HAEMOSTATIC FACTORS AND INTRALUMINAL THROMBUS THICKNESS
IN ABDOMINAL AORTIC ANEURYSM. IS SECONDARY FIBRINOLYSIS RELEVANT?
2Department of Vascular and General Surgery and Angiology, Pomeranian Medical University, Szczecin, Poland
INTRODUCTION
According to Subcommittee on Reporting Standards for Arterial Aneurysm, abdominal aortic aneurysm (AAA) is defined as a permanent dilatation of the abdominal aorta of at least 30 mm or 1.5 times the expected normal aortic diameter (1). The AAA is characterized by chronic inflammation and the extensive disruption of extracellular matrix (ECM), mainly elastin, by serine-dependent, cysteine-dependent and metallodependent proteases, resulting in a reduction in tensile strength (2-4). The AAA rupture has a very high mortality rate (up to 90% in women) (5, 6) representing one of the commonest causes of death (6). Aneurysm size has not only been consistently associated with the AAA growth (7, 8), but it is recognized as one of the strongest predictors of the risk of rupture with the risk increasing substantially for aneurysms with diameter over 55 mm (9). However, other risk factors for rupture of AAAs, such as gender (10-12), current smoking (10-12), hypertension (9, 10), increased wall tension and stress (6), aneurysm expansion rate (13), intraluminal thrombus (ILT) size and growth (6, 14, 15) have been proposed as well.
There has been a running debate over the causative relationship between ILT formation and degradation of the AAA wall or even whether such a relationship exists (2). Although the ILT is present in the majority of abdominal aortic aneurysms (around 75% of all AAAs) (16), its role remains still largely undefined. Many researchers agree that the presence of the ILT is disadvantageous, through being a source of proteases (17, 18), or causing inflammatory response, wall thinning, weakening and local hypoxia (16, 19). Specifically, the AAA wall covered by ILT is thinner, contains more inflammatory cells, less smooth muscle cells, and exhibits severely degraded extracellular matrix proteins, especially elastin, when compared to AAA wall not covered by mural thrombus (19). Despite controversies, there is now growing evidence that ILT participate in the AAA dilatation or the growth rate (15, 19-24), the risk of AAA rupture (6, 14, 15, 24) as well as the cardiovascular events (22).
The ILT formation and growth seems to be an active, dynamic process, in which a variety of haemodynamic and biochemical factors such as blood particles (red blood cells, white blood cells, platelets) transit time, clotting of blood particles and coagulation (25). Indeed, activated state of both coagulation and fibrinolysis has been reported in patients with AAA in general (20) as well as in patients with small aneurysms (4). Parry et al. (4) suggested that continual thrombus remodelling may explain increased levels of fibrinogen, thrombin-antithrombin complexes, prothrombin fragments 1 and 2 and D-dimer in patients with abdominal aneurysms.
Till now, many circulating markers of abdominal aneurysm presence and progression have been investigated and those markers are grouped into several categories such as matrix degrading enzymes, thrombosis associated proteins, lipids and inflammatory proteins (26). Haemostatic markers have been investigated in relation to the AAA size (20, 21, 27-29), growth (3, 29-31) as well as ILT size (20, 21, 28, 32). However, the results of these studies seem to be uncertain and inconsistent. For instance, t-PA was reported to predict the AAA growth (3), but this conclusion has not been corroborated by others yet (27, 29). Recently, Davies et al. (32), using soluble P-selectin (sP-selectin) as a marker, first examined the relationship between platelet activation and the AAA thrombus volume. Despite the previous observation of elevated levels of sP-selectin in patients with AAA suggesting platelet activation (33), no correlation was found between intraluminal thrombus volume and sP-selectin (32).
As the results obtained so far are inconsistent and equivocal, we conducted a study to verify previous observations of the association of haemostatic markers with aortic abdominal aneurysm and thrombus size. The first aim of the present study was to compare the haemostatic parameters of fibrinolysis such as tissue plasminogen activator (t-PA), plasminogen activator inhibitor (PAI-1), fibrinogen (Fb), D-dimer and the thrombotic markers including prothrombin fragments 1 and 2 (F1+2) and thromboxane B2 (TXB2) in patients with AAA and controls. The purpose was to investigate the relationship between those parameters and both maximum aneurysm diameter and intraluminal thrombus thickness.
