MELATONIN AND OMENTIN: INFLUENCE FACTORS
IN THE OBSTRUCTIVE SLEEP APNOEA SYNDROME?
INTRODUCTION
The obstructive sleep apnoea syndrome (OSAS) is a disease with increasing worldwide incidence. Approximately 2% of women and 4% of men are affected by this disease in Western countries (1-4). Newly issued studies even refer to a higher prevalence of OSAS. OSAS was observed in up to 20–30% of the study collective (5, 6). Due to its frequent co-occurrence with the metabolic syndrome the majority of the patients arise in the industrial nations. OSAS is characterized by repeated airway collapse (repetitive obstruction of the upper airways) during sleep (2), which results in an episodic hypoxaemia, oxidative stress (7) disturbed sleep at night (8) and in consequence in excessive sleepiness during the day (4). These changes cause neural, cardiovascular and metabolic alterations (9).
While intermittent nocturnal blood pressure peaks and pulse accelerations of OSA patients implicate a higher risk for cardiovascular diseases by themselves, also metabolic and inflammatory processes have emerged as critical in the pathogenesis of atherogenesis and various cardiovascular disorders. Increased levels of several circulating markers of inflammation including tumour necrosis factor a (TNF-a), interleukin 6 (IL-6), IL-8 and C-reactive protein have been reported as associated with future cardiovascular risk (4). Patients with OSAS exhibit elevated levels of C-reactive protein, tumour necrosis factor-a and interleukin-6 (9). Intermittent hypoxia, the hallmark of OSAS, results in activation of pro-inflammatory transcription factors such as nuclear factor kappa B (NF-kappa B) and activator protein (AP)-1 (4). Inflammatory cells such as monocytes and lymphocytes are activated and release pro-inflammatory mediators which may cause endothelial dysfunction and damage. Another result of OSAS is activation of peripheral sympathetic nervous system and the hypothalamic-pituitary-adrenal axis as well as insulin sensitivity (9, 10).
Previous studies have shown that OSAS is associated with insulin resistence and type 2 diabetes mellitus, two disorders which by themselves contribute to cardiovascular diseases (11). Impaired glucose metabolism was observed in 60.5% of patients with sleep apnoea (DM in 30.2% and IGT in 30.2%) (12).
OSAS is in generally associated with increased mortality (13) and contribues to an impairment of quality of life (14). The golden standard in therapy remains the nocturnal continuous positive airway pressure therapy (CPAP) (2). Obesity is the most frequent predisposing condition of OSAS (15). Meanwhile there is a strong evidence that overweight plays an important role in the pathogenesis of OSAS. Weight loss is considered a key intervention to reduce the severity of OSAS or to avoid the necessarity of CPAP therapy at all (16, 17).
Accumulating evidence indicates that adipose tissue is an active endocrine organ that produces various bioactive substances (18, 19). These are also known as adipocytokines or adipokines. An increase in adipose tissue, as often observed in OSA patients, is linked with an over- or under-expression of many adipokines. In the last years more and more adipose tissue hormones were discovered. Noteworthy in this context are leptin, adiponectin, visfatin, vaspin, apelin, chemerin and recently omentin (20). They mediate various effects regarding energy expenditure, weight gain, angiogenesis, blood vessel reaction, type 2 diabetes, atherogenesis (all key points in the metabolic syndrome) and many more (21, 22). Recently, they also come to interest for a better understanding of the OSAS pathogenesis. There is an accumulating data that the adipokines may influence the severity of OSAS or their plasma levels showed differences before and under CPAP therapy (23, 24). Visfatin levels for example were associated with characteristics of sleep architecture (25). Further, nCPAP treatment diminishes leptin in obese OSA patients and adiponectin levels in obese and non-obese patients with OSAS (26). In the present study we focused on omentin-1.
