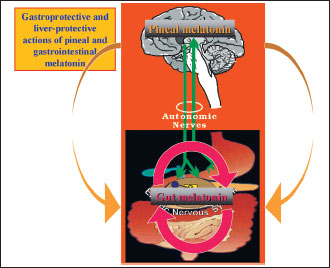 |
Fig. 2. In addition of pineal melatonin released on circadian manner, about 500 times of this indoleamine is generated and released by the EE cells of the gastrointestinal tract (GIT) to serve as local protector of the GIT mucosa and the liver (Adapted with permission from ref. 89). |
Melatonin also plays an important role in regulation (partly via the brain gut axis) and in direct protection of the mucosa of gastrointestinal tract (GIT) being generated and released by the EE cells of the GIT in enormous amounts to.portal circulation to protect the liver and biliary tract from various irritants.
It has repeatedly been found to provide gastroprotective effects against ethanol, cold-stress, asprin, and ischemia-reperfusion induced gastric lesions (86, 87). A three-month human trial has revealed that oral administration of melatonin, combined with modification of diet and exercise, decreased plasma liver enzyme levels in NASH (nonalcoholic steatohepatitis) patients greater than diet and exercise alone (88). Another recent human trial has found that administration of exogenous melatonin was successful in attenuating proinflammatory cytokine levels (89) (Fig. 2).
A large number of scientists seem to have come to the realization that light exposure at inappropriate times, i.e., at night, may not be as innocuous as assumed. Again, the reader is reminded that throughout evolution all animals including humans were exposed to regularly repeating periods of light and darkness. This cycle induced the evolution of a master clock in the brain so as to use that information for the physiological well being of the organism. With the invention of the light bulb by Thomas Alva Edison in 1879, the lighting environment in which we live changed drastically. What is now best described as light pollution has significantly impacted both physiology and behavior. Leisure activities and work schedules have spread throughout the 24 hours. This has led to severe disruptions of circadian rhythms at both the cellular and organismal levels. Likewise, the quantity of melatonin produced on a daily basis has been substantially depleted. Such perturbations are likewise not physiologically trivial and to consider these perturbations to be insignificant would seemingly be naive considering what we currently know about the significance of circadian rhythmicity and of the importance of melatonin.
Circadian rhythmicity is an integral component of normal and optimal physiology. Not only the SCN, but cells throughout the organism are equipped with internal clocks that control the molecular events that occur during each 24-hour period. These events appear to be precisely timed and in modern societies the intrinsic time keepers are provided with misinformation which, when prolonged, likely has ramifications in terms of increased pathologies. It would seem judicious to take better care of the circadian network by providing appropriate photoperiodic cues, which currently seemed to be almost totally ignored. In addition, large amounts of this indoleamine is produced in the mucosa of GIT that seems to serve as local antioxidant and protective substance for the gut and liver against a variety of noxious agents, particularly the bacteria and their toxins, introduced into the gut with each meal.
Acknowledgements: A preliminary raport of this work was presented at the International Symposium on Biological Clocks: Pathophysiology and Clinical Aspects, Cracow, Poland, June 25th, 2010.
Conflicts of interest: None declared.
- Mazzoccoli G. The timing clockwork of life. J Biol Regul Homeost Agents 2011; 25: 137-143.
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 2011; 108: 1657-1662.
- Schwartz WJ. Circadian rhythms: a tale of two nuclei. Curr Biol 2009; 19: R460-R462.
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 2010; 72: 551-577.
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery in health and disease. Nat Rev Neurosci 2003; 4: 649-661.
- Erren TC, Reiter RJ. Defining chronodisruption. J Pineal Res 2009; 46: 245-247.
- Green CB, Takahashi J, Bass J. The meter of metabolism. Cell 2008; 134: 728-742.
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system. Annu Rev Physiol 2010; 72: 517-549.
- Sack RL, Lewy AJ, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab 1992; 75: 127-134.
- Sack RL, Brandes RW, Kendall AR, Lewry AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Eng J Med 2000; 343: 1070-1077.
- Barnard AR, Nolan PM. When clocks go bad: neurobehavioral consequences of disrupted circadian timing. PLoS Genet 2008; 4:e1000040.
- Rüger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord 2009; 10: 245-260.
- Erren TC, Pape HC, Reiter RJ, Piekarski C. Chronodisruption and cancer. Naturwissenschaften 2008; 95: 357-382.
- Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocr Metab 2007; 18: 4-11.
- Challet E. Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007; 148: 5648-5655.
- Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int 2001; 18: 801-808.
- Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol 2011; 519: 1492-1504.
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev 2010; 90: 1547-1581.
- Ecker JL, Dumitrescu ON, Wong KY, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 2010; 67: 49-60.
- Davies WL, Foster RG, Hankins MW. Focus on molecules: melanopsin. Exp Eye Res 2010; Aug 7: (epub ahead of print).
- Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci 2008; 31: 27-36.
- Vandewalle G, Schmidt C, Albouy G, et al. Brain responses to violet, blue and green monochromatic light exposures in humans: prominent role of blue light and the brain stem. PLoS One 2007; 2: e1247.
- Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol 2007; 27: 195-204.
- Canteros NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, Swanson LW. The retinohypothalamic tract: comparison of axonal projection patterns from four major targets. Brain Res Rev 2011; 65: 150-183.
- Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience 2006; 137: 1285-1297.
- Moore RY, Speh JC. Serotonin innervation of the primate suprachiasmatic nucleus. Brain Res 2004; 1010: 169-173.
- Grone BP, Chang D, Bourgin P, et al. Acute light exposure suppresses circadian rhythms in clock gene expression. J Biol Rhythms 2011; 26: 78-81.
- Maywood ES, O'Neill JS, Reddy AB, et al. Genetic and molecular analysis of the central and peripheral circadian clockwork of mice. Cold Spring Harb Symp Quant Biol 2007; 78: 85-94.
- Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008; 134: 317-328.
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 2008; 320: 949-953.
- Maywood ES, Chesham JE, Meng QJ, Nolan PM, Loudon AS, Hastings MH. Tuning of the period of the mammalian circadian clock: additive and independent effects of CK, 1e Tau, and Fbx13Afh mutations on mouse circadian behavior and molecular pacemaking. J Neurosci 2011; 31: 1539-1544.
- Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. Circadian clocks are resounding in peripheral tissues. PLoS Comput Sci 2006; 2: e16.
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 2010; 72: 517-549.
- Gerstner JR. The aging clock: 'BMAL'icious toward learning and memory. Aging 2010; 2: 251-254.
- Farnell YF, Shende VR, Neuendorff N, Allen GC, Earnest DJ. Immortalized cell lines for real-time analysis of circadian pacemaker and peripheral oscillator properties. Eur J Neurosci 2011; 33: 1533-1540.
- Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Invest Med 2001; 49: 30-40.
- Hanifin JP, Brainard GC. Photoreception for circadian, neuroendocrine and neurobehavioral regulation. J Physiol Anthropol 2007; 26: 87-94.
- Smale L, Cassone VM, Moore RY, Morin LP. Paraventricular nucleus projects mediating pineal melatonin and gonadal responses to photoperiod in the hamster. Brain Res Bull 1989; 22: 263-269.
- Moore RY. Neural contral of pineal function in mammals and birds. J Neural Transm 1978(Suppl): 47-58.
- Karasek M. Ultrastructure of the mammalian pineal gland: its comparative and functional aspects. Pineal Res Rev 1983; 1: 1-48.
- King TS, Steinlechner S. Pineal indolalkylamine synthesis and metabolism: kinetic considerations. Pineal Res Rev 1985; 3: 69-114.
- Maronde E, Saade A, Ackermann K, et al. Dynamics in enzymatic protein complexes offer a novel principle for the regulation of melatonin synthesis in the human pineal gland. J Pineal Res 2011; 51: 145-155.
- Vaughan GM, Pelham RW, Pang SF, et al. Nocturnal elevation of plasma melatonin and urinary 5-hydroxyindoleacetic acid in young men: attempts at modification by brief changes in environmental lighting and sleep and by autonomic drugs. J Clin Endocrinol Metab 1976; 42: 752-764.
- Tricoire H, Locatelli A, Chemineau P, Malpaux B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 2002; 143: 84-90.
- Tan DX, Manchester LC, Sanchez-Barcelo E, Mediavilla MD, Reiter RJ. Significance of high levels of endogenous melatonin in mammalian cerebrospinal fluid and in the central nervous system. Curr Neuropharmacol 2010; 8: 162-167.
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 1991; 12: 151-180.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210: 1267-1269.
- Stevens RG, Blask DE, Brainard GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect 2007; 115: 1357-1362.
- Erren TC, Reiter RJ. A generalized theory of carcinogenesis due to chronodisruption. Neuroendocrinol Lett 2008; 29: 815-821.
- Filipski E, Levi F. Circadian disruption in experimental cancer processes. Integr Cancer Ther 2009; 8: 299-302.
- Reiter RJ, Tan DX, Sanchez-Barcelo E, Mediavilla MD, Gitto E, Korkmaz A. Circadian mechanisms in the regulation of melatonin synthesis: disruption with light at night and the pathophysiological consequences. J Exp Integr Med 2011; 1: 13-22.
- Wehr TA. The duration of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrinol Metab 1991; 73: 1276-1280.
- Gallup Organization. Sleep in America. Princeton, WJ, Gallup Organization, 1995.
- Kripke D, Simons R, Garfinkel L, Hammond E. Short and long sleep and sleeping pills: is increased mortality associated? Arch Gen Psychiatr 1979; 36: 103-116.