MATERIAL AND METHODS
Subjects and study design
The study group included 36 patients who underwent elective repair of AAA, at the Department of Vascular and General Surgery and Angiology of Pomeranian Medical University in Szczecin, Poland. There were 27 (75%) men and 9 (25%) women with a mean age of 71 years (range 56 to 84 years). Coexisting diabetes mellitus (type 2) was found in 7 patients, arterial hypertension in 23. There were 2 patients with the history of myocardial infarction and stroke and 1 with deep vein thrombosis. Seventeen patients were active smokers. Four were treated with statin (simvastatin) and one with antiplatelet drug (acetylsalicylic acid). The control group consisted of 30 subjects with normal infrarenal aortic diameter who were treated in vascular outpatient clinic. Normal diameter was defined as maximum infrarenal aortic diameter less than 30 mm. There were 21 (70%) men and 9 (30%) women with a mean age of the subjects of 63 years (range 63 to 51 years). Coexisting diabetes mellitus was found in 7 patients, arterial hypertension in 17. There were 6 patients with the history of myocardial infarction, 5 with the history of stroke and 1 with deep vein thrombosis. Eighteen were active smokers. Eight control individuals were treated with statin (simvastatin, atorvastatin and rosuvastatin) and 3 with antiplatelet drug (acetylsalicylic acid). General exclusion criteria were a known history of renal dysfunction, liver dysfunction and haematological disorders. The study was approved by the institutional ethics committee and conformed to the Helsinki declaration. Patients and control subjects gave their approval by written informed consent before entering the study.
Blood sampling
Peripheral venous blood samples were taken from controls and each patient preoperatively and before any blood product transfusion or heparin administration. Whole blood was collected into tubes with 3.2% sodium citrate for analysis of tissue plasminogen activator (t-PA), D-dimer, fibrinogen (Fb), prothrombin fragment 1+2 (F1+2) and thromboxane B2 (TXB2); tubes containing buffered citrate solution, theophylline, adenosine and dipyridamole (BD Vacutainer® Coagulation Tube CTAD) for plasminogen activator inhibitor (PAI-1) measurement and tubes with clot activator for analysis of lipids profile (S-Monovette with clot activator, Sarstedt, Germany). Platelet poor plasma and serum were prepared from whole blood by centrifugation (3000g for 15 minutes) within 30 minutes of collection. Samples were then aliquoted and stored at –80°C until assays were performed. All samples were studied within 2 months of collection. Samples from study and control groups were determined in the same series to avoid bias due to assay variability.
Measurements
Plasma concentrations of t-PA were determined using AssayMax Human tPA ELISA kit (AssayPro) according to the manufacturers’ instructions. Manufacturer’s normal range: 0.031–2.000 ng/mL. The AssayMax Human PAI-1 ELISA kit (AssayPro) was used for detection of PAI-1 in plasma. Manufacturer’s normal range: 0.078–5.000 ng/mL. Serum D-dimer concentration was evaluated by quick ELFA test (VIDAS D-Dimer Exclusion II TM, bioMerieux, France), using a quantitative automatic analyser. Threshold value for D-dimer was 500 ng/mL, according to the manufacturer’s recommendations. Fibrinogen concentration was assessed quantitatively by von Clauss method (Q.F.A. Thrombin (bovine), HemosILTM). Manufacturer’s normal range: 1.81–3.84 g/L. The concentrations of F1+2 was measured by ELISA using commercially available reagents - enzyme-linked immunosorbent assay kit for prothrombin fragment 1+2 (F1+2) (USCN Life Science). Manufacturer’s normal range: 0.625–40.000 ng/mL. Total cholesterol (TC), HDL-cholesterol (HDL-C), triglycerides (TG), and LDL-cholesterol (LDL-C) were measured with enzymatic colorimetric tests on the ARCHITECT cSystemTM analyzer (Abbott Diagnostics). LDL-cholesterol (LDL-C) was also calculated using the Friedewald’s formula.
Assay of thromboxane B2 concentrations
The mixture of 2 mL of 100% acetonitrile (Sigma-Aldrich, St, Louis, MO, USA), cooled down at 4°C, and 1 mL of platelet poor plasma, defrosted at room temperature, was vigorously mixed for three minutes. Next, the mixture was first cooled down to –20°C over 10 minutes and then centrifuged (3824g, 10 min, 4°C). Four millilitres of 1 mM HCl was added to the supernatant and acidified with 1 M HCl to pH 3. The samples were equilibrated with 100% actonitrile and 20% acetonitrile in 1 mM HCl and then applied to the Bakerbond speTM (RP-18, Mallinckrodt Baker, Inc. Phillipsburg, NJ, USA) columns. Three millilitres of methanol with ethyl acetate mixture (1:1; v/v) was used to conduct elution and the eluate was dried under the nitrogen stream. The TXB2 concentrations were determined by ELISA with the use of the reagent set thromboxane B2 express EIA kit (Cayman Chemical) as indicated by the manufacturer’s protocol. Results were expressed as picogram per mL (pg/mL). The minimum detectable concentration was 15.6 pg/mL TXB2.
Imaging
The aortic size was confirmed in all patients and controls by computed tomography (CT) and the maximal aortic diameter was recorded. A significant ILT was present in all the AAA studied. Circulating aortic channels were measured at the level of maximal dilatation on CT scan and the ILT thickness was calculated as follows: maximal aortic diameter-circulating channel (34).
