Presence of autoantibodies against cytoplasmic
proteins of neutrophil (c-ANCA) is a common finding in human diseases characterized
by vasculitis. The main target for c-ANCA is proteinase-3 (PR3), a serine proteinase
found in azurophili granules and secretory vesicles of the neutrophil (1). In
patients with some autoimmune diseases like granulomatosis with polyangitis
(Wegener's), PR3 expression on the neutrophil surface is high and correlates
with disease severity (2). In healthy subjects expression of PR3 on resting
neutrophil is low, medium or bimodal when both types of expression are present,
but is inducible by exposure of neutrophil to proinflammatory cytokines,
e.g.
tumor necrosis factor (TNF-

).
This causes translocation of PR3 to the cell membrane, where it becomes available
for c-ANCA binding (3). Neutrophil is well known as an important player in inflammation,
but recent studies show that their role extends well beyond the traditional
function as a professional phagocyte. It has been shown that under appropriate
experimental conditions, human neutrophil can synthesize and secret a number
of chemokines such as CXCL8, CXCL1, IP-10, MIG or MIP-1ß, which not only
can modulate neutrophil behavior in an autocrine or paracrine action, but can
also promote other immune cells activation and recruitment (4).
The classical model of ANCA-associated neutrophil activation assumes direct
recognition of PR3
via Fab region of cANCA antibody and interaction of
the Fc part of the antibody with immunoglobulin gamma receptors (Fc

Rs)
on neutrophil surface. This causes neutrophil activation, degranulation, generation
of reactive oxygen intermediates and finally transmigration through the endothelial
cell layer of the vessel (5). Although there is a lot of evidence supporting
this mechanism, some important questions still remain unanswered. Development
of new molecular biology techniques such as microarrays can contribute to better
understanding of this processes. Despite the fact that some whole blood gene
expression studies in patients with several types of ANCA-associated vasculitides
have been already performed, none of them focused on specific neutrophil gene
profile following c-ANCA stimulation (6). The disease is difficult to treat
and may follow a rapidly progressing pulmonary-renal syndrome with alveolar
haemorrhage and necrotizing glomerulonephritis (7). Any pharmacological targets
in Wegener's granulomatosis, including molecules up-regulated by anti-PR3 autoantibodies
in neutrophils, are of importance for development of new therapeutic strategies
for the disease.
MATERIALS AND METHODS
Immunoglobulin G (IgG) purification
Antibodies were extracted from pooled serum samples stored at the collection of the local diagnostic laboratory as reference sera. Their originated from six well defined patients suffering granulomatosis with polyangiitis (anti-PR3 IgG>200 mU/L; anti-MPO<20 mU/L). The total IgG fraction was purified by ammonium sulfate precipitation followed by removal of other proteins using negative affinity adsorption (Melon Gel IgG Purification kits, Thermo Scientific, Rockford, USA). To remove a possible endotoxin contamination, samples were cleaned up using AffinityPak Endotoxin Removal Column (Pierce, Rockford, USA). Purity of IgG samples was assessed by SDS-PAGE electrophoresis. Concentration of total IgG following extraction and purification was determined by immunonephelometry (Siemens Dade Behring BN II Nephelometer, Munnich, Germany) and specific anti-PR3 IgG level was assessed by ELISA (anti-PR3 ELISA kit, EUROIMMUN Medizinische Labordiagnostika, Luebeck, Germany).
Neutrophil-enriched granulocyte isolation and stimulation
The study received ethical approval from the Bioethical Committee of Jagiellonian
University. For enrollment of healthy blood donors, informed consent was obtained,
and this non-interventional phase 1
in vitro study was performed in respect
to Declaration of Helsinki considering confidentiality and lack of interest
conflicts. Granulocytes were isolated from citrated blood of healthy donors
(n=12, average age 30 years, 2 males and 10 females ) using dextran sedimentation
and Histopaque (Sigma-Aldrich Chemical Co, St Louis, USA) centrifugation followed
by hypotonic lysis of erythrocytes. Purity of neutrophil fraction was determinated
by flow cytometry (>98%) and cells
viability was verified by tryphan
blue exclusion staining (>95%). Immediately after isolation, granulocytes were
resuspended in Hanks balanced salts solution (HBSS) with calcium and magnesium
containing 5% fetal bovine serum. Before stimulation experiments, neutrophils
were primed with 2 ng/mL recombinant TNF-a (R&D Systems, Minneapolis, MN, USA)
for 15 minute at 37°C. Primed neutrophils (3.5x10
6/well)
were incubated with purified human IgG fraction containing native anti-PR3 (200
µg/ml) for 4 h at 37°C. To evaluate gene expression in neutrophils after anti-PR3
IgG stimulation, six independent experiments were performed. In each experiment
neutrophils simulations were made in duplicates for two donors in parallel.
RNA isolation, reverse transcription and genes expression
Total cellular RNA was isolated using total RNA kit (A&A Biotechnology, Gdynia,
Poland) as recommended by the manufacturer. Reverse transcription was done using
high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City,
CA, USA). cDNA from each experiment was pooled for two donors, two duplicates
each, and relative expression of specific mRNA for genes studied was quantified
using two low density expression arrays by 5'nuclease assay (TaqMan Low-density
array inflammation panel; custom made panel - Applied Biosystems) using 7900HT
fast real time PCR system (Applied Biosystems). Data were normalized to ribosomal
18S rRNA used as the endogenous control. Relative quantities were calculated
with use of 2-