Omentin is a novel 38-40 kDa protein preferentially produced by visceral adipose tissue with insulin-sensitizing effects (27, 28). Omentin-1 is the major circulating isoform (29). In vitro experiments revealed that treatment with recombinant omentin-1 enhances insulin-mediated glucose uptake in human subcutanous and omental adipocytes (30). While it is highly expressed in human visceral fat tissue, circulating omentin levels are reduced in obese subjects (19, 31, 32) (negative correlation with the body mass index) (29). Decreased levels of omentin-1 are also associated with insulin resistance (33, 34), type 2 diabetes mellitus (35) and coronary artery disease (31) arterial stiffness and carotid plaque (36, 37) or in other words correlated inversely with the metabolic syndrome (19). Reduced omentin levels were also reported in several proinflammatory states such as Crohn disease, rheumatoid arthritis (38), psoriasis (29) and polycystic ovary syndrome (34). In contrast Alcelik et al. demonstrates elevated omentin levels in patients with end stage renal disease receiving hemodialysis (39). Further, omentin causes vasodilatation of blood vessels and attenuates C-reactive protein-induced angiogenesis potentially via the nuclear factor B signaling pathway (28). It is believed, that it has anti-inflammatory (40), anti-atherogenic, anti-cardiovascular disease (41) and antidiabetic properties (27). Omentin therefore was thought to have beneficial effects on the metabolic syndrome and could potentially be used as a biologic marker. Only one study to OSAS and omentin-1 is found in literature up to now, but in this context no CPAP therapy was established. This study showed decreased omentin 1 levels in OSA patients in comparison to a healthy control group (42).
While melatonin regulates the human cycle of sleep and wakefulness (8, 43), it's plasma levels were also analysed in this study. Melatonin is a hormone produced in the pineal gland that has a strong impact on circadian rhythm. Levels of melatonin are under the control of the suprachiasmatic nucleus and vary according the daily cycle (8, 44, 45). It's secretion is light dependent (light exposure during the night results in a rapid suppression of melatonin) and has a peak at about 2.00 a.m. The lowest concentration was found in the afternoon (1). Interestingly, melatonin is released in a high amount in the gastrointestinal tract independently from light exposure, but in dependence from the food intake. Newly issued studies underlined a potential role of melatonin for the treatment or prevention of obesity (46). It has also been claimed that melatonin has sleep-promoting properties (47). Therefore Hernandez et al. investigated the nocturnal melatonin plasma levels in patients with OSAS during diagnostic polysomnography and one day after CPAP-therapy. The data could demonstrate that OSA patients have an abnormal melatonin secretion pattern. Patients with OSAS showed an absence of a nocturnal serum melatonin peak before and under therapy (8). Certainly there are some important limitations of this study. A comparison of melatonin levels of OSA patients only after one day of CPAP therapy might be misleading. So we decided to wait three months under CPAP therapy and then compare the blood melatonin levels.
PATIENTS AND METHODS
Subjects
All persons studied gave written informed consent, the study protocol was approved by the local ethics committee.
Ten patients (n=10, male) with newly diagnosed symptomatic obstructive sleep apnea syndrome and apnoea/hypopnoea index (AHI >10/h and Epworth sleepiness scale (ESS) >10 points) were enrolled in the study. In order to ensure and quantify the severity of the OSAS a diagnostic polysomnography in the sleep laboratory was performed as a first step. The AHI as well as the daytime sleepiness were assessed. Before diagnostic measurement subjects underwent a complete medical history, clinical chemistry and physical examination to rule out diabetes, active infections, hepatitis, cancer or other serious medical conditions. None of the patients or control subjects had any significant comorbidities that may have affected their sleep-wake behaviour or circadian system. Hypertension was present in 6 patients and asthma in 1 patient (very mild form, no need for medicaments since 3 years, no symptoms since 3 years, no reported sleep disturbance). Hypertension was treated with a single or combined therapy of calcium antagonists, diuretics and ACE inhibitors, patients on b-blockers were excluded. Due to their possible effects on melatonin metabolism patients with anti-depressive, anti-epileptic or sedative medication were also excluded of the study as those with an active abuse of alcohol.
Inclusion criteria: middle-aged men and women (58.9±10.2 years) with symptoms of suggestive OSAS i.e. daytime sleepiness, snoring, breathing interruptions reported by partners, and ESS >10 points. They should have an apnoen/hypopnoea index >10/h and therefore an indication for CPAP therapy. No severe comorbidity was reported.
Exclusion criteria: already CPAP treated patients, patients with severe comorbidities which might have influenced the circadian system or sleep-wake behaviour. Patients with abnormal kidney or liver function, insomnia, infectious or endocrinous disease. Also patients with active alcohol abuse were excluded.