Statistical analysis
Continuous variables were tested for normality with a one-sample Kolmogorov-Smirnov test. Normally distributed variables were presented as mean and standard deviation, while log-normal (TXB2, PAI-1) as geometric mean. The 95% confidence interval for the mean on the logarithmic scale was antilogged to give a 95% confidence interval for the geometric mean on the natural scale. The differences in means and proportions were tested with Student’s t test and χ2 test, respectively. As the AAA and control groups differed significantly in age, all comparisons between those two groups were age-adjusted with either analysis of covariance (ANCOVA) for a difference in means or logistic regression for a difference in proportions. Bivariate Pearson correlation was used to examine the relationship between factors and AAA size or ILT thickness. A multiple linear regression was run to assess the simultaneous effects of predictors with the standard method of entry (“enter” method). Apart from standardised coefficients, partial and semi partial coefficients were calculated along with tolerance as the measure of multicolinearity. All analyses were performed using STATISTICA version 10 (StatSoft, Inc.) with α level set to 0.05.
RESULRS
Haemostatic and biochemical parameters
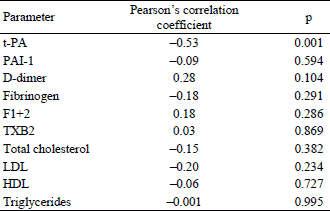
In Table 1 the clinical, biochemical and haemostatic characteristics of patients with the abdominal aortic aneurysm (AAA) and control individuals is presented. The two groups differed significantly with respect to age, as the patients with the AAA were significantly older than control individuals (p=0.00009). Therefore, further comparisons between the AAA patients and controls were carried out with adjustment for age. There was no significant difference in clinical characteristics between groups, including smoking, diabetes mellitus, hypertension, myocardial infarction, stroke and treatment with statins. Lipid profile was similar in both groups except LDL level which was significantly higher in the AAA patients. Among haemostatic factors, t-PA and D-dimer levels, but not PAI-1, were significantly higher in subjects with the AAA. The two groups did not differ in remaining haemostatic factors such as TXB2, fibrinogen, prothrombin 1+2 (Table 1).
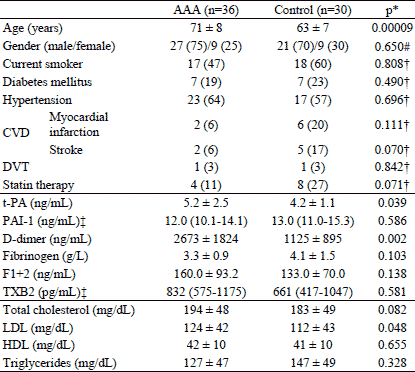
† logistic regression adjusted for age; ‡ log normal data reported as geometric mean and antilogged 95% confidence intervals in parentheses;
CVD - cardiovascular disease; DVT - deep vein thrombosis; LDL - low-density lipoprotein; HDL - high-density lipoprotein; TXB2 - thromboxane B2; F1+2 - prothrombin fragments 1 and 2; PAI-1 - plasminogen activator inhibitor; t-PA - tissue plasminogen activator.
Aneurysm size and intraluminal thrombus thickness
The mean maximum aortic diameter in patients with the AAA was 59±12 mm (range 42–100). The mean ILT thickness was 32±10 mm (range 8–56). Age was not significantly correlated with either aneurysm size (r=0.12, p=0.470) or ILT thickness (r=0.05, p=0.775). There was a strong significant correlation between thickness of intraluminal thrombus and maximum aneurysm size (r=0.69, p<0.0001, Fig. 1). The maximum aneurysm size did not differ with respect to sex, smoking status, diabetes mellitus, hypertension as well as the treatment with statins (Table 2). There were only two patients with the history of previous myocardial infarction or stroke in the AAA group and they were excluded from analysis. To examine the relationship between haemostatic parameters and maximum aneurysm diameter a Pearson’s bivariate correlation was carried out. The correlation coefficients and p values are shown in Table 3. There was a moderate, negative relationship between t-PA and aneurysm diameter (Fig. 2). No other correlations were observed.
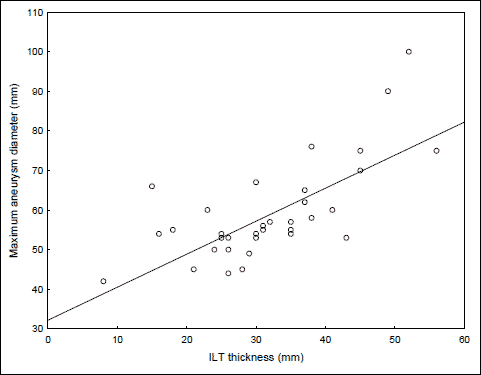 |
Fig. 1. Correlation between intraluminal thrombus (ILT) thickness and maximum aneurysm size. |


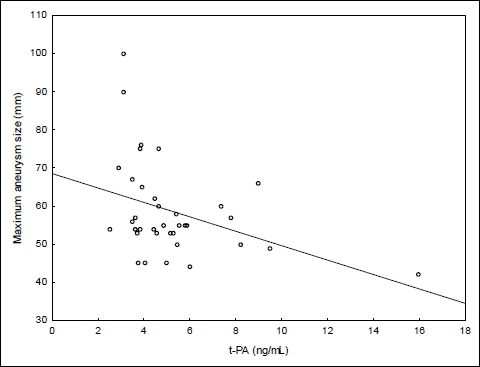 |
Fig. 2. Correlation between t-PA and maximum aneurysm size. |
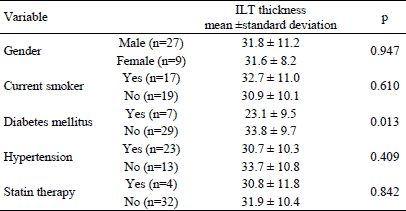
The same analysis was run using intraluminal thrombus thickness as the dependent variable. Similarly, the ILT thickness did not differ with respect to sex and clinical characteristics except the difference of ILT thickness between diabetic and non-diabetic patients (Table 4). The ILT thickness was significantly smaller in patients with diabetes mellitus. Bivariate correlation analysis (Table 5) revealed significant moderate and negative relation of t-PA with ILT thickness (Fig. 3).