- National Center for Health Statistics. Quick stats: percentage of adults who report an average of 6 hours of sleep per 24 hour period by sex and age group - United States, 1985-2004. Biol Psychiatr 2000; 47: 921-927.
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med 2008; 9(Suppl 1): S23-S28.
- Reiter RJ. Melatonin: that ubiquitously-acting pineal hormone. Neus Physiol Sci 1990; 6: 223-227.
- Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res 2010; 181: 127-151.
- Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1993; 1: 57-60.
- Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res 2010; 48: 297-310.
- Jou MJ, Peng TI, Hsu LF, et al. Visualization of melatonin's multiple mitochondrial levels of protection against mitochondrial Ca2+ mediated permeability transition and beyond in rat brain astrocytes. J Pineal Res 2010; 48: 20-38.
- Mukherjee D, Roy SC, Bandyopadhyay A, et al. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J Pineal Res 2010; 48: 251-262.
- Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol 2009; 44: 175-200.
- Acuna-Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem 2011; 11: 221-240.
- Reiter RJ, Richardson BA, Johnson LY, Ferguson BN, Dinh DT. Pineal melatonin rhythm: reduction in aging Syrian hamsters. Science 1980; 210: 1372-1373.
- Reiter RJ, Craft CM, Johnson LY, et al. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology 1981; 109: 1295-1297.
- Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM. Human melatonin production decreases with age. J Pineal Res 1986; 3: 379-388.
- Harman D. Aging: overview. Ann NY Acad Sci 2001; 928: 1-21.
- Chahbouni M, Escames G, Venegas C, et al. Melatonin treatment normalized plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res 2010; 48: 282-290.
- Akbulut KG, Gonul B, Akbulut H. Exogenous melatonin decreases age-induced lipid peroxidation in the brain. Brain Res 2008; 1238: 31-35.
- Molpeceres V, Mauriz JL, Garcia-Mediavilla MV, Gonzalez P, Barrio JP, Gonzalez-Gallego J. Melatonin is able to reduce the apoptotic liver changes induced by aging via inhibition of the intrinsic pathway of apoptosis. J Gerontol A Biol Sci Med Sci 2007; 62: 687-695.
- Cardinali DP, Furio AM, Brusco JI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Curr Neuropharmacol 2010; 8: 218-227.
- Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308: 1043-1045.
- Garaulet M, Madrid JA. Chronobiology, genetic and metabolic syndrome. Curr Opin Lipidol 2009; 20: 127-134.
- Reiter RJ, Tan DX, Korkmaz A. The circadian melatonin rhythm and its modulation: possible impact in hypertension. J Hypertens (Suppl) 2009; 27: S17-S20.
- Reiter RJ, Tan DX, Korkmaz A, Ma S. Obesity and metabolic syndrome: association with chronodisruption, sleep deprivation and melatonin suppression. Ann Med 2011; Jun 13: (epub ahead of print).
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res 2010; 49: 14-22.
- Jung-Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res 2010; 48: 9-19.
- Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol 2011; 62: 13-19.
- Bron R, Furness JB. The rhythm of digestion: keeping time in the gastrointestinal tract. Clin Exp Pharmacol Physiol 2009; 36: 1041-1048.
- Reiter RJ, Tan DX, Korkmaz A, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncogen 2007; 13: 303-328.
- Blask DE. Melatonin, sleep disturbances and cancer risk. Sleep Med Rev 2009; 13: 357-364.
- Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther 2009; 8: 337-346.
- Wood PA, Yang X, Hrushesky WJ. Clock genes and cancer. Integr Cancer Ther 2009; 8: 301-308.
- Reiter RJ, Tan DX, Erren TC, Fuentes-Broto L, Paredes SD. Light-mediated perturbations of circadian timing and cancer risk: a mechanistic analysis. Integr Cancer Ther 2009; 8: 354-360.
- Brzozowski I, Ptak-Belowska A, Pawlik M, et al. Mucosal strengthening activity of central and peripheral melatonin in the mechanism of gastric defense. J Physiol Pharmacol 2009; 60: 47-56.
- Konturek PC, Celinski K, Slomka M, et al. Melatonin and its precursor L-tryptophan prevent acute gastric mucosal damage induced by aspirin in humans. J Physiol Pharmacol 2008; 59(Suppl 2): 67-75.
- Gonciarz M, Gonciarz Z, Bielanski W, et al. The pilot study of a 3-month course of melatonin treatment of patients with nonalcoholic steatohepatitis: Effect on plasma levels of liver enzymes, lipids and melatonin. J Physiol Pharmacol 2010; 61: 705-710.
- Konturek SJ, Konturek PC, Brzozowski T, Bubenik GA. Role of melatonin in the upper gastrpomtestinal tract. J Physiol Pharmacol 2008; 58(Suppl 6): 23-52.