Additionally to the routine clinical chemistry the hormone melatonin was measured in four hour intervals (2, 6, 10 a.m. and 2, 6, 10 p.m.) including the night of diagnostic polysomnography. The dedicated blood samples for omentin 1 were taken in between, at 0, 8 a.m. and 4 p.m.. Normal sleep-wake rhythms were retained, the average sleep time duration lay between six and eight hours. All patients and controls reported the same sleep and wake rhythms (sleeping time from 23.00–24.00 h to 6.00-7.00 h the next day). The patients were accommodated in noise and light shielded single rooms (<50 lux during sleep). They all had breakfast between 7.00 and 8.00 a.m. Breakfast (as well as lunch and dinner) was standardized. Breakfast for example contained two bread rolls with butter, jam, a slice of ham and a pot of coffee. During hospital stay (at daytime) the patients mostly remained in their rooms, intensive physical activity was forbidden, to go out for a walk (2 hours) was allowed.
Blood drawings were performed throughout an indwelling superficial forearm catheter especially to do not disturb the sleep. During taking blood samples the light was not turned on; a pocket lamp was used. In the daytime blood withdrawings were performed in sedentary posture, during night in horizontal posture.
nCPAP titration and therapy
The following night was used to establish a sufficient nCPAP therapy with an individually different pressure. Manual titration was started from an initial pressure level of 5 mbar and elevated according to further ocurring events of snoring, hypopnea or apnea. The pressure was increased in steps of 0.5 mbar under polysomnographic control by an experienced sleep laboratory technician at intervals of at least 10 minutes.
Before demission with the minimal effective pressure (11.2±2.0 mbar) each patient was instructed to use the nCPAP therapy regularly each night for at least six hours to ensure a therapeutic effect. In different studies adherence is defined up to 4 to 5 hours of CPAP therapy nightly use (48). This duration time is ment to be (at least in part) effective to ameliorate OSA symptoms such as daytime sleepiness (ESS), daily, cardiometabolic and emotional functioning, social interactions and life quality in general (49, 50). Also mortality rates decrease under CPAP treatment in OSA patients with moderate or high adherence (>6 hours of usage) (51).
All patients had a return visit after four weeks of CPAP treatment during which time CPAP use was reviewed and problems in adherence were addressed. Furthermore patients were encouraged to call the research coordinator at any time during the study period if they experienced problems with the therapy, equipment or mask fitting.
After three months of therapy all examinations were repeated (polysomnography under nCPAP therapy, questionnaire, clinical chemistry and measurements of hormones/adipokines) und compared with the initial results. To control CPAP adherence we collected and downloaded data from CPAP device including hours of use. So we could visualize the average CPAP usage per night (5.40±0.59 h).
As a control group for melatonin ten healthy unrelated volunteers (n=9 male and n=1 female) with no sleep disorder were recruited. As melatonin levels are age-dependend we tried to match probands and patients regarding age (53.6±7.7 years versus 58.9±10.2 years, p>0.05). Another eight probands (n=2 male and n=6 female) for analysing omentin-1 plasma levels were also obtained for the study, this was necessary because of the lack of residual material of the initial control group. In analogy to the ten patients with OSAS they underwent the same examinations (clinical chemistry, questionnaire etc.). To exclude subjects with OSAS the volunteers were measured under study conditions with Apnoe Screen (ApnoeScreen Pro, viaSYS Healthcare GmbH, Hochberg, Germany). Following variables were recorded: nasal airflow measured by oronasal thermistors, snoring detected by a microphone, oxyhaemoglobin saturation and pulse using a finger oxymeter and absolute position transducer. Finally, a comparison of the data of the volunteers and the patients regarding circadian rhythm of melatonin and omentin-1 was made.
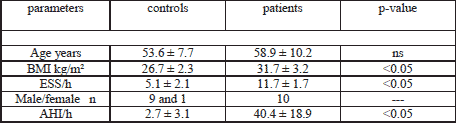
The detailed characteristics of the healthy controls and the OSA patients are given in Table 1.
Samples
The samples were collected in ethylendiamine tetraacetate-coated polypropylene tubes, centrifuged immediately at 3.000 rpm for 20 min at 0°C, and the clear plasma supernatant was then stored at –80°C until plasma melatonin and omentin-1 levels were measured as follows:
Measurement of serum melatonin
Plasma melatonin concentration was determined using human RIA kit (RIA-3972, DRG Instruments GmbH, Marburg, Germany) as described previously (52). The cross-reactivity of the antiserum used in the assay showed negligible cross-reactivity with any product related to the melatonin metabolism such as serotonin (<0.01), DL-tryptophan, DL-methoxytryptophan (<0.01), 5-methoxytryptamine (0.08) or N-acetylserotonin (0.08). The detection limit for melatonin was 2 pg/mL, and the intra- and interassay variations were CV 12.1-12.3% and 12.3-16.2% respectively.