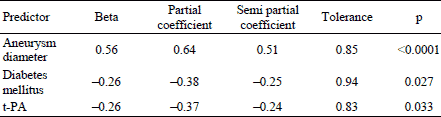
Given the presence of the relationship between ILT thickness and maximum aneurysm size, diabetes mellitus and t-PA we also performed multiple linear regression analysis with ILT thickness as the dependent variable and t-PA, maximum aneurysm size and diabetes mellitus as the independent predictors. The model accounted for 63.2% of variance in ILT thickness with all predictors being significant (Table 6).
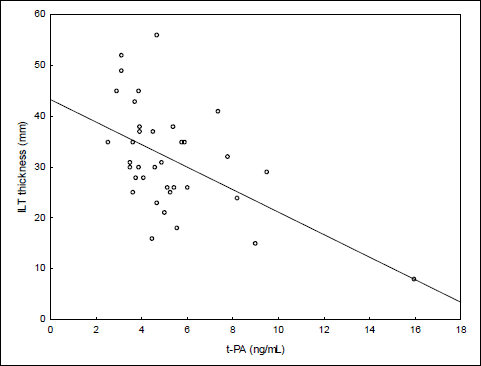 |
Fig. 3. Correlation between t-PA and intraluminal thrombus thickness. |
DISCUSSION
In the present study we confirmed a well-known observation (4, 20, 27, 35) of the increased fibrin turnover, judged by significantly elevated D-dimer (35) in patients with abdominal aortic aneurysms as compared to control individuals. Interestingly, in previous studies, despite the observed elevation in D-dimer, t-PA did not differ between patients with the AAA (non-ruptured aneurysms) and controls (4, 27, 35). Parry et al. (4) suggested that t-PA, exclusively expressed within aneurysm wall (36) may not be released to circulation thereby explaining the lack of difference in t-PA levels between groups. The observation of slow release of t-PA from luminal pole of ILT into conditioned media, probably due to the strong affinity of t-PA for fibrin (36), seems to support this hypothesis. In accordance with results received by Wanhainen et al. (37), in this study we found elevated t-PA in plasma among patients with the AAA compared to controls. According to Wanhainen et al. (37) elevated t-PA in patients with the AAA supports the hypothesis that the fibrinolytic system is important in the pathogenesis of the AAA.
Another finding of the present study is a significant and positive correlation between intraluminal thrombus (ILT) thickness and the abdominal aortic aneurysm maximum diameter. This is in broad agreement with the observations made by other authors (20, 21), but not all (32), and may suggest an important role of ILT in aneurysm growing and progression. The studies, however, report antagonistic effects of ILT on the AAA wall. As a matter of fact, two aspects should be considered regarding the role of the intraluminal thrombus on the AAA growth rate: mechanical and biochemical. Computational studies using different material properties of thrombus and idealized as well as patients-specific geometrics showed that thrombus lowers the AAA wall stress and this effect is stronger for thicker and stiffer (high shear modulus) thrombi (23, 38, 39). These findings must be confronted with numerous reports on the positive correlation between intraluminal thrombus volume and the aneurysmal size or growth (15, 19-23) as well as the risk of rupture (15). Speelman et al. (23) suggested that diameter growth of AAA is not instigated by the stress in the wall, but rather the wall weakening is the basis for the AAA growth explaining the increased growth for AAAs with a relative large thrombus. To date, many studies have attempted to determine the causative relationship between ILT formation and degradation of the AAA. Kazi et al. (19) showed that thrombus covered wall is thinner and exhibits more signs of proteolytic activity with more degraded elastin. Recently, Khan et al. (40) found a significant positive correlation of ILT thickness with active MMP-9 and TIMP-1 levels in the adjacent AAA wall. On the other hand, Folkesson et al. (41) provided evidence that there are spatial differences within the ILT in the activity of MMP-9 and neutrophil derived elastase (NE), two proteases suggested to be involved in the degradation of the AAA wall (18). Interestingly, the enzymatic activity was either absent or very low in the abluminal as compared to luminal layer of the thick multilayer ILT (41). Thus, the authors proposed other mechanisms that possibly contribute to degradation of the AAA wall such as hypoxia concomitant with neovascularisation and mobilization of mast cells (41). However, the previous studies conducted in our clinic showed that the activity of elastase and MMP-9 within the AAA wall were significantly higher beneath the thin section (<10 mm) of the eccentric ILT compared to thick one (>25 mm) (42). Thus, it is likely that thin thrombus-covered wall receives both greater mechanical and greater biochemical stress.