 Ct
Ct
method, which reflects the ratio between abundance of transcripts after stimulation
to the one at base. Two other transcripts,
i.e. glyceraldehyde dehydrogenase-
GAPDH
and beta-actin -
ACTB might be used as supplementary internal controls
but showed greater variability than 18S rRNA control. Results were presented
in comparison to TNF-

primed but non anti-PR3 stimulated granulocytes.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4.0 package (GraphPad
Software Inc, San Diego, CA). Because of small size of the studied group and
distribution of variables data which departed from the normal one, all comparisons
were done using Wilcoxon's signed rank test for between the groups and Mann-Whitney's-U
test for paired ones. For the same reason descriptive statistics was presented
as medians and interquartile ranges (25
th-75
th
percentile) of the fold-change in mRNA abundance. A conventional heat map of
genes activated in cells by anti-PR3 was assembled using Matrix2png software,
a freely available internet a tool for visualization of matrix data (8).
RESULTS
We stimulated TNF-

primed neutrophil-enriched healthy donors granulocytes with anti-PR3. These
was a purified IgG fraction obtained from clinically diagnosed patients affected
by c-ANCA positive granulomatosis with vasculitides syndrome and verified for
the presence of native anti-PR3 idiotype but negative for anti-MPO. Control
experiments did not reveal any activity of the IgG fraction obtained by the
same method from the sera of healthy donors, using the same experimental setup.
Following exposure of the cells to specific c-ANCA for 4 h we used a commercial
TaqMan low-density array to analyze expression of 147 genes involved in several
pathways of inflammatory and immune response. We chose a single 4 hour time
point on the basis of initial experiments (data not shown) and a literature
queries on genes' expression profiles. Out of 147 measured transcripts, 128
genes expressed detectable levels of mRNA, while only 19 were not detectable
or showed very low expression detected in some donors. By comparison of the
expression profile between anti-PR3 IgG stimulated and non-stimulated cells,
we observed up-regulation (>2 fold change in mRNA abundance) of 13 genes (
CCL2,
CXCL2, VCAM1, MMP9, PLCB4, PDE4C, PLA2G4C, RAC1, RHOA, IRAK1, CACNA1D, CACNB2,
PTGDR), further 11 genes were up-regulated only in some donors (
IL13,
PF4, IL2RG, ITGB1, CD83, PLA2G7, ALOX12, AXNA1, AXNA5, LTA4H, MCR2) yet
two others (
HRH3 and
PLA2G2D) were up-regulated in a few samples
and undetectable in others (
Fig. 1). Full list of analyzed genes and