Measurement of serum omentin-1
Plasma omentin-1 levels were measured by a commercially available ELISA kit (Human Ometin ELISA kit, Enzo Life Sciences GmbH, Lorrach, Germany). The sensitivity ranges from 0.5 to 32 ng/ml. All samples and standards were analysed in duplicate within the same assay.
Sleep studies
The polysomnographies were performed according to the recommendations of the American Thoracic Society (53) and the German Sleep Society (54, 55). Sleep parameters were determined using the criteria of Rechtschaffen and Kahles (56) and microarousals were defined in accordance with the definitions of the American Sleep Disorders Association (ASDA) (57). All variables were recorded on a computer (SleepLabTM, Jaeger amd Toennies, Wurzburg, Germany). These data included submental electromyography, snoring detected by a microphone, electrocardiography, thoracic and abdominal movements, bilateral electrooculography, electroencephalography, nasal airflow measured by oronasal thermistors and nasal canulas during diagnostic polysomnographics and by an pneumotachograph during CPAP studies and oxyhaemoglobin saturation using a finger oxymeter (Microspan 3040GTM, Jaeger and Toennies, Wurzburg, Germany). The polysomnographic measurements were analyzed manually by an experienced sleep lab technician.
Statistical analysis
The statistical calculations were performed by using Microsoft Exel. Sleep variables and hormone measures were compared before and after CPAP treatment by the paired Student's t test. All reported p values are two sided with significance set at p<0.05. All the quantitative variables are expressed as mean ±S.D.
RESULTS
In contrast to daytime sleepiness (ESS 11.7±1.7 versus 5.1±2.1 points, p<0.05), BMI (31.7±3.2 versus 26.7±2.3 kg/m2, p<0.05) and AHI (40.4±18.9 versus 2.7±3.1/h, p<0.05) the patients and controls did not differ significantly regarding age (58.9±10.2 versus 53.6±7.7, p>0.05). From the 10 patients at the beginning 3 refused CPAP therapy in the first 4 weeks due to discomfort. The other 7 subjects used the therapy as initially instructed (at least 6 hours per night) and were controlled after 3 months. The CPAP treatment significantly reduced the previously observed obstructions. In consequence the initial AHI was normalized (42.1±16.2 versus 4.7±6.0 /h, p<0.05) and the mean SaO2 increased (91.2±4.1 versus 94.6±1.7%, p<0.05). Analogous the subjective sleepiness was ensured (ESS 11.8±1.8 versus 5.0±2.0 , p<0.05).
Melatonin
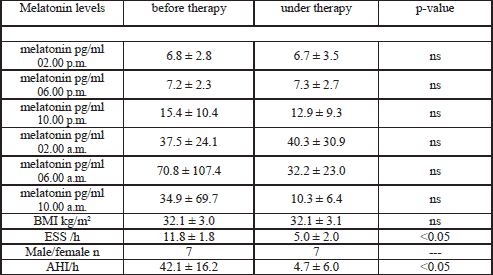
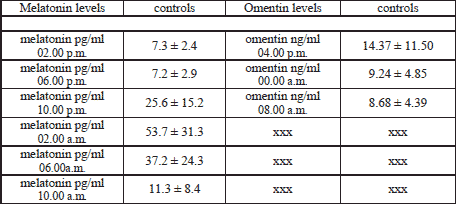
The plasma melatonin levels measured at 2, 6, 10 a.m. and 2, 6, 10 p.m. did not differ significantly in the 3 analysed study groups (controls, patients before and under therapy). But by interest the newly diagnosed patients showed a later melatonin peak as the volunteer group. Melatonin peaked in the patients group at 6.00 a.m., whereas the healthy volunteers at 2.00 a.m.. After three months of CPAP therapy the patients developed an equal melatonin rhythm as the controls (Table 2 and Fig. 1).