In the present study we also found a negative relationship between t-PA and maximum AAA diameter as well as ILT thickness. So far, many studies have investigated the relationship between haemostatic markers and aneurysm geometrics or intraluminal thrombus size (20, 21, 27, 28) as well as the AAA growth (30, 31, 43), frequently providing inconsistent or conflicting findings. To the best of our knowledge, this is the first report demonstrating the negative correlation between plasma t-PA level and ILT thickness. Aho et al. (28), although using a different measure of ILT size (volume of the thrombus), did not observe such a relationship. Assuming the aortic aneurysm associated with larger ILT progresses faster (15, 19, 22), our observation seems to contradict the findings obtained by Lindholt et al. (43) who found a positive correlation between the aneurysmal progression rate and plasma t-PA antigen.
Reasons for these discordant results are unclear. Apart from its fibrinolytic activity in plasma, plasmin takes part in tissue degenerative processes, including the destruction of aortic matrix (elastin and collagen) in abdominal aneurysm either directly or indirectly via activation of matrix metalloproteases and the macrophages to destroy extracellular matrix (44). The changes in aortic media structure are accompanied by impaired contractile function (45). Recently Czerski et al. (46) confirmed in a large animal model that the damage of elastin and collagen fibers plays a significant, although not exclusive, role in the AAA development. This could partially explain the positive relationship between t-PA and annual growth of the AAA. Within ILT, however, plasmin activity is not distributed evenly, as plasmin-antiplasmin complexes and D-dimers were mostly released by the thrombus luminal layer with plasminogen/plasmin antigen and plasmin activity exhibiting a negative gradient from the luminal to the abluminal side (36). This seems to mostly be in agreement with recent findings of Folkesson et al. (41) who documented the negative gradient of MMP-9 form the luminal to the abluminal side and only luminal, newly formed fresh layer contained active MMP-9. Therefore our observation of negative relationship between plasma t-PA and the thickness of ILT may represent a deficiency of secondary fibrinolysis within the outer layer of the ILT leading to a disruption of thrombotic/fibrinolysis balance and accelerated formation of intraluminal thrombus.
In this view, the potentially opposite activities of t-PA and plasmin should be considered. Regarding the pathogenesis of the AAA, these components of the fibrinolytic system exhibit a Janus-faced behavior with two contrasting aspects. First, the t-PA, a major plasminogen activator, constitutes an important component of the system involved in destruction of aortic matrix (44). The second face of t-PA and plasmin appears in the context of fibrinolytic activity. Taking part in the process of secondary fibrinolysis and remodelling of ILT mainly within its luminal layer, t-PA and plasmin are capable of slowing down the growth of mural thrombus which is generally viewed as the predictor of aneurysm progression. The net effect of this complex and contrasting t-PA activity may be difficult to estimate and the overall result may also be modulated by a variety of patient-specific biochemical and biomechanical factors, including ILT location, geometrics, architecture and spatial differences in fibrinolytic and proteolytic activities both in the intraluminal thrombus and within the aneurysm wall. Recently, Scott et al. (47) examined clot structure in patients with AAA and showed that dense and smaller pored thrombi are more resistant to fibrinolysis and correlate with aneurysm size.
Interestingly, the correlation between t-PA and ILT thickness remained significant in a multiple regression model in which maximum AAA size and diabetes mellitus were entered as the co-variables. As all predictors in multiple regression analysis were significant, t-PA, aneurysm size and diabetes mellitus seem to relate to ILT thickness simultaneously. It is likely that the interplay between abdominal aneurysm and its associated thrombus fits a reciprocal causation model. Initially, a dilatation of abdominal aorta encourages formation of thrombus which then affects the aneurysm both biomechanically and biochemically and accelerates its expansion. Basciano et al. (25) showed that ILT volume changes correlate positively with blood particle transit time, and both were found to be the highest in the middle, the most dilated, axial region of abdominal aneurysm. Diabetes has been shown to have an inverse relationship with aortic diameter (48). In the present study the patients with diabetes had significantly thinner ILTs compared to non-diabetic patients, however, the mechanism of this relation remains unclear. One of the possible protective mechanisms of diabetes is the formation of advanced glycation end products (AGEs) which may form covalent cross-links between proteins, including elastin and collagen in the vessel wall, thereby leading to an increased stiffness of the aneurysm wall more resistant to proteolysis (48). This could be offered as the possible explanation for our observation of thinner ILT in diabetic patients, however, no difference in maximum aneurysm diameter was found between patients with and without diabetes.