their corresponding protein products are presented in a
Table 1 along
with relative changes in their expression.
 |
Fig. 1. Gene expression profile
in neutrophil-enriched granulocytes stimulated with anti-PR3 IgG. Green
color represents down-regulated genes, black color - genes with no change
of expression and red color up-regulated genes. Undetectable genes were
marked in white. To ensure that observed gene activation was specific
for anti-PR3 neutrophil activation, similar experiments with IgG isolated
using the same method from healthy volunteer were performed. No activation
of neutrophils was observed following exposure to these IgG preparations
(data not shown). Measurements were done on the cells pooled from two
healthy, anti-PR3 negative donors. |
| Table 1. List of
analyzed genes and their relative mRNA abundance changes following incubation
of neutrophils with purified anti-PR3 (* significantly up-regulated genes,
p<0.05, Wilcoxon signed rank test). |
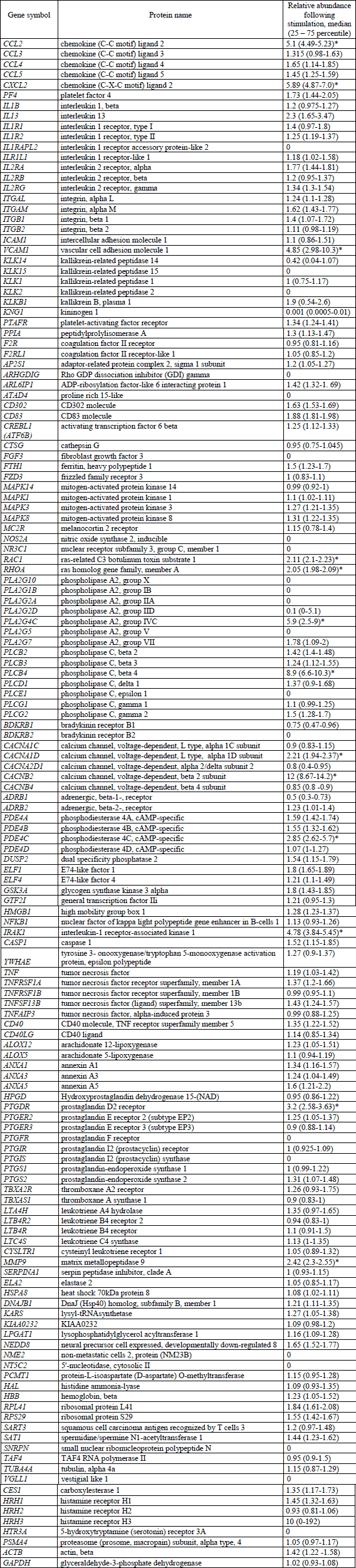 |
DISCUSSION
Polymorphonuclear leukocytes (neutrophils) are pivotal as an component of innate
immune system. In this preliminary study we aimed to examine a molecular background
of c-ANCA associated neutrophil activation. The majority of the studies addressing
c-ANCAs induced neutrophil activation focused on reactive oxygen species production,
chemotaxis and release of cytokines and chemokines. However, a little is still
known about molecular mechanisms involved in regulation of neutrophils activation.
Classical pathway of c-ANCA neutrophil activation assumes that both fragments
of IgG, Fab binding to PR3 and Fc activating the immunoglobulin Fc

Rs,
are required for complete granulocyte activation. Neutrophil predominantly expresses
two types of Fc

Rs:
RIIa (CD32) and RIIIb (CD16b), which have a different affinity for specific
subclasses of IgG (9). Because PR3 is a protein not having a transmembrane domain
(10), neutrophil activation caused by anti-PR3 Fab binding requires probably
association with another, still unknown membrane docking protein. According
to the current knowledge, the interaction between IgG and Fc

Rs
leads to activation of several pathways, like calcium signaling, phosphatidylinositol
3-kinase AKT and MAPK signaling pathways activation. Up-regulation of some genes
like
CACNA1D,
CACNB2 or
RAC1,
RHOA and
IRAK1
observed by us supports this findings, however up-regulation of
PDE4C, PTGDR
or
PLCB4 suggests that G-protein signaling system may also contribute
to the signal transduction in c-ANCA mediated neutrophil activation. In addition,
we observed an increase in the expression of NF-