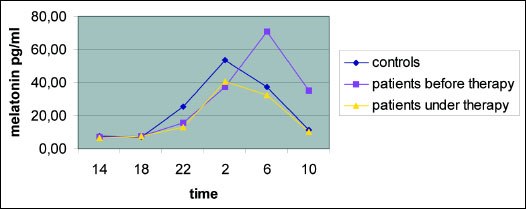 |
Fig. 1. Plasma melatonin levels of the patients and the controls before and under CPAP therapy. Blue - controls; yellow - patients under three months of CPAP therapy; pink - patients at diagnosis without any therapy; initially patients showed a later melatonin peak at 6 a.m. in contrast to controls (they peaked at 2 a.m.); this partially normalized under CPAP therapy. |
Omentin-1
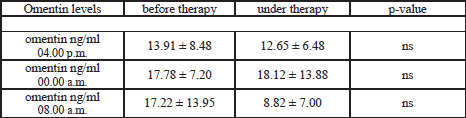
The control group showed significant lower plasma omentin-1 levels in comparison to the untreated patient group regarding 0 and 8 a.m. (9.24±4.85 respectively 8.68±4.39 versus 17.78±7.20 respectively 17.22±13.95 ng/ml, p<0.05). After three months of CPAP treatment the plasma omentin-1 level of the patient group was similar to the probands at 8 a.m. (Fig. 2) (8.82±7.00 versus 8.68±4.39 ng/ml, p>0.05).
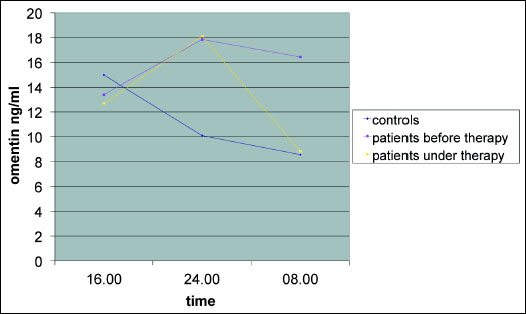 |
Fig. 2. Plasma omentin-1 levels (ng/ml) of the patients and the controls before and under CPAP therapy. Blue - controls; yellow - patients under three months of CPAP therapy; pink - patients at diagnosis without any therapy; patients at the beginning showed partially higher omentin-1 levels than controls; this observation "normalized" under CPAP at 8 a.m. |
DISCUSSION
The incidence of obesity and therefore the incidence of the obstructive sleep apnea syndrome is rising rapidly in industrialized and developing countries. Since many years continuous positive airway pressure therapy (CPAP) remains the golden standard in therapy. While weight loss has undoubtedly a positive therapeutic effect, each OSA patient is instructed to try to loose adequately weight to improve this disease pattern.
As several adipokines are associated with obesity, and obesity itself is related to the obstructive sleep apnea syndrome, we analysed in this context omentin-1. For so long, recent studies suggest that omentin may play a protective role in coronary atherosclerosis and other obesity-related cardiovascular disorders (37).
Very recently the first study to omentin-1 and OSAS was also published. In this context a comparison of omentin levels in native OSA patients and healthy controls was made. A CPAP therapy was not yet established. The data showed decreased omentin levels in the patient group, the values of the healthy volunteers were clearly higher (42). In addition Auguet et al. could show that omentin-1 plasma levels were decreased in morbidly obese women (BMI >40) in comparison to healthy controls (1.97±2.15 ng/ml) (19).
In contrast to these findings our obese OSA patient collective has higher omentin plasma levels than the normal weight control group. After three months of CPAP therapy they partially normalized showing comparable values as the controls at 08.00 a.m. However, at midnight the plasma levels of omentin were still higher than in the control group. Our findings indicate that the obstructive sleep apnoea syndrom is associated with a significantly increased plasma level of omentin. The reason why our obese OSA collective showed the increased levels in contrast to the other available study is not clear. A possible explanation for our controversial findings might be the presence of nonalcoholic fatty liver disease (NASH) in our patients. It is already known that OSA patients show a high prevalence of NASH since it is postulated that intermittent hypoxia present in OSAS may play a role in the NASH pathogenesis (58). Turkay at al. (59) for example reported a presence of NASH of 66% in an OSAS collective. Conversly, OSAS was present in 71% of NASH patients. Other data revealed NASH in OSA patients up to scarce 70% (60). This hypothesis is supported by Yilmaz et al. (61) who showed elevated omentin levels in patients with biopsy-proven nonalcoholic fatty liver disease. In the present study we cannot verify that our OSAS patients suffered from NASH, because we did not perform an ultrasound in this study by routine.