We found no relationship between TXB2 and either AAA size or ITL thickness. Platelet inhibition has been reported to inhibit the progression of AAA in animal (49) and human studies (50). Recently, Davies et al. (32) failed to find an association between plasma soluble P-selectin, a marker of in vivo platelet activation, and AAA thrombus volume. “Irreversible” platelet aggregation is the result of the platelet release reaction, which relates to the release of arachidonic acid metabolites, mainly endoperoxidases and thromboxanes (51). Thromboxane A2 is the major cyclooxygenase product of human platelets and is a strong platelet activator (52). In the current study, we used a thromboxane B2 as the marker of platelet activation. Thromboxane B2 is a stable metabolite of thromboxane A2 (53, 54), and its derivative 11-dehydro-TXB2 was shown to be a sensitive index of in vivo platelet activation (55). Davies et al. (32) suggested that the lack of association between sP-selectin and mural thrombus volume might be explained by differences in the proportion of patients with hypercholesterolemia and treated with statins, as the high content of cholesterol in the platelets membrane may render them more sensitive to stimulation (56). However, although the AAA group in the current study exhibited a significantly higher LDL level compared to control individuals, there were no differences in TXB2 between the two groups. The relationship between platelets aggregation and the release of thromboxanes is not linear. Lands et al. (57) showed that the relationship between aggregation and thromboxane generation is not a simple dose-response association. Whereas the 50% aggregation occurred with relatively low thromboxane generation (up to 50 ng/mL), aggregations of 50 to 95% produced TXB2 concentrations between 50–500 ng/mL (57). This phenomenon may explain the lack of relationship between TXB2 level and ILT thickness, as platelets seem to generate more TXB2 than it is needed for aggregation.
Our study has several limitations. The study and control groups were not perfectly matched with respect to demographic and clinical (statins) characteristics. Compared to control individuals a smaller fraction of patients with AAA was treated with statins. Although the use of statins inhibits the secretion of pro-inflammatory mediators (58), and simvastatin attenuates the activity of matrix MMP-9 in aneurysmal aortic tissue (59), in this study, statin and non-statin users did not differ with respect to AAA diameter and ILT thickness. Moreover, relationships between t-PA and maximum aneurysm size as well as between t-PA and ILT thickness remained largely unchanged among non-statin users (data not shown). Therefore, the uneven use of statins is unlikely to negatively affect main conclusions drawn from the study. Furthermore, it must be noted, that methods of thrombus size measurement vary across studies, with some using the thrombus volume (21, 32) and others, the maximum thickness of thrombus (20) or the arc of aneurysm wall covered by thrombus (15). Here, a thrombus load was represented by the thickness of ILT taken at the level of maximal aortic dilatation on 2D computed tomography. Although it is reasonable to assume that this measure is a good proxy for the total ILT volume, to our knowledge, the consistency between different measurements of ILT load have not been examined. Therefore, the thrombus thickness at the level of maximum AAA diameter may not correlate with total volume of mural thrombus. Another limitation stems from the fact that a t-PA mass (t-PA antigen) rather than its active form, was measured. The t-PA exists in plasma in multiple forms - active t-PA, t-PA/PAI-1 complex and t-PA/C1-inhibitor complex (60). Hence, an elevated t-PA antigen may be a reflection of high levels of circulating PAI-1, resulting in a large portion of t-PA being captured by PAI-1 and thereby rendering it inactive (61). Indeed, some studies have reported the association of increased t-PA antigen level with myocardial infarction (61), carotid atherosclerosis (62) and peripheral vascular disease (63). A number of explanations have been suggested for a high total t-PA antigen as a risk factor (62, 64). Chandler et al. (64) suggested that higher levels of PAI-1 in blood convert a greater fraction of the secreted t-PA in t-PA/PAI-1 complex which is cleared more slowly than active t-PA, and the total level of t-PA rises even though t-PA activity is falling.
In conclusion, the higher plasma concentrations of t-PA and D-dimer in particular support the hypothesis that the secondary fibrinolysis plays an important role in the pathogenesis of the aortic abdominal aneurysm formation. In addition, our main result, the strong negative correlation between t-PA plasma level and ILT thickness, suggests that thrombotic/fibrinolysis imbalance may also favour accelerated formation of intraluminal thrombus and possibly aneurysm progression.
Acknowledgement: This work was supported by a grant from the Polish Research Committee (KBN) in 2010-2013 (no. NN402 523739).
Conflict of interests: None declared.
REFERENCES
- Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991; 13: 452-458.
- Yoshimura K, Ikeda Y, Aoki H. Innocent bystander? Intraluminal thrombus in abdominal aortic aneurysm. Atherosclerosis 2011; 218: 285-286.
- Lindholt JS, Jorgensen B, Shi GP, Henneberg EW. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2003; 25: 546-551.
- Parry DJ, Al-Barjas HS, Chappell L, Rashid T, Ariens RA, Scott DJ. Haemostatic and fibrinolytic factors in men with a small abdominal aortic aneurysm. Br J Surg 2009; 96: 870-877.
- Noel AA, Gloviczki P, Cherry K, Jr, et al. Ruptured abdominal aortic aneurysms: the excessive mortality rate of conventional repair. J Vasc Surg 2001; 34: 41-46.