B-dependent
proinflammatory chemokines
CCL2I and
CXCL2 (11). It is of interest,
that if confirmed by the chemokines measurements in serum of patients or the
culture supernatant, this would strongly suggest, that activated neutrophil
can participate in further recruitment of other inflammatory cells, like monocytes
(12). A bioinformatic database search (13) on the transcipts consistently up-regulated
in neutrophils by the native anti-PR3 IgG showed their clustering into five
major functional pathways of: chemokine signaling (
PLCB4, CCL2, CXCL2, RAC1,
RHOA; p<0.001), leukocyte transendothelial migration (
VCAM1, MMP9, RAC1,
RHOA; p=0.0017), vascular smooth muscle contraction (
PLCB4, RHOA, CACNA1D;
p=0.023), neurotrophin signaling pathway (
IRAK1, RAC1, RHOA, p=0.028)
and Wnt signaling pathway (
PLCB4, RAC1, RHOA; p=0.04). Among these, chemokine
signaling, leukocyte transendothelial migration and vascular smooth muscle contraction
are known to contribute in granulomatosis with polyangiitis. Out of the four
transcripts, not linked to the pathways, cytosolic phospholipase A
2
(
PLA2G4C) and prostaglandin D
2 receptor
(
PTGDR) are related to prostaglandin D
2
mediated vasoconstriction and vascular leak, while voltage dependent calcium
channel subunit 2 (
CACNB2) and phosphodiesterase 4C (
PDE4C) have
direct impact on activation status of neutrophil by mediating calcium entry
and decreasing cytosolic cAMP level. It was recently demonstrated, that superoxide
anion radical mimics vasoconstriction induced by activated neutrophils (14).
Oxidative burst is one of the main innate defense mechanism of neutrophils against
bacterial pathogens. Thus, up-regulation of
CACNB2 and
PDE4C in
parallel with pro-inflammatory signaling would suggest, that anti-PR3 can sensitize
neutrophil to oxidative burst response.
Circulating neutrophils are the major source of matrix metaloproteases (MMPs),
however,
MMP9 gene is expressed rather during the early stage of neutrophil
maturation, and synthesized MMP9 protein is stored in cytoplasmic granules (15).
However, Nagaoka
et al. showed that under specific inflammatory conditions,
MMP9 expression level can increase in neutrophils due to
de novo production
following the gene activation (16). Our results seem to support these observation.
In our experiments, anti-PR3 stimulated neutrophils showed increased levels
of MMP9 mRNA.
In addition to up-regulation of genes involved into known Fc

Rs-dependent
signaling cascades, we detected an increased expression of vascular cell adhesion
molecule-1 (VCAM-1) transcripts. Vascular cell adhesion molecule-1 is expressed
on activated endothelial cells (17, 18) and further upregulated by proinflammatory
stimuli, reactive oxygen species, or by anti-PR3 antibodies. By the interaction
with integrin receptors present on neutrophil surface, VCAM-1 can initiate both
rolling-type and a firm adhesion interactions between leukocytes and endothelia
(19). Our observation, that neutrophils are also capable of expression of mRNA
for VCAM-1 is surprising, and suggests that docking of granulocyte to vascular
endothelial cells may be reinforced upon specific anti-PR3 activation of the
granulocyte itself, allowing for cellular aggregates. It is probably of importance
that we did not observe this transcript in non-stimulated granulocytes in most
of analyzed donors (
Fig. 2). mRNA for VCAM-1 was easy detectable following
stimulation with anti-PR3, and two donors, whose VCAM-1 mRNA was detected in
non-stimulated cells, responded with 10-fold up-regulation in stimulated neutrophils.
 |
Fig. 2. Change in vascular
cell adhesion molecule-1 (VCAM-1) transcripts abundance following anti-PR3
simulation of neutrophil-enriched granulocytes. Results are presented
as real-time polymerase chain reaction threshold cycle standardized to
18S mRNA (VCAM1CT-18SCT).
Median value of  Ct
VCAM-1 in non stimulated neutrophils is significantly higher than in anti-PR3
antibody stimulated cells (28.44 vs. 26.11; p<0.05). Ct
VCAM-1 in non stimulated neutrophils is significantly higher than in anti-PR3
antibody stimulated cells (28.44 vs. 26.11; p<0.05). |
In summary, we demonstrated that c-ANCA mediated activation of neutrophils has
a profound impact on mRNA expression of most genes involved into inflammatory
response. Changes in mRNA suggest signal transduction not only
via Fc