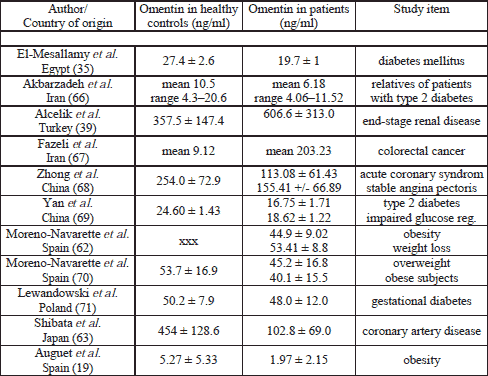
Similar to Auguet et al. the plasma omentin levels of the lean control group were 8.68±4.39 ng/ml at the morning (Auguet demonstrated mean plasma omentin levels of 5.27±5.33 ng/ml). It is noteworthy that in various studies quite different levels of omentin-1 in the healthy control group were described (the range lay in between 5 to 454 ng/ml) (19, 35, 62, 63) As we recognized this fact, we conducted a intensive literature search in Pubmed, Table 5 reflects the findings more in detail.
In the end perhaps we have to challenge the results to omentin in general. Dependent of the omentin plasma values in a so-called healthy group (and there is a great margin of deviation) the cited studies sometimes show an increase or a decrease of omentin in the patient group. The values were mostly not matchable although the same enzyme and the same examination method (ELISA) was used. So, perhaps we have to admit that omentin is influenced by such a great number of different diseases and circumstances that we cannot easily interprete it's values in a certain collective? Maybe a comparison is only possible in the same group of people observed over a determined period of time? So once again more data regarding this problem is needed.
As other studies before we also analysed melatonin in OSA patients. Althought melatonin plasma levels of the volunteer and patient group (neither at diagnosis nor under three months CPAP therapy) did not show significant differences. As a trend we could demonstrate that the newly diagnosed OSA patients had their melatonin peak later at 6.00 a.m in comparison to a 2.00 a.m. peak of the healthy control group. Similar to our data, Brzecka et al. (64) observed in an OSA collective in 24% of patients a prolonged peak melatonin secretion to early morning hours. Thus, in this study, in most of patients (66%) there was a peak melatonin excretion at 2.00 a.m. (as in a normal collective). Also analogous to our results, another study proved the highest melatonin levels of OSA patients at 6.00 a.m (8).
In contrast to these findings Wilkner at al. (65) could not demonstrate differences between melatonin levels in OSAS patients before and after CPAP. The interval between the two measurement points and established CPAP therapy was only four weeks. So maybe this was not enough CPAP duration time for a sufficient and reproducable change in melatonin plasma levels. Ulfberg et al. (1) showed that OSA patients presented higher melatonin levels in the afternoon than control subjects without any sleep disorder breathing. An observation which we cannot confirm regarding our data.
In conclusion our data indicate that: 1) omentin-1 seems to play a role in OSAS; 2) OSA patients demonstrate an altered circadian rhythm of melatonin showing a delayed peak of this hormone in the night toward the morning hours. However, the exact role of melatonin should be analysed in further larger prospective studies.
Without doubt there are several limitations of our study: we had a quite small study collective, so additional studies should be performed to verify the data; we do not have a reasonable explanation for the higher omentin-1 levels in the patient group in comparison the the healthy probands; the discussed NASH is only a hypothesis and not proven by our data; in addition it must be supposed that there are many more influence factors in omentin plasma levels as assumed up to now regarding the wide range of omentin values in so-called healthy subjects (also in other studies). As omentin is expressed by the visceral (omental) and not subcutanous fat we should have performed measurements of the waist circumference as well. We had a relative high standard deviation of our values, as other studies to omentin before; therefore again further studies are needed.
Conflict of interests: None declared.
REFERENCES
- Ulfberg J, Micic S, Strom J. Afternoon serum-melatonin in sleep disordered breathing. J Intern Med 1998; 244 : 163-168.
- Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, Llamas-Carreras JM, Solano-Reina E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med Oral Patol Oral Cir Bucal 2012; 17: e925-e929.
- Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010; 7: 677-685.
- Hecht L, Mohler R, Meyer G. Effects of CPAP-respiration on markers of glucose metabolism in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Ger Med Sci 2011; epub 2011 Aug 8. doi: 10.3205/000143.
- Mahboub B, Afzal S, Alhariri H, Alzaabi A, Vats M, Soans A. Prevalence of symptoms and risk of sleep apnea in Dubai, UAE. Int J Gen Med 2013; 6: 109-114.
- Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010; 11: 441-446.