- Vorp DA, Vande Geest JP. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol 2005; 25: 1558-1566.
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT, UK Small Aneurysm Trial Participants. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004; 110: 16-21.
- Vega de Ceniga M, Gomez R, Estallo L, Rodriguez L, Baquer M, Barba A. Growth rate and associated factors in small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2006; 31: 231-236.
- Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: a comprehensive review. Exp Clin Cardiol 2011; 16: 11-15.
- Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg 1999; 230: 289-297.
- Fillinger M. Who should we operate on and how do we decide: predicting rupture and survival in patients with aortic aneurysm. Semin Vasc Surg 2007; 20: 121-127.
- Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 2003; 37: 724-732.
- Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: the impact of size, gender, and expansion rate. J Vasc Surg 2003; 37: 280-284.
- Stenbaek J, Kalin B, Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2000; 20: 466-469.
- Wolf YG, Thomas WS, Brennan FJ, Goff WG, Sise MJ, Bernstein EF. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J Vasc Surg 1994; 20: 529-538.
- Vorp DA, Lee PC, Wang DH, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 2001; 34: 291-299.
- Swedenborg J, Eriksson P. The intraluminal thrombus as a source of proteolytic activity. Ann N Y Acad Sci 2006; 1085: 133-138.
- Fontaine V, Jacob MP, Houard X, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol 2002; 161: 1701-1710.
- Kazi M, Thyberg J, Religa P, et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg 2003; 38: 1283-1292.
- Yamazumi K, Ojiro M, Okumura H, Aikou T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg 1998; 175: 297-301.
- Shindo S, Matsumoto H, Kubota K, et al. Is the size of an abdominal aortic aneurysm associated with coagulopathy? World J Surg 2005; 29: 925-929.
- Parr A, McCann M, Bradshaw B, Shahzad A, Buttner P, Golledge J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg 2011; 53: 28-35.
- Speelman L, Schurink GW, Bosboom EM, et al. The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J Vasc Surg 2010; 51: 19-26.
- Koole D, Zandvoort HJA, Schoneveld A, et al. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J Vasc Surg 2013; 57: 77-83.
- Basciano C, Kleinstreuer C, Hyun S, Finol EA. A relation between near-wall particle-hemodynamics and onset of thrombus formation in abdominal aortic aneurysms. Ann Biomed Eng 2011; 39: 2010-2026.
- Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation 2008; 118: 2382-2392.
- Wallinder J, Bergqvist D, Henriksson AE. Haemostatic markers in patients with abdominal aortic aneurysm and the impact of aneurysm size. Thromb Res 2009; 124: 423-426.
- Aho PS, Niemi T, Piilonen A, Lassila R, Renkonen R, Lepantalo M. Interplay between coagulation and inflammation in open and endovascular abdominal aortic aneurysm repair - impact of intra-aneurysmal thrombus. Scand J Surg 2007; 96: 229-235.
- Flondell-Site D, Lindblad B, Kolbel T, Gottsater A. Markers of proteolysis, fibrinolysis, and coagulation in relation to size and growth rate of abdominal aortic aneurysms. Vasc Endovascular Surg 2010; 44: 262-268.
- Lindholt JS, Jorgensen B, Fasting H, Henneberg EW. Plasma levels of plasmin-antiplasmin-complexes are predictive for small abdominal aortic aneurysms expanding to operation-recommendable sizes. J Vasc Surg 2001; 34: 611-615.
- Golledge J, Muller R, Clancy P, McCann M, Norman PE. Evaluation of the diagnostic and prognostic value of plasma D-dimer for abdominal aortic aneurysm. Eur Heart J 2011; 32: 354-364.
- Davies RS, Abdelhamid M, Vohra RK, Bradbury AW, Adam DJ. The relationship between aortic aneurysm sac thrombus volume on coagulation, fibrinolysis and platelet activity. Thromb Res 2012; 130: 463-466.
- Blann AD, Devine C, Amiral J, McCollum CN. Soluble adhesion molecules, endothelial markers and atherosclerosis risk factors in abdominal aortic aneurysm: a comparison with claudicants and healthy controls. Blood Coagul Fibrinolysis 1998; 9: 479-484.
- Houard X, Touat Z, Ollivier V, et al. Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovasc Res 2009; 82: 532-541.
- Skagius E, Siegbahn A, Bergqvist D, Henriksson AE. Fibrinolysis in patients with an abdominal aortic aneurysm with special emphasis on rupture and shock. J Thromb Haemost 2008; 6: 147-150.
- Houard X, Rouzet F, Touat Z, et al. Topology of the fibrinolytic system within the mural thrombus of human abdominal aortic aneurysms. J Pathol 2007; 212: 20-28.
- Wanhainen A, Nilsson TK, Bergqvist D, Boman K, Bjorck M. Elevated tissue plasminogen activator in patients with screening-detected abdominal aortic aneurysm. J Vasc Surg 2007; 45: 1109-1113.
- Mower WR, Quinones WJ, Gambhir SS. Effect of intraluminal thrombus on abdominal aortic aneurysm wall stress. J Vasc Surg 1997; 26: 602-608.