Rs
signaling system, but also with involvement of other pathways, like G-proteins.
Neutrophil is a very sensitive cell, responding to many environment changes.
Our results documented also a substantial variability of anti-PR3 responses
across donors, despite the fact that experiments were done on total RNA pooled
from two individuals. Nevertheless, an important finding of increased VCAM-1
mRNA following anti-PR3 stimulation seems constant for all replicates of our
experiments. Whether this is reflected by expression of this adhesion molecule
on the cell surface, will require flow cytometry confirmation. The role of VCAM-1
in endothelium and smooth muscle cells is studied extensively. It contributes
to the pathomechanism of atheromatosis, promoting inflammatory cells infiltration
to the vascular wall. It would be highly interesting to test, if a specific
granulocyte vasculitis can develop due to presence of circulation anti-PR3 autoantibodies
by a mechanism of mutual granulocyte activation and recruitment due to their
expression of VCAM-1.
Conflict of interests: None declared.
REFERENCES
- Hu N, Westra J, Kallenberg CG. Membrane-bound proteinase 3 and its receptors: relevance for the pathogenesis of Wegener's granulomatosis. Autoimmun Rev 2009; 8: 510-514.
- Muller Kobold AC, Kallenberg CG, Tervaert JW. Leucocyte membrane expression of proteinase 3 correlates with disease activity in patients with Wegener's granulomatosis. Br J Rheumatol 1998; 37: 901-907.
- Harper L, Radford D, Plant T, Drayson M, Adu D, Savage CO. IgG from myeloperoxidase-antineutrophil cytoplasmic antibody-positive patients stimulates greater activation of primed neutrophils than IgG from proteinase 3-antineutrophil cytosplasmic antibody-positive patients. Arthritis Rheum 2001; 44: 921-930.
- Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010; 49: 1618-1631.
- Rarok AA, Limburg PC, Kallenberg CG. Neutrophil-activating potential of antineutrophil cytoplasm autoantibodies. J Leukoc Biol 2003; 74: 3-15.
- Alcorta DA, Barnes DA, Dooley MA, et al. Leukocyte gene expression signatures in antineutrophil cytoplasmic autoantibody and lupus glomerulonephritis. Kidney Int 2007; 72: 853-864.
- Zycinska K, Wardyn KA, Zielonka TM, Otto M. The role of ANCA and anti-GBM antibodies in pulmonary-renal syndrome due to Wegener's granulomatosis. J Pharmacol Physiol 2007; 58(Suppl. 5): 839-846.
- Pavlidis P, Noble WS. Matrix2png: a utility for visualizing matrix data. Bioinformatics 2003; 19: 295-296.
- Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol 2001; 22: 510-516.
- van der Geld YM, Limburg PC, Kallenberg CG. Proteinase 3, Wegener's autoantigen: from gene to antigen. J Leukoc Biol 2001; 69: 177-190.
- Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes. Brain Res 2009; 1287: 47-57.
- Soehnlein O, Zernecke A, Weber C. Neutrophils launch monocyte extravasation by release of granule proteins. Thromb Haemost 2009; 102: 198-205.
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44-57.
- Bauer V, Sotnikova R, Drabikova K. Effects of activated neutrophils on isolated rings of rat thoracic aorta. J Physiol Pharmacol 2011; 62: 513-520.
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 2003; 28: 12-24.
- Nagaoka I, Hirota S. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm Res 2000; 49: 55-62.
- Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989; 59: 1203-1211.
- Bryk D, Zapolska-Downar D, Malecki M, Hajdukiewicz K, Sitkiewicz D. Trans fatty acids induce a proinflammatory response in endothelial cells through ROS-dependent nuclear factor- B activation. J Physiol Pharmacol 2011; 62: 229-238.
- Chen C, Mobley JL, Dwir O, et al. High affinity very late antigen-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J Immunol 1999; 162: 1084-1095.