- Franco CM, Lima AM, Ataide L Jr., et al. Obstructive sleep apnea severity correlates with cellular and plasma oxidative stress parameters and affective symptoms. J Mol Neurosci 2012; 47: 300-310.
- Hernandez C, Abreu J, Abreu P, Castro A, Jimenez A. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J 2007; 30: 496-500.
- Alves ED, Ackel-D'Elia C, Luz GP, et al. Does physical exercise reduce excessive daytime sleepiness by improving inflammatory profiles in obstructive sleep apnea patients? Sleep Breath 2013; 17: 505-510.
- Dempsey JA, Veasey SC, Morgan BJ, O´Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010; 90: 47-112.
- Bulcun E, Ekici M, Ekici A. Disorders of glucose metabolism and insulin resistance in patients with obstructive sleep apnoea syndrome. Int J Clin Pract 2012; 66: 91-97.
- Tamura A, Kawano Y, Watanabe T, Kadota J. Relationship between the severity of obstructive sleep apnea and impaired glucose metabolism in patients with obstructive sleep apnea. Respir Med 2008; 102: 1412-1416.
- Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J 2005; 25: 514-520.
- Lacasse Y, Godbout C, Series F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J 2002; 19: 499-503.
- Quercioli A, Mach F, Montecucco F. Inflammation accelerates atherosclerotic processes in obstructive sleep apnea syndrome (OSAS). Sleep Breath 2010; 14: 261-269.
- Salvador J, Iriarte J, Silva C, Gomez Ambrosi J, Diez Caballero A, Fruhbeck G. The obstructive sleep apnoea syndrome in obesity: a conspirator in the shadow. Rev Med Univ Navarra 2004; 48: 55-62.
- Nerfeldt P, Nilsson BY, Mayor L, Udden J, Friberg D. A two-year weight reduction program in obese sleep apnea patients. J Clin Sleep Med 2010; 6: 479-486.
- Shibata R, Ouchi N, Takahashi R, et al. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr 2012; 4: 37.
- Auguet T, Quintero Y, Riesco D, et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet 2011; 12: 60.
- Kalisz M, Baranowska B, Bik W. Do novel adipokines play a causative or only modulating role in the pathogenesis of obesity and metabolic disorders? Neuro Endocrinol Lett 2012; 33: 11-55.
- Castan-Laurell I, Dray C, Attane C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine 2011; 40: 1-9.
- Declercq V, Enns J, Yeganeh A, Taylor C, Zahradka P. Modulation of cardiovascular function by adipokines. Cardiovasc Hematol Disord Drug Targets 2012; 13: 59-72.
- Feng X, Li P, Zhou C, Jia X, Kang J. Elevated levels of serum chemerin in patients with obstructive sleep apnea syndrome. Biomarkers 2012; 17: 248-253.
- Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit 2011; 17: CR159-CR164.
- Trakada G, Steiropoulos P, Nena E, et al. Plasma visfatin levels in severe obstructive sleep apnea-hypopnea syndrome. Sleep Breath 2009; 13: 349-355.
- Sanchez-de-la-Torre M, Mediano O, Barcelo A, et al. The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath 2012; 16: 649-656.
- Zhou JY, Chan L, Zhou SW. Omentin: linking metabolic syndrome and cardiovascular disease. Curr Vasc Pharmacol 2012; epub. Jun 22.
- Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med 2012; 20: 143-148.
- Ismail SA, Mohamed SA. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br J Dermatol 2012; 167: 436-439.
- Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 2006; 290: E1253-E1261.
- Shibata R, Ouchi N, Kikuchi R, et al. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis 2011; 219: 811-814.
- de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007; 56: 1655-1661.
- Brunetti L, Nisio C, Recinella L, et al. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides 2011; 32: 1866-1871.
- Choi JH, Rhee EJ, Kim KH, Woo HY, Lee WY, Sung KC. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur J Endocrinol 2011; 165: 789-796.
- El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med 2011; 28: 1194-1200.
- Yoo HJ, Hwang SY, Hong HC, et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol 2011; 10: 103.
- Shang FJ, Wang JP, Liu XT, et al. Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers 2011; 16: 657-662.
- Cantarini L, Simonini G, Fioravanti A, et al. Circulating levels of the adipokines vaspin and omentin in patients with juvenile idiopathic arthritis, and relation to disease activity. Clin Exp Rheumatol 2011; 29: 1044-1048.
- Alcelik A, Tosun M, Ozlu MF, et al. Serum levels of omentin in end-stage renal disease patients. Kidney Blood Press Res 2012; 35: 511-516.