- Wang DH, Makaroun MS, Webster MW, Vorp DA. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg 2002; 36: 598-604.
- Khan JA, Abdul Rahman MN, Mazari FA, et al. Intraluminal thrombus has a selective influence on matrix metalloproteinases and their inhibitors (tissue inhibitors of matrix metalloproteinases) in the wall of abdominal aortic aneurysms. Ann Vasc Surg 2012; 26: 322-329.
- Folkesson M, Silveira A, Eriksson P, Swedenborg J. Protease activity in the multi-layered intra-luminal thrombus of abdominal aortic aneurysms. Atherosclerosis 2011; 218: 294-299.
- Wiernicki I, Stachowska E, Safranow K, et al. Enhanced matrix-degrading proteolytic activity within the thin thrombus-covered wall of human abdominal aortic aneurysms. Atherosclerosis 2010; 212: 161-165.
- Lindholt JS. Activators of plasminogen and the progression of small abdominal aortic aneurysms. Ann NY Acad Sci 2006; 1085: 139-150.
- Jean-Claude J, Newman KM, Li H, Gregory AK, Tilson MD. Possible key role for plasmin in the pathogenesis of abdominal aortic aneurysms. Surgery 1994; 116: 472-478.
- Gnus J, Czerski A, Ferenc S, et al. in vitro study on the effects of some selected agonists and antagonists of alpha(1)-adrenergic receptors on the contractility of the aneurysmally-changed aortic smooth muscle in humans. J Physiol Pharmacol 2012; 63: 29-34.
- Czerski A, Bujok J, Gnus J, et al. Experimental methods of abdominal aortic aneurysm creation in swine as a large animal model. J Physiol Pharmacol 2013; 64: 185 192.
- Scott DJ, Prasad P, Philippou H, et al. Clot architecture is altered in abdominal aortic aneurysms and correlates with aneurysm size. Arterioscler Thromb Vasc Biol 2011; 31: 3004-3010.
- Shantikumar S, Ajjan R, Porter KE, Scott DJ. Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2010; 39: 200-207.
- Dai J, Louedec L, Philippe M, Michel JB, Houard X. Effect of blocking platelet activation with AZD6140 on development of abdominal aortic aneurysm in a rat aneurysmal model. J Vasc Surg 2009; 49: 719-727.
- Lindholt JS, Sorensen HT, Michel JB, Thomsen HF, Henneberg EW. Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc Endovascular Surg 2008; 42: 329-334.
- Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J 2001; 22: 1561-1571.
- FitzGerald GA, Healy C, Daugherty J. Thromboxane A2 biosynthesis in human disease. Fed Proc 1987; 46: 154-158.
- Tohgi H, Tamura K, Kimura B, Kimura M, Suzuki H. Individual variation in platelet aggregability and serum thromboxane B2 concentrations after low-dose aspirin. Stroke 1988; 19: 700-703.
- Kim HS, Zhang YH, Fang LH, Yun YP, Lee HK. Effects of tetrandrine and fangchinoline on human platelet aggregation and thromboxane B2 formation. J Ethnopharmacol 1999; 66: 241-246.
- Gresele P, Catalano M, Giammarresi C, et al. Platelet activation markers in patients with peripheral arterial disease - a prospective comparison of different platelet function tests. Thromb Haemost 1997; 78: 1434-1437.
- Tomizuka T, Yamamoto K, Hirai A, Tamura Y, Yoshida S. Hypersensitivity to thromboxane A2 in cholesterol-rich human platelets. Thromb Haemost 1990; 64: 594-599.
- Lands WE, Culp BR, Hirai A, Gorman R. Relationship of thromboxane generation to the aggregation of platelets from humans: effects of eicosapentaenoic acid. Prostaglandins 1985; 30: 819-825.
- Korybalska K, Kawka E, Breborowicz A, Witowski J. Atorvastatin does not impair endothelial cell wound healing in an in vitro model of vascular injury. J Physiol Pharmacol 2012; 63: 389-395.
- Evans J, Powell JT, Schwalbe E, Loftus IM, Thompson MM. Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur J Vasc Endovasc Surg 2007; 34: 302-303.
- Chandler WL, Jascur ML, Henderson PJ. Measurement of different forms of tissue plasminogen activator in plasma. Clin Chem 2000; 46: 38-46.
- Fernhall B, Szymanski LM, Gorman PA, Milani J, Paup DC, Kessler CM. Fibrinolytic activity is similar in physically active men with and without a history of myocardial infarction. Arterioscler Thromb Vasc Biol 1997; 17: 1106-1113.
- Salomaa V, Stinson V, Kark JD, Folsom AR, Davis CE, Wu KK. Association of fibrinolytic parameters with early atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation 1995; 91: 284-290.
- Killewich LA, Gardner AW, Macko RF, et al. Progressive intermittent claudication is associated with impaired fibrinolysis. J Vasc Surg 1998; 27: 645-650.
- Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation 1997; 96: 761-768.
A c c e p t e d : June 10, 2013