- Kazama K, Usui T, Okada M, Hara Y, Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-alpha-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol 2012; 686: 116-123.
- Northcott JM, Yeganeh A, Taylor CG, Zahradka P, Wigle JT. Adipokines and the cardiovascular system: mechanisms mediating health and disease. Can J Physiol Pharmacol 2012; 90: 1029-1059.
- Wang Q, Feng X, Zhou C, Li P, Kang J. Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Ann Clin Biochem 2013; 50: 230-235.
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 2011; 62: 139-150.
- Stehle JH, Saade A, Rawashdeh O, et al., A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res 2011; 51: 17-43.
- Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol 2011; 62: 13-19.
- Rastmanesh R, de Bruin PF. Potential of melatonin for the treatment or prevention of obesity: an urgent need to include weight reduction as a secondary outcome in clinical trials of melatonin in obese patients with sleep disorders. Contemp Clin Trials 2012; 33: 574-575.
- Anton-Tay F, Diaz JL, Fernandez-Guardiola A. On the effect of melatonin upon human brain. Its possible therapeutic implications. Life Sci I 1971; 10: 841-850.
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008; 5: 173-178.
- Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas Cl, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath 2012; 16: 563-569.
- Tasali E, Chapotot F, Leproult R, Whitmore H, Ehrmann DA. Treatment of obstructive sleep apnea improves cardiometabolic function in young obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2011; 96: 365-374.
- Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128: 624-633.
- Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M. The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J Physiol Pharmacol 2010; 61: 577-580.
- Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. American Thoracic Society. Official statement adopted March 1944. Am J Respir Crit Care Med 1994; 150: 1738-1745.
- Penzel T, Hajak G, Hoffmann RM, et al. Empfehlungen zur Durchführung und Auswertung polygraphischer Ableitungen im diagnostischen Schlaflabor. EEG-EMG 1993; 24: 65-70.
- Ficker JH, Wiest GH, Lehnert G, Wiest B, Hahn EG. Evaluation of an auto-CPAP device for treatment of obstructive sleep apnoea. Thorax 1998; 53: 643-648.
- Rechtschaffen A, Kahles A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service, US Government Printing Office 1968.
- Bonnet M, Carley D, Carskadon M, et al. EEG arousals: scoring rules and examples. Sleep 1992; 15: 174-184.
- Souza MR, Diniz Mde F, Medeiros-Filho JE, Araujo MS. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol 2012; 49: 89-96.
- Turkay C, Ozol D, Kasapoglu B, Kirbas I, Yildirim Z, Yigitiglu R. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respir Care 2012; 57: 244-249.
- Musso G, Olivetti C, Cassader M, Gambino R. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis 2012; 32: 49-64.
- Yilmaz Y, Yonal O, Kurt R, et al. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol 2011; 46: 91-97.
- Moreno-Navarrete JM, Catalan V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 2010; 7: 27.
- Shibata R, Ouchi N, Kikuchi R, et al. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis 2011; 219: 811-814.
- Brzecka A, Piesiak P, Zareba-Bogdal E, Zierkiewicz G, Plamieniak Z. Rhythm of melatonin excretion in obstructive sleep apnea syndrome (in Polish). Pneumonol Alergol Pol 2001; 69: 650-654.
- Wikner J, Svanborg E, Wetterberg L, Rojdmark S. Melatonin secretion and excretion in patients with obstructive sleep apnea syndrome. Sleep 1997; 20: 1002-1007.
- Akbarzadeh S, Nabipour I, Assadi M, et al. The normoglycemic first-degree relatives of patients with type 2 diabetes mellitus have low circulating omentin-1 and adiponectin levels. Cytokine 2012; 58: 295-299.
- Fazeli MS, Dashti H, Akbarzadeh S, Assadi M, et al. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine 2013; 62: 81-85.
- Zhong X, Zhang H, Tan H, et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin 2011; 32: 873-878.
- Yan P, Liu D, Long M, Ren Y, Pang J, Li R. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2011; 119: 257-263.
- Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernandez-Real JM. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity 2011; 19: 1552-1559.
- Lewandowski K, Nadel I, Lewinski A, et al. Positive correlation between serum omentin and thrombospondin-1 in gestational diabetes despite lack of correlation with insulin resistance indices. Ginekol Pol 2010; 81: 907-912.
A c c e p t e d : June 26, 